Published online Mar 7, 2011. doi: 10.3748/wjg.v17.i9.1174
Revised: October 12, 2010
Accepted: October 19, 2010
Published online: March 7, 2011
AIM: To assess the long-term efficacy of seton drainage with infliximab maintenance therapy in treatment of stricture for perianal Crohn’s disease (CD).
METHODS: Sixty-two patients with perianal CD who required surgical treatment with or without infliximab between September 2000 and April 2010 were identified from our clinic’s database. The activities of the perianal lesions were evaluated using the modified perianal CD activity index (mPDAI) score. The primary endpoint was a clinical response at 12-15 wk after surgery as a short-term efficacy. Secondary endpoints were recurrence as reflected in the mPDAI score, defined as increased points in every major element. The clinical responses were classified as completely healed (mPDAI = 0), partially improved (mPDAI score decreased more than 4 points), and failure or recurrence (mPDAI score increased or decreased less than 3 points).
RESULTS: There were 43 males and 19 females, of whom 26 were consecutively treated with infliximab after surgery as maintenance therapy. Complete healing was not seen. Failure was seen in 10/36 (27.8%) patients without infliximab and 4/26 (15.4%) patients with infliximab (P = 0.25). Partial improvement was seen in 26/36 (72.2%) patients without infliximab and 22/26 (88.5%) patients with infliximab (P = 0.25). Short-term improvement was achieved in 48/62 (77.4%) patients. Although the mPDAI score improved significantly with surgery regardless of infliximab, it decreased more from baseline in patients with infliximab (50.0%) than in those without infliximab (28.6%), (P = 0.003). In the long-term, recurrence rates were low regardless of infliximab in patients without anorectal stricture. In patients with anorectal stricture, cumulative recurrence incidences increased gradually and exceeded 40% at 5 years regardless of infliximab. No efficacy of infliximab treatment was found (P = 0.97). Although the cumulative rate of ostomy creation was also low in patients without stricture and high in patients with stricture, no protective efficacy was found with infliximab treatment (P = 0.6 without stricture, P = 0.22 with stricture).
CONCLUSION: Infliximab treatment was demonstrated to have short-term efficacy for perianal lesions. Long-term benefit with infliximab was not proven, at least in patients with anorectal stricture.
- Citation: Uchino M, Ikeuchi H, Bando T, Matsuoka H, Takesue Y, Takahashi Y, Matsumoto T, Tomita N. Long-term efficacy of infliximab maintenance therapy for perianal Crohn’s disease. World J Gastroenterol 2011; 17(9): 1174-1179
- URL: https://www.wjgnet.com/1007-9327/full/v17/i9/1174.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i9.1174
In recent years, infliximab (IFX), an IgG1 murine-human chimeric monoclonal antibody against tumor necrosis factor-alpha, has been recognized to be an effective treatment for active perianal fistula with Crohn’s disease (CD)[1]. However, in the ACCENT II trial, the closure of fistulas after the initial three IFX infusions occurred in 63% of patients at week 14, but decreased to 36% at week 54 with every 8 wk maintenance therapy and a high abscess formation rate (15%) was also seen[2].
The placement of setons and drains prior to IFX therapy is generally recognized as the best combination therapy, and it allows for control of fistula healing and prevention of abscess formation[3]. However, the efficacy of IFX with surgical treatment in perianal CD is controversial, and mainly short-term efficacy has been suggested[4,5]. Moreover, most lesions of perianal CD are complicated by anorectal stricture that could affect the high recurrence rate and the risk for further ostomy creation. Although a further, large and long-term prospective study is needed for proper evaluation, the aim of this study was to assess the short and long-term efficacy of seton drainage in relation to IFX maintenance therapy with or without anorectal stricture for perianal CD retrospectively in advance of a longer duration prospective study.
The clinical records of 131 patients with a perianal CD lesion who required surgical treatment between September 1995 and April 2010 were reviewed retrospectively. The surgical indications for all patients were limited to only a perianal lesion. Of these, 62 patients were identified from our out patients clinical records that were appreciable in detail of symptoms for assigning a modified perianal CD activity index (mPDAI) score, and these demographics and relevant medical details were recorded retrospectively[6].
Patients on concomitant therapy that included corticosteroid and immunosuppressive agents were excluded from this study. Patients who did not use these agents postoperatively or used them after recurrence were included. Most patients were treated with oral antibiotics, including metronidazole, ciprofloxacin, or cefcapene pivoxil hydrochloride hydrate, before surgery as conservative treatment and after surgery as combination therapy. All patients were given a second-generation cefem intraoperatively.
Fistulas were classified by the method described by Parks et al[7]. At surgery, fistula tracts were thoroughly curetted, the abscess cavities were sufficiently drained and rubbed with a surgical spoon. Soft silastic or Teflon-coated vessel tape was inserted along the fistula tracts and tied loosely to facilitate drainage where appropriate as seton drainage. Anal dilation with the index finger was also performed in patients with stricture at surgery. Setons were removed in the outpatient clinic if the infection resolved on MRI findings and left in place if there were continuing signs of infection. The patients with incidental lesions as superficial fistulas were treated with conventional fistulotomy and excluded from this study.
Initial IFX infusions (5 mg/kg) were administered within 2 wk from seton drainage (0 wk), and consecutive infusions were administered 2 wk and 6 wk later. This was continued as maintenance therapy every 8 wk. The patients who were given episodic infusions or could not be maintained on consecutive infusions due to adverse effects were not included in this study. Anal dilatation was performed in all patients with anorectal stricture preceding initial IFX infusion.
The activities of perianal lesions were evaluated using the perianal CD activity index (PDAI)[6]. The PDAI score is a 5-point index that detects changes in perianal status. The five major elements were the degrees of discharge, pain or restriction of living activity, restriction of sexual activity, type of perianal disease, and induration. Scores ranged from 0 to 20, with higher scores indicating more severe disease activity. Since the mPDAIs were reviewed from clinical records retrospectively in this study, the element of sexual function was not included, and the score ranged from 0 to 16.
The primary endpoint was a clinical response at 12-15 wk after surgery. The effective response was defined as at least a one point or greater reduction in every major element from baseline at week 12 as a short-term efficacy (a decrease of more than 4 points on the mPDAI score). Secondary endpoints were recurrence as reflected in the mPDAI score, defined as increased points in every major element (an increase of more than 4 points). The clinical responses were classified as completely healed (mPDAI = 0), partially improved (mPDAI score decreased more than 4 points), and failure (mPDAI score increased or decreased less than 3 points). Anorectal stricture was defined as the forefinger could not pass through during digital examination due to fibrotic changes.
Unless otherwise indicated, all numerical data are expressed as the median and range. Differences in patient’s characteristics and mPDAI improvement rates with or without IFX were analyzed using the χ2 test or the Mann-Whitney U-test. Evaluation of the change in mPDAI score was analyzed using Wilcoxon’s signed rank test. The cumulative maintained response rates or ostomy creation rates were compared using a log-rank test with Kaplan-Meier analysis. All statistical analyses were performed with SPSS for Windows, version 11.0.1. Two-tailed P values less than 0.05 were considered to indicate statistically significant differences.
Sixty-two patients who underwent surgery for perianal CD could be followed up at our outpatient clinic consecutively. There were 43 males and 19 females, and their median age at surgery was 27.0 (12-58) years. Of these, 26 patients were treated with IFX after surgery as maintenance therapy from April 2004. Patients’ characteristics are shown in Table 1. There was a high frequency of patients with anorectal stricture with or without IFX. Adverse events occurred in 7 patients, with infusion reactions as minor events. No major events related to IFX were seen in this series.
| Surgery + infliximab (n = 26) | Surgery alone (n = 36) | P value | |
| Male:Female | 16:10 | 27:9 | 0.26 |
| Age at initial surgery (median years and range) | 27.5 (16-55) | 27.5 (16-41) | 0.73 |
| Duration from onset of perianal lesion (median month and range) | 45 (2.5-275.5) | 120 (26.5-339.5) | < 0.01 |
| Duration from initial surgery for perianal lesion | 28.2 (0.57-62.4) | 74.5 (7.4-218.9) | < 0.01 |
| (median month and range) | |||
| Types of fistula | |||
| Intrasphincteric | 7 (26.9) | 4 (11.1) | 0.11 |
| Transsphincteric | 12 (46.2) | 16 (44.4) | 0.89 |
| Suprasphincteric | 4 (15.4) | 8 (22.2) | 0.5 |
| Extrasphincteric | 3 (11.5) | 8 (22.2) | 0.28 |
| Anorectal stricture | 19 (73.1) | 21 (58.3) | 0.23 |
| Current smoking | 8 (30.8) | 16 (44.4) | 0.28 |
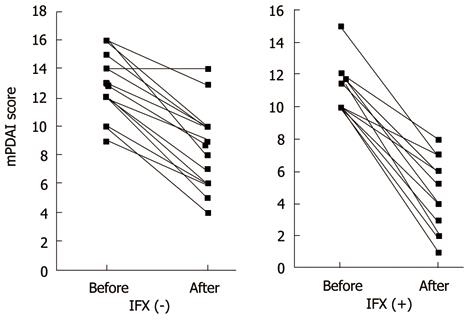
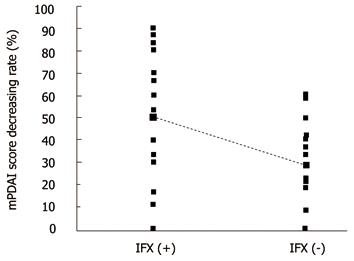
Complete healing was not seen in any patients. Failure was seen in 10/36 (27.8%) patients without IFX and 4/26 (15.4%) patients with IFX (P = 0.25). Partial improvement was seen in 26/36 (72.2%) patients without IFX and 22/26 (88.5%) patients with IFX (P = 0.25). Short-term improvement was achieved in 48/62 (77.4%) patients. The mPDAI scores improved significantly with surgery regardless of IFX treatment: from 13.5 (9-16) before surgery to 9.5 (4-14) after surgery without IFX; from 11 (8-15) before surgery to 6 (1-10) with IFX (P < 0.01) (Figure 1). The rates of improving mPDAI score from baseline were greater in patients with IFX - 50.0% (0-90%) - than in those without IFX -28.6% (0%-60%); (P = 0.003) (Figure 2).
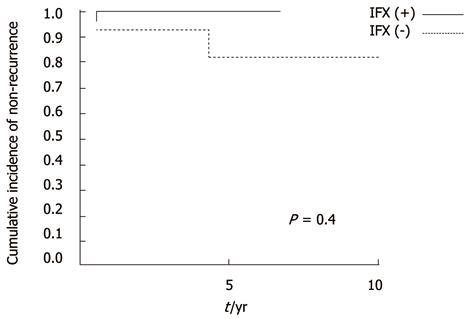
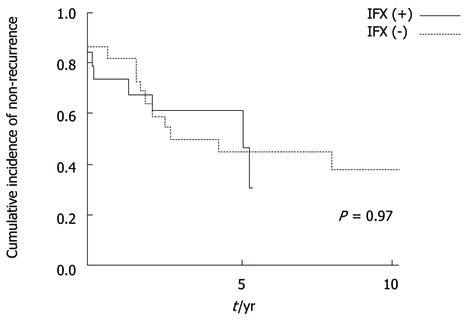
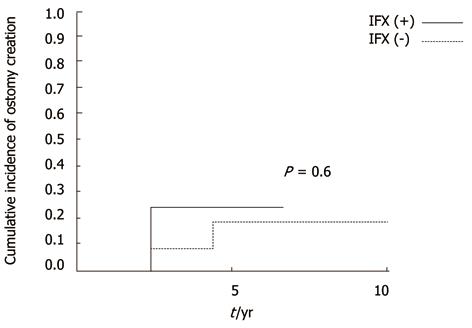
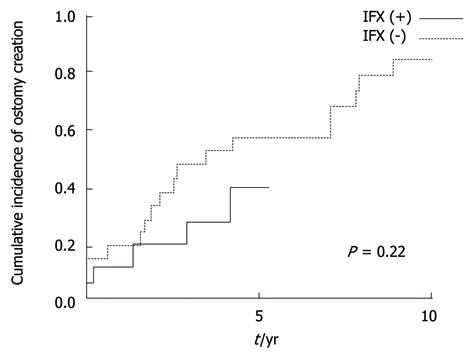
In the long-term, recurrences were low regardless of IFX in patients without anorectal stricture (not significant, P = 0.4) (Figure 3). However, in patients with stricture, cumulative recurrence incidences increased gradually and exceeded 40% at 5 years in both groups (Figure 4). IFX treatment was not found to be effective (P = 0.97). Although the cumulative rate of ostomy creation was also low in patients without stricture and high in patients with stricture, no protective efficacy was found with IFX treatment (P =0.6 without stricture, P = 0.22 with stricture) (Figures 5 and 6).The patients’ present statuses are shown in Table 2. Of these, 12 patients required abdominoperineal resection (APR), and 14 patients required Hartmann’s procedure. There were no significant differences in rates and duration from initial surgery to ostomy creation with or without IFX treatment.
| IFX (-) | IFX (+) | P value | |
| APR | 9/36 | 3/26 | 0.19 |
| Duration from initial surgery to APR, median yr (range) | 2.5 (0-4.3) | 3.9 (1.0-4.1) | 0.79 |
| Hartmann's procedure | 10/36 | 4/26 | 0.25 |
| Duration from initial surgery to Hartmann, median yr (range) | 2.3 (0-7.0) | 0.7 (0-2.9) | 0.22 |
Two patients without IFX treatment were diagnosed as having carcinoma in anorectal fistula and required APR. They had an 8.1- and 10.2-year history of perianal CD, respectively. Both cases demonstrated highly advanced disease and recurred within 2 years after APR.
CD is a chronic inflammatory bowel disease of unknown etiology, characterized by fissuring ulcers and segmental transmural inflammation of the gastrointestinal tract. Perianal fistulas are a frequent manifestation of CD, and the prevalence ranges from 20% to 50%[8]. Medical approaches, with metronidazole, ciprofloxacin, azathioprine, or 6-mercaptopurine, and surgical approaches have been involved in combination therapy[9-11]. These therapies have been suggested to have a short-term benefit. Furthermore, management with loose setons, advancement flaps, fibrin glue, or collagen plugs has been reported to have a short-term benefit[12]. Though the conventional fistulotomy is generally avoided for recurrence and fecal incontinence, surgical treatment with loose setons has shown good results[13,14]. However, although the ideal goal for treatment of perianal CD lesion is fistula closure and prevention of recurrence, diversion with ostomy creation or APR might often be necessary in patients with refractory lesions.
Recently, IFX treatment has become well established. However, its efficacy for perianal CD has been controversial. Gaertner et al[4] reported that surgery for perianal CD resulted in complete healing in approximately 60% of patients regardless of the use of IFX. However, the time to healing was shorter and the healing rate was improved with surgery plus IFX. Hyder et al[5] also showed the efficacy of IFX in combination with surgery in 85% of cases as measured by PDAI. Although both studies evaluated the short-term efficacy of IFX plus surgery, the medium- or long-term results are less impressive. Also in Japan, IFX in combination with surgery has been recognized as the one of the main treatments for perianal CD, although the results are controversial. Although there are many variables and confounding factors that were not controlled in this retrospective study such as concomitant antibiotics administration, surgery plus IFX treatment was demonstrated to have a short-term benefit compared to surgery alone in improving the rate of PDAI. It is considered that IFX has additional effects which include a greater decrease in the degree of discharge and a reduced healing period though the pain, restriction of living activity and indurations could be improved a little with surgery alone. However, a long-term benefit with maintenance IFX was not proven, at least in patients with anorectal stricture who were excluded from the ACCENT II study, even with the anal dilatation in this series[2]. Though the surgery with IFX treatment had no efficacy for preventing the recurrence and the comparison of ostomy creation was not significant, the p value was 0.08 in regard to ostomy creation in this study. The efficacies of avoidance or time extension for further ostomy creation should be considered in a further long-term and large prospective randomized trial.
On the other hand, prolonged inflammation in a perianal fistula will cause another problem. Although the causative relationship between anorectal fistula and CD has yet to be determined, the epithelial regeneration and hyperplasia are predisposed to malignant changes, and perianal fistula might lead to carcinogenesis due to constant regeneration[15]. The carcinomas complicating CD have strikingly similar clinicopathologic features to ulcerative colitis with chronic inflammation. The increased risk of carcinoma in areas of prolonged inflammation, such as perianal lesions, has generally been recognized, and surveillance colonoscopy has been recommended for carcinoma screening in CD[16,17]. However, such surveillance would be difficult for patients with severe pain and stricture of the anorectal CD lesion. In general, sudden changes in clinical symptoms, such as increased discharge, severe pain, or progressive stricture, could raise the suspicion of malignancies. Unfortunately, these malignancies are almost all advanced carcinomas when these symptoms change. Lad et al[18] suggested that contrast-enhanced MRI would be helpful for the diagnosis of carcinoma, but subsequent biopsy would be necessary. Finally, examination under anesthesia may be needed for accurate and early diagnosis, though there is a possibility of missing the diagnosis of occult carcinoma.
In Japan, the incidence of prolonged perianal CD has increased and the condition is associated with a risk of carcinogenesis due to long-standing inflammation[19]. While the numbers of CD patients with carcinomas are increasing, and the establishment of an effective surveillance protocol is desired, it will be difficult to develop for the above reasons. Furthermore, Sjödahl et al[20] suggested that the rectal remnant should be considered a risk factor for carcinogenesis. Prophylactic proctectomy may be justified in patients with persistent or recurrent perianal CD. Even if the long-term efficacy of IFX maintenance therapy is proven, it will be necessary to consider whether the prolonged anorectal CD lesion increases carcinogenesis.
In conclusion, surgery with IFX treatment was demonstrated to have short-term efficacy. However, a long-term benefit with maintenance IFX was not proven, at least in patients with anorectal stricture. Maintenance IFX therapy for patients with anorectal stricture may be contraindicated, even if anal dilatation is performed. Although a long-term prospective randomized trial is needed, surgery with IFX treatment had less efficacy for decreasing the recurrence rate and prophylactic proctectomy may be justified in patients with persistent or recurrent perianal CD, especially in patients with anorectal stricture, for early resolution of occult carcinogenesis.
In recent years, infliximab (IFX), an IgG1 murine-human chimeric monoclonal antibody against tumor necrosis factor-alpha, has been recognized to be an effective treatment for active perianal fistula with Crohn’s disease (CD).
In the ACCENT II trial, the closure of fistulas after the initial three IFX infusions occurred in 63% of patients at week 14, but closure decreased to 36% at week 54 with every 8 wk maintenance therapy and there was a high abscess formation rate (15%). The placement of setons and drains prior to IFX therapy is generally recognized as the best combination therapy, and it allows for control of fistula healing and prevention of abscess formation. However, the efficacy of IFX with surgical treatment in perianal CD is controversial, and mainly short-term efficacy has been suggested. Perianal lesions with stricture are recognized to have high recurrence potential, and this is thought to be a contraindication for combination therapy. The aim of this study was to assess the short and long-term efficacy of seton drainage in relation to IFX maintenance therapy with or without anorectal stricture for perianal CD.
The management with loose setons, advancement flaps, fibrin glue, or collagen plugs for perianal CD has been reported to have a short-term benefit. Though the conventional fistulotomy is generally avoided for recurrence and fecal incontinence, surgical treatment with loose setons has shown good results. However, although the ideal goal for treatment of perianal CD lesion is fistula closure and prevention of recurrence, diversion with ostomy creation or abdominoperineal resection may often be necessary in patients with refractory lesions. This study was designed to access data on the long-term efficacy of combination therapy. Although a further, large and long-term prospective study is needed for proper evaluation, the aim of this study was to assess retrospectively the short and long-term efficacy of seton drainage in relation to IFX combination therapy in advance of a prospective study that might take place over a longer period.
Sixty-two patients were identified from our out patients clinical records that were appreciable in detail of symptoms for assigning a modified perianal CD activity index (mPDAI) score, and the demographics and relevant medical details were recorded retrospectively. To evaluate the efficacies of combination therapy, the authors reviewed the clinical records in regard to anorectal stricture.
The activities of perianal lesions were evaluated using the perianal CD activity index (PDAI). The PDAI score is a 5-point index that detects changes in perianal status. The five major elements were the degrees of discharge, pain or restriction of living activity, restriction of sexual activity, type of perianal disease, and induration. Scores ranged from 0 to 20, with higher scores indicating more severe disease activity. Since the mPDAIs were reviewed retrospectively from clinical records in this study, the element of sexual function was not included, and the score ranged from 0 to 16.
Surgery with IFX treatment was demonstrated to have short-term efficacy. However, a long-term benefit with maintenance IFX was not proven, at least in patients with anorectal stricture. Maintenance IFX therapy for patient swith anorectal stricture may have to be contraindicated, even if anal dilatation isperformed. Prophylactic proctectomy may be justified in patients with persistent or recurrent perianal CD. Even if the long-term efficacy of IFX maintenance therapy is proven, it will be necessary to consider whether the prolonged anorectal CD lesion increases carcinogenesis.
Peer reviewer: Ron Shaoul, MD, Director, Pediatric Gastroenterology and Nutrition Unit, Meyer Children’s Hospital, Rambam Medical Center, POB 9602, Haifa 31096, Israel
S- Editor Sun H L- Editor Wang XL E- Editor Ma WH
| 1. | Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, van Hogezand RA, Podolsky DK, Sands BE, Braakman T, DeWoody KL. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med. 1999;340:1398-405. |
| 2. | Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, Kamm MA, Korzenik JR, Lashner BA, Onken JE. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876-885. |
| 3. | Spradlin NM, Wise PE, Herline AJ, Muldoon RL, Rosen M, Schwartz DA. A randomized prospective trial of endoscopic ultrasound to guide combination medical and surgical treatment for Crohn’s perianal fistulas. Am J Gastroenterol. 2008;103:2527-2535. |
| 4. | Gaertner WB, Decanini A, Mellgren A, Lowry AC, Goldberg SM, Madoff RD, Spencer MP. Does infliximab infusion impact results of operative treatment for Crohn’s perianal fistulas? Dis Colon Rectum. 2007;50:1754-1760. |
| 5. | Hyder SA, Travis SP, Jewell DP, McC Mortensen NJ, George BD. Fistulating anal Crohn's disease: results of combined surgical and infliximab treatment. Dis Colon Rectum. 2006;49:1837-1841. |
| 6. | Irvine EJ. Usual therapy improves perianal Crohn’s disease as measured by a new disease activity index. McMaster IBD Study Group. J Clin Gastroenterol. 1995;20:27-32. |
| 8. | Present DH. Crohn’s fistula: current concepts in management. Gastroenterology. 2003;124:1629-1635. |
| 9. | Bernstein LH, Frank MS, Brandt LJ, Boley SJ. Healing of perineal Crohn's disease with metronidazole. Gastroenterology. 1980;79:357-365. |
| 10. | O'Brien JJ, Bayless TM, Bayless JA. Use of azathioprine or 6-mercaptopurine in the treatment of Crohn's disease. Gastroenterology. 1991;101:39-46. |
| 11. | Present DH, Korelitz BI, Wisch N, Glass JL, Sachar DB, Pasternack BS. Treatment of Crohn’s disease with 6-mercaptopurine. A long-term, randomized, double-blind study. N Engl J Med. 1980;302:981-987. |
| 12. | van Koperen PJ, Safiruddin F, Bemelman WA, Slors JF. Outcome of surgical treatment for fistula in ano in Crohn's disease. Br J Surg. 2009;96:675-679. |
| 13. | Williams JG, MacLeod CA, Rothenberger DA, Goldberg SM. Seton treatment of high anal fistulae. Br J Surg. 1991;78:1159-1161. |
| 14. | Sohn N, Korelitz BI, Weinstein MA. Anorectal Crohn’s disease: definitive surgery for fistulas and recurrent abscesses. Am J Surg. 1980;139:394-397. |
| 15. | Traube J, Simpson S, Riddell RH, Levin B, Kirsner JB. Crohnâs disease and adenocarcinoma of the bowel. Dig Dis Sci 1980; 25: 939-944 . . |
| 16. | Siegel CA, Sands BE. Risk factors for colorectal cancer in Crohn’s colitis: a case-control study. Inflamm Bowel Dis. 2006;12:491-496. |
| 17. | Friedman S, Rubin PH, Bodian C, Goldstein E, Harpaz N, Present DH. Screening and surveillance colonoscopy in chronic Crohn’s colitis. Gastroenterology. 2001;120:820-826. |
| 18. | Lad SV, Haider MA, Brown CJ, Mcleod RS. MRI appearance of perianal carcinoma in Crohn’s disease. J Magn Reson Imaging. 2007;26:1659-1662. |
| 19. | Higashi D, Futami K, Kawahara K, Kamitani T, Seki K, Naritomi K, Egawa Y, Hirano K, Tamura T, Tomiyasu T. Study of colorectal cancer with Crohn’s disease. Anticancer Res. 2007;27:3771-3774. |
| 20. | Sjödahl RI, Myrelid P, Söderholm JD. Anal and rectal cancer in Crohn's disease. Colorectal Dis. 2003;5:490-495. |