Published online Feb 21, 2011. doi: 10.3748/wjg.v17.i7.938
Revised: October 20, 2010
Accepted: October 27, 2010
Published online: February 21, 2011
AIM: To investigate intraperitoneal transplantation of microencapsulated hepatic-like cells from human umbilical cord blood for treatment of hepatic failure in rats.
METHODS: CD34+ cells in umbilical cord blood cells were isolated by magnetic cell sorting. In the in vitro experiment, sorted CD34+ cells were amplified and induced into hepatic-like cells by culturing with a combination of fibroblast growth factor 4 and hepatocyte growth factor. Cultures without growth factor addition served as controls. mRNA and protein levels for hepatic-like cells were analyzed by reverse transcription-polymerase chain reaction, immunohistochemistry and immunofluorescence. In the in vivo experiment, the hepatic-like cells were encapsulated and transplanted into the abdominal cavity of acute hepatic failure (AHF) rats at 48 h after D-galactosamine induction of acute hepatic failure. Transplantation with PBS and unencapsulated hepatic-like cells served as controls. The mortality rate, hepatic pathological changes and serum biochemical indexes were determined. The morphology and structure of microcapsules in the greater omentum were observed.
RESULTS: Human albumin, alpha-fetoprotein and GATA-4 mRNA and albumin protein positive cells were found among cultured cells after 16 d. Albumin level in culture medium was significantly increased after culturing with growth factors in comparison with culturing without growth factor addition (P < 0.01). Compared with the unencapsulated group, the mortality rate of the encapsulated hepatic-like cell-transplanted group was significantly lower (P < 0.05). Serum biochemical parameters, alanine aminotransferase, aspartate aminotransferase and total bilirubin in the encapsulated group were significantly improvement compared with the PBS control group (P < 0.01). Pathological staining further supported these findings. At 1-2 wk post-transplantation, free microcapsules with a round clear structure and a smooth surface were observed in peritoneal lavage fluid, surviving cells inside microcapsules were found by trypan blue staining, but some fibrous tissue around microcapsules was also detected in the greater omentum of encapsulated group by hematoxylin and eosin staining.
CONCLUSION: Transplantation of microencapsulated hepatic-like cells derived from umbilical cord blood cells could preliminarily alleviate the symptoms of AHF rats.
- Citation: Zhang FT, Wan HJ, Li MH, Ye J, Yin MJ, Huang CQ, Yu J. Transplantation of microencapsulated umbilical-cord-blood-derived hepatic-like cells for treatment of hepatic failure. World J Gastroenterol 2011; 17(7): 938-945
- URL: https://www.wjgnet.com/1007-9327/full/v17/i7/938.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i7.938
Substantial efforts have been made with regard to cell transplantation as an effective supporting system for hepatic failure and assisted therapies. However, immunological rejection has always been an important problem for cell transplantation. Alginate-poly-l-lysine-alginate (APA) microcapsules have proven to be effective in protecting enclosed target cells from immune rejection following transplantation into experimental animals, thereby eliminating the problems of immunosuppressive therapy[1-3].
Extensive studies have also been conducted on the core of this therapy, namely the cell sources. The investigated cells have included liver stem cells, embryonic stem cells, human umbilical cord blood (UCB) cells and bone marrow stem cells. Human UCB cells have some advantages that other cells do not have. The frequencies of UCB hematopoietic stem/progenitor cells exceed those from bone marrow and peripheral blood. In our previous study, we confirmed the differentiation of mononuclear cells (MNCs) from human UCB into hepatocytes in three different ways, namely co-culture with injured liver cells, growth factor-assisted culture, and MNC transplantation in animal models of liver injury[4]. In the present study, we found that CD34+ cells derived from human UCB could be converted into hepatic-like cells that generate hepatocyte lineage cells. Furthermore, we encapsulated the hepatic-like cells using an alginate method and transplanted them into acute hepatic failure (AHF) rats to evaluate the effects of encapsulated hepatic-like cell transplantation.
UCB (more than 80 samples) from full-term deliveries were obtained from the Obstetrics Department of Peking University Shenzhen Hospital. UCB cells were harvested after written inform consent was obtained. The study protocol was approved by the Ethics Committee of Peking University Shenzhen Hospital. MNCs were isolated from the UCB samples by density-gradient centrifugation at 2000 r/min for 35 min using Ficoll-Hypaque (Huajing, Shanghai, China). CD34+ subpopulations were isolated using a Miltenyi Direct CD34 Progenitor Cell Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany). The specific steps were as follows: (1) isolated MNCs were resuspended in a final volume of 300 μL of PBS that contained 5 g/L bovine serum albumin (BSA); (2) 100 μL of FcR Blocking Reagent and 100 μL of CD34 Micro Beads per 1 × 108 total cells were sequentially added, mixed well and incubated for 30 min in a refrigerator at 4°C; (3) cells were passed through a magnetic column twice and purified; and (4) CD34+ cells were collected, resuspended in 100 μL PBS, incubated with 10 μL CD34-phycoerythrin for 10 min at 4°C and identified by flow cytometry.
Freshly isolated CD34+ cells were primarily cultured in Dulbecco’s modified Eagle’s medium - low glucose (DMEM-LG, Gibco, Carlsbad, CA, USA), amplified for 3-5 d with a combination of 12.5 μg/mL thrombopoietin (TPO) (R&D Systems, Minneapolis, MN, USA), 50 ng/mL stem cell factor (SCF) (R&D Systems) and 50 ng/mL Flt-3 (R&D Systems); then induced into hepatic-like cells by culturing in DMEM-LG that contained 50 mL/L fetal bovine serum (Gibco), 100 U/mL penicillin, 100 μg/mL streptomycin, 4.7 μg/mL linoleic acid, 1 × insulin-transferrin-selenium and 1 × 10-4 mol/L L-ascorbic acid 2-P supplemented with 100 ng/mL fibroblast growth factor (FGF)4 (R&D Systems) and 20 ng/mL hepatocyte growth factor (HGF; Sigma, St. Louis, MO, USA). CD34+ cells were incubated in 24-well plates at 37°C in a 5% CO2 atmosphere. Culture medium was replaced every 3 d. Cultured cells were collected after 8 and 16 d. Cultures without growth factors served as controls.
Total mRNA was extracted from collected cells using Trizol (Mrcgene, Cincinnati, OH, USA). mRNA was reverse-transcribed and the resulting cDNA was amplified using the primer sets shown in Table 1 and a RobusT I reverse transcription-polymerase chain reaction (RT-PCR) Kit (Finnzymes, Espoo, Finland). Reverse transcriptase reaction was run at 48°C for 45 min and PCR was initiated with pre-denaturation at 94°C for 2 min, followed by 35 cycles of 30 s at 94°C, annealing at 58°C for 30 s and extension at 72°C for 30 s, with 72°C for 7 min for final extension. The PCR products were separated on a 1.2% agarose gel.
| Gene | Primer (5’-3’) | Amplicon(bp) | |
| Forward primer | Reverse primer | ||
| ALB | CTTTCAAAGCATGGGCAGTAG | GCAGCAGCACGACAGAGTAA | 411 |
| GATA-4 | ACCTGGGACTTGGAGGATAG | GACAAGGACATCTTGGGAAA | 250 |
| AFP | TGAGCACTGTTGCAGAGGAG | CTGAGACAGCAAGCTGAGGA | 308 |
Cytospins prepared from cells were fixed with 4% paraformaldehyde and 0.15% picric acid in PBS at room temperature for 20 min, then permeabilized and blocked with 10% goat serum and 0.1% Triton X-100 in PBS at room temperature for 10 min. The cells were sequentially incubated with a mouse anti-human albumin antibody (R&D Systems) for 30 min, a biotinylated peroxidase-conjugated secondary antibody (Zymed, South San Francisco, CA, USA) for 10 min, and diaminobenzidine for 10 min. Between the above steps, cells were washed with 0.1 mol/L PBS that contained 1 g/L BSA.
Culture media were collected for the quantitative determination of human albumin by ELISA using a Human Albumin ELISA Kit (Alpha Diagnostics International, San Antonio, TX, USA) according to the manufacturer’s instructions.
Cells collected after 16 d induction were washed with PBS and resuspended in the alginate. The alginate-cell mixture was passed though a microcapsule generator and extruded into 40 mL 1.1% CaCl2 solution. The airflow rate was adjusted for the regulation of the microcapsule diameter between 300 and 800 μm. The capsules and CaCl2 solution were then transferred to 50-mL conical tubes. After removal of the supernatant, the capsules were gently mixed with the wash solution and allowed to settle for 2 min. Before transplantation, a few drops of encapsulated cells were placed on a slide, stained with 0.4% Trypan blue, covered with a cover glass and lightly pressed to force cells out of the microcapsules. Numbers of living cells were counted and expressed as percentages.
Sprague-Dawley rats were purchased from the Experimental Animal Center of Southern Medical University (Guangzhou, China). The Scientific Committee at Peking University Shenzhen Hospital approved the use of animals for experimental purposes. Forty-eight hours before transplantation, the Sprague-Dawley rats (weight: 180-250 g) were intraperitoneally injected at 1.4 g/kg with a 10% D-galactosamine solution in normal saline. On the day of the experiment, microencapsulated cells at a density of 2 × 106 cells/mL were prepared and transplanted into the abdominal cavity of rats. Transplantation with PBS only or unencapsulated hepatocyte-like cells were performed for the establishment of control groups. As UCB samples are not delivered on the same day, animal experiments were carried out by batch and the transplantation of cells performed also on different days. The mortality rate, hepatic pathological changes and serum biochemical indexes were determined.
We obtained total 135 AHF rats 48 h after injection of D-galactosamine. They were divided into three groups on the day of the transplantation. Namely, encapsulated group (transplantation with encapsulated hepatic-like cells, n = 55), unencapsulated group (transplantation with unencapsulated hepatic-like cells, n = 40), PBS group (transplantation with PBS, n = 40). Among these, 76 AHF rats were determined for hepatic pathological changes and serum biochemical indexes (encapsulated group, n = 36; unencapsulated group, n = 20; PBS group, n = 20). The remaining 59 rats were determined for mortality rate (encapsulated group, n = 19; unencapsulated group, n = 20; PBS group, n = 20).
The liver and greater omentum from all three groups were fixed in 4% buffered formaldehyde overnight. After paraffin embedding, 4-5-μm thick serial sections were stained with hematoxylin and eosin (HE) and observed under the light microscope.
Data were expressed as the mean ± SD. Mortality rate analysis was determined by Fisher’s exact test. Serum biochemical index statistical analysis was performed by ANOVA using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). Differences with P values < 0.05 were considered statistically significant.
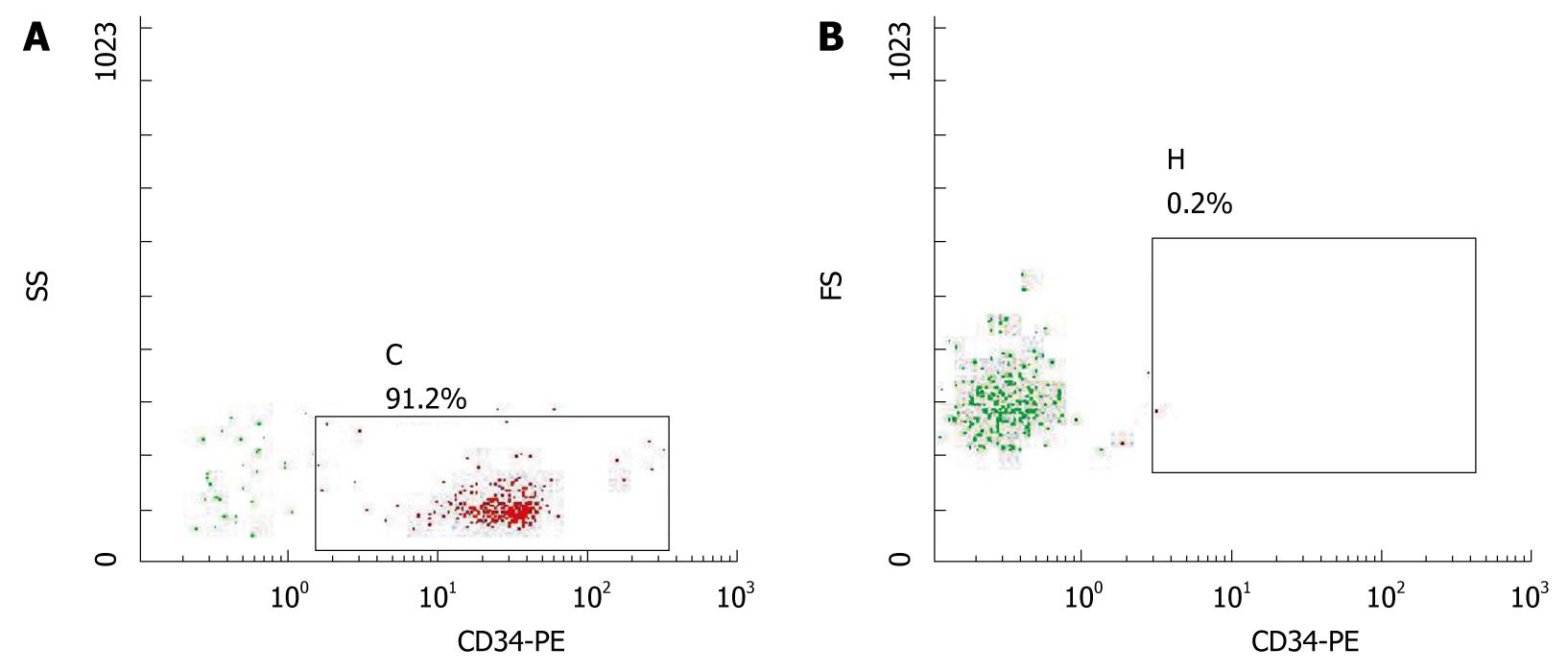
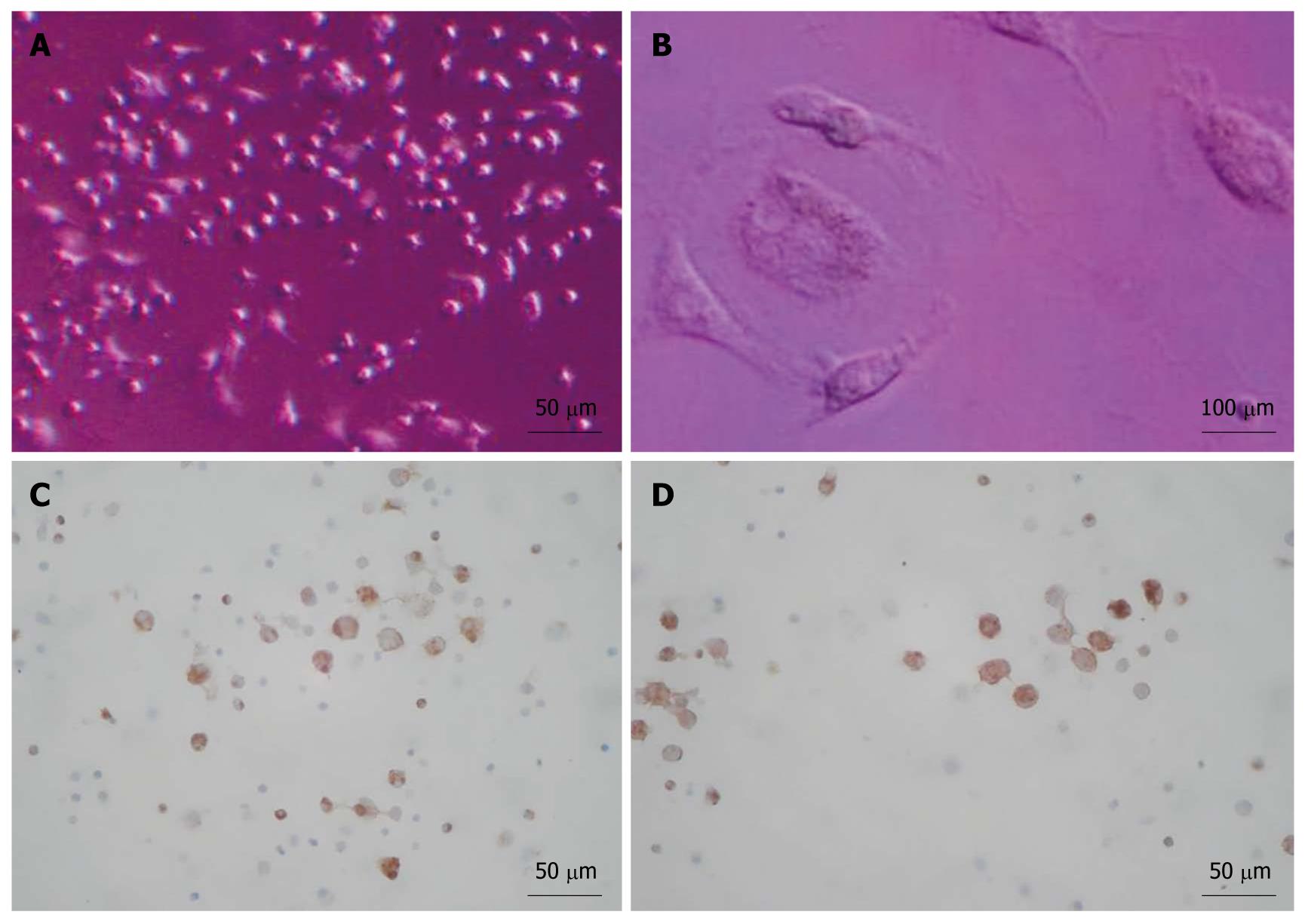
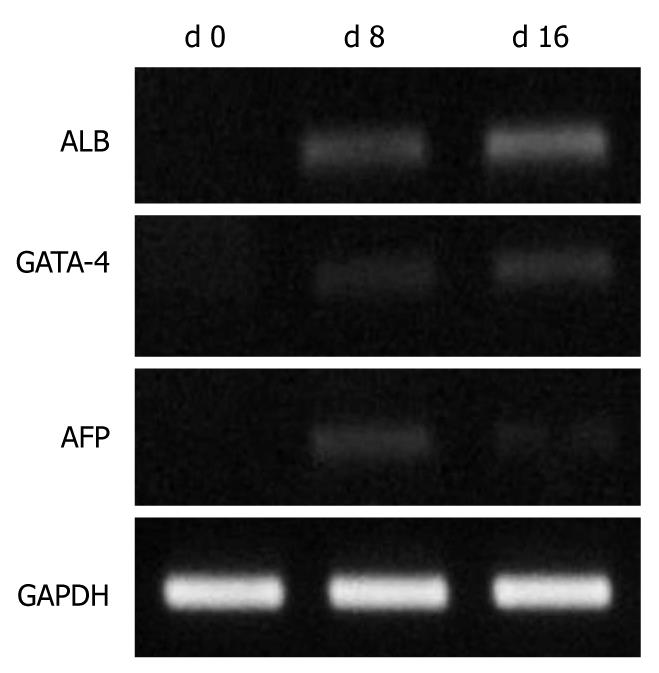
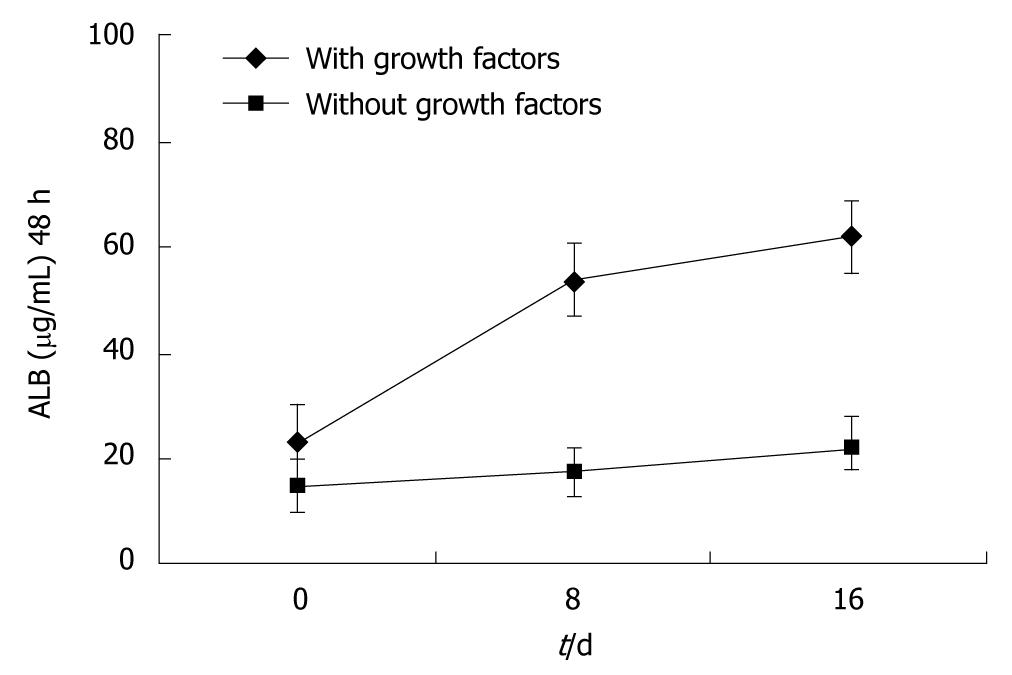
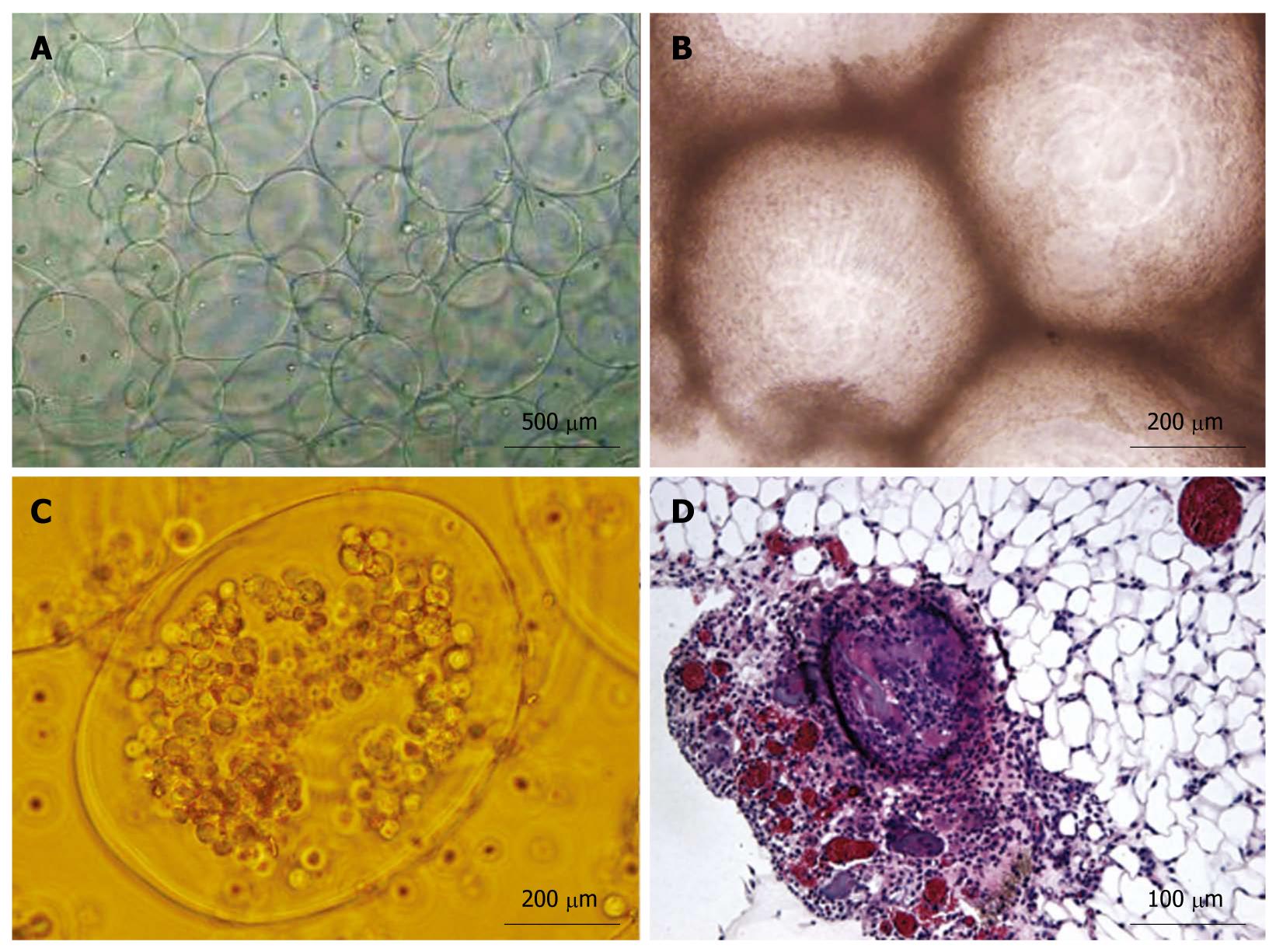
Approximately 3 × 105-9 × 105/mL sorted cells were obtained using the CD34 immunomagnetic bead method, and 91% of them expressed CD34 by flow cytometry analysis (Figure 1). CD34+ cells were firstly amplified 20-fold by a combination of TPO, SCF and Flt-3, and then they were cultured with HGF and FGF4. At 16 d, they developed larger volumes, richer cytoplasts, and binucleated structures, as observed under a Hoffman microscope (Figure 2). The RT-PCR showed no human albumin, α-fetoprotein (AFP) and GATA-4 mRNA expression in CD34+ cells before the induction procedure. The expression of albumin and GATA-4 mRNA increased with the culture time after the addition of growth factors, whereas the amount of AFP mRNA expression peaked after 8 d and reduced at 16 d (Figure 3). Cells that expressed albumin and AFP were verified by immunocytochemical staining and ELISA (Figures 2 and 4). The percentage of albumin- and AFP-positive cells at 16 d was 30% and 24%, respectively. The albumin product in culture medium was significantly increased after culturing with HGF and FGF4 in comparison with control groups (P < 0.01).
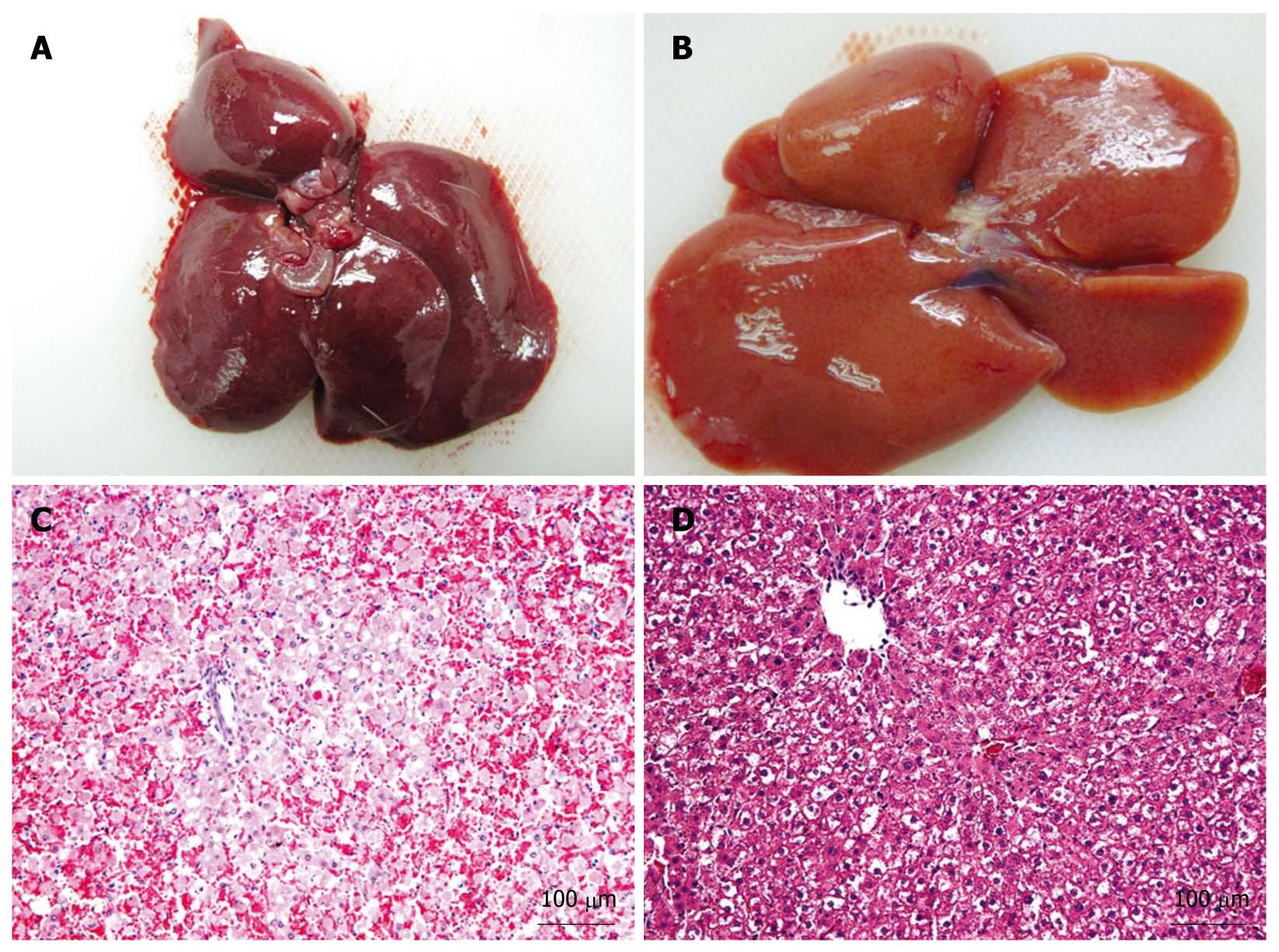
The APA microencapsulation technique was used to encapsulate hepatic-like cells. The percentage of living cells was > 80%, as determined by trypan blue staining. The AHF animal model was successfully established using Sprague-Dawley rats by the injection of D-galactosamine. Pathological section of the AHF liver revealed that the structure of the hepatic lobules was destroyed and the hepatic cord was disordered, with large areas of denatured and necrotic hepatocytes, and infiltrating lymphocytes were found on the portal area at 48 h after injection. On the day of the experiment, microencapsulated cells at a density of 2 × 106 cells/mL were prepared and transplanted into the abdominal cavity of AHF rats. The mortality rate and hepatic pathological changes were determined. At 48 h after transplantation, HE staining of the encapsulated group revealed that the hepatic lobules were still intact; denaturation was the major change in hepatocytes and the area of necrosis nidus was small, and congestion and hemorrhage were almost undetectable (Figure 5). The mortality rate at 48 h after transplantation in three groups was 42.1% (encapsulated group), 65% (unencapsulated group) and 75% (PBS group), respectively. Compared with the unencapsulated group, the mortality rate of the encapsulated group was significantly lower (P < 0.05). In addition, the serum biochemical indexes of ALT, AST and total bilirubin in the microencapsulated group differed significantly from those in the PBS group (P < 0.01) at 48 h after transplantation, but there were no differences between the encapsulated and the unencapsulated group (Table 2). At 1-2 wk post-transplantation, free microcapsules with a round clear structure and a smooth surface were observed in peritoneal lavage fluid, surviving cells in microcapsules were found by trypan blue staining, but some fibrous tissues around microcapsules were also detected in the greater omentum of encapsulated group by HE staining (Figure 6).
| 48 h after injection D-GalN | 48 h after transplantation | 7 d after transplantation | |||||
| All 3 groups | Encapsulated group | Unencapsulated group | PBS group | Encapsulated group | Unencapsulated group | PBS group | |
| ALT (U/L) | 3242.3 ± 2403.24 | 93.93 ± 63.45b | 126.1 ± 54.35 | 245.9 ± 67.87 | 42.25 ± 11.86 | 45.07 ± 10.56 | 47.27 ± 11.08 |
| AST (U/L) | 4237.20 ± 1372.07 | 168.87 ± 89.33b | 275.7 ± 52.74 | 439.7 ± 133.01 | 162.6 ± 54.29 | 124.52 ± 24.61 | 114.83 ± 16.50 |
| TBIL (μmol/L) | 5.57 ± 1.86 | 1.73 ± 1.01a | 2.23 ± 1.98 | 3.50 ± 1.23 | 1.90 ± 0.52 | 2.72 ± 0.96 | 3.72 ± 1.18 |
With the continued increase in people with hepatic failure from cirrhosis and hepatocarcinoma, cell transplantation as an effective therapy is becoming a matter of concern for more scientists. Cell transplantation could offer metabolic support when liver function is damaged, and extend the waiting time for a liver donor[5,6]. Hepatic cell transplantation via the peritoneum or spleen has shown good prospects in clinical and animal experiments. However, the cell sources for transplantation and the requirement for long-term immunosuppression have caused stagnation in this field.
There have been some intriguing studies that have described adult stem cells displaying plasticity in recent years. These studies have led us to consider that using adult stem cells might cure diseases such as AHF[7,8]. Human UCB cells are enriched in hematopoietic stem/progenitor cells that exceed those in the bone marrow and peripheral blood. In comparison with bone marrow stem cells, UCB stem cells are even more immature and with lower immunogenicity. In our previous study, we confirmed that the conversion of UCB MNCs into hepatocytes by three different ways, namely co-culture with injured liver cells, growth-factor-assisted culture, and MNC transplantation in AHF animal models[4]. In the present study, we explored the possibility that CD34+ cells derived from human UCB could be converted into hepatic-like cells. At present, the curative effect of hepatic-like cells derived from CD34+ cells in the bone marrow has already been confirmed by in vivo animal experiments[9-12]. This showed that an AHF model was initially set up using immunodeficient mice, and CD34+ cells enriched by immunobeads were injected through the tail vein or portal vein into the model animals. Expression of differentiation markers of donor cells in recipient livers at different times after transplantation was determined by fluorescence in situ hybridization, immunohistochemistry and molecular biological techniques. It was found that stress-induced signals, such as increased expression of stromal-cell-derived factor 1, matrix metalloproteinase-9 and HGF, recruits human CD34+ progenitors with hematopoietic and/or hepatic-like potential to the liver of NOD/SCID mice[13]. Furthermore, another study has confirmed that FGF, leukemia inhibitory factor, SCF, HGF, FGF4 and oncostatin M contribute to the proliferation and/or differentiation of hepatic cells in different ways, and that combinations of these factors, especially HGF and FGF4, are necessary for human UCB cells to convert into albumin-producing cells[14].
With a combination of HGF and FGF4, we have established a 16-d culture system to induce CD34+ cell differentiation. The culture system with HGF and FGF4 displays the capability to convert the CD34+ cells from human UCB into cells with hepatocyte phenotypes, as confirmed by RT-PCR, immunohistochemical staining, and ELISA. Moreover, the positive ratio of albumin-containing cells by immunocytochemical staining was about 30%, which is consistent with the study of Kakinuma et al[14]. All these indicate that after proliferation and differentiation, we could obtain many transplantable hepatic-like cells.
Although the lower immunogenicity of UCB stem cells has advantages in heterogenic transplantation, untreated UCB cells can sometimes cause serious immune rejection. How to resolve this problem is therefore a key point for further studies. Microencapsulation offers a possibility to overcome the difficulty. This technique uses microcapsules such as APA microcapsules to coat target cells or organs, and is beneficial for heterogenic transplantation because its biocompatible and semi-permeable membranes are capable of intercepting substances with molecular weights above 11 × 104. Since Lim et al[15] first presented the concept of bio-microcapsules in 1980, artificial cell microcapsules as an effective barrier system for immunoprotection have been successfully applied in diabetes, parkinsonism, spinal cord injury, and peripheral nerve regeneration[15,16].
Our study examined coated hepatic-like cells derived from UCB by the APA microencapsulation technique. The obtained microcapsules exhibited a good smooth surface and integrated appearance. Furthermore, living cells inside the microcapsules were > 80% as determined by trypan blue staining. The mortality rate of AHF rats transplanted with microencapsulated hepatic-like cells significantly decreased in comparison with AHF rats transplanted with unencapsulated cells. In addition, there were significantly better outcomes in serum biochemical indexes such as ALT, AST and total bilirubin in the encapsulated group than in the PBS group, but no differences were observed between the encapsulated and the unencapsulated groups. Liver pathological staining supported these findings. The reason why the latter two groups showed no difference requires further exploration, although it is possibly related to the lower number of encapsulated cells. There have been some studies to support the notion that microcapsules provide the encapsulated cells with a good living space, and can significantly increase their survival time, therefore, we could theoretically reduce the number of transplanted cells[17]. Our data suggest that the transplantation of microencapsulated hepatic-like cells could offer a metabolic support to AHF rats in the short term, but it is not sufficient to interrupt or repair the damage of the recipient hepatocytes.
In our study, the pathological staining clearly showed liver recovery at 7 d after induction of AHF with D-galactosamine. At 2 wk post-transplantation, the morphological form of free microcapsules could be observed in the peritoneal lavage fluid, and showed round clear structures and smooth surfaces, and some microcapsule fragments were observed as well. HE staining revealed that some microcapsules attached to the greater omentum exhibited lymphocyte invasion surrounded with fibrous tissues. Although transplantation of microencapsulated hepatic-like cells could preliminarily alleviate the symptoms of AHF rats, their short lifespan and varying stability are still problems for the further use of the technique. The improvement in the airflow encapsulation system might be considered to yield sufficient uniformity in the size of microcapsules[18].
Transplantation of microencapsulated cells could provide a temporary metabolic support to AHF patients and/or be a transitional treatment, because its mechanism is not only related to the immunosuppressive and substitution effects of the transplanted cells, but is also associated with liver repair promoted by the transplanted cells. This new approach could provide a potential alternative for severe liver diseases.
With the continued increase in people with hepatic failure from cirrhosis and hepatocarcinoma, cell transplantation could offer metabolic support when liver function is damaged, and extend the waiting time for a liver donor. However, the cell sources for transplantation and the requirement for long-term immunosuppression have caused stagnation in this field.
Alginate-poly-l-lysine-alginate (APA) microcapsules have been proved effective in protecting enclosed target cells from immune rejection following transplantation into experimental animals. Many studies have been conducted on the cell sources such as liver stem cells, embryonic stem cells, umbilical cord blood (UCB) cells and bone marrow stem cells.
The research team led by Professor Yu has established an artificial cell microcapsules platform, which is based on APA microcapsule technology and stem cell differentiation, to study the therapeutic effects of intraperitoneal transplantation of microencapsulated hepatic-like cells derived from UCB cells on AHF in rats. The effective immunoprotectivity of artificial cell microcapsules has been observed in this study, which suggests that the transplantation of microencapsulated hepatic-like cells could offer a metabolic support to AHF rats in the short term, but it is not yet sufficient to interrupt or repair the damage of the recipient hepatocytes.
Transplantation of microencapsulated cells could provide a temporary metabolic support to AHF patients and/or be used as a transitional treatment. This new approach could provide a potential alternative for severe liver diseases.
UCB was obtained from full-term deliveries at the Obstetrics Department of Peking University Shenzhen Hospital. Hepatic-like cells were induced from UCB CD34+ cells by culturing with FGF4 and HGF. Alginate-poly-l-lysine-alginate microcapsules have biocompatibility and semi-permeable membranes, and can intercept substances with molecular weights > 1.1 × 105.
Zhang et al reported that CD34+ cells sorted from human UCB cells were cultured for 16 d in a specific medium and could differentiate into hepatocyte-like cells. When the hepatocyte-like cells were encapsulated by alginate and intraperitoneally transplanted into rats with galactosamine-induced AHF, the number of surviving rats increased compared to that of control rats at 2 d after transplantation. Although the differentiation of CD34+ cells derived from UCB to hepatocyte-like cells has been reported, it is interesting to use the peritoneal injection of alginate-encapsulated hepatocyte-like cells for the alleviation of AHF. If the preserved UCB cells are used for the treatment of AHF and related diseases, it will be beneficial to the patients.
Peer reviewer: Toshihiro Mitaka, MD, Professor, Department of Pathophysiology, Cancer Research Institute, Sapporo Medical University, 060-85567 Sapporo, Japan
S- Editor Sun H L- Editor Kerr C E- Editor Ma WH
| 1. | Sun Y, Ma X, Zhou D, Vacek I, Sun AM. Normalization of diabetes in spontaneously diabetic cynomologus monkeys by xenografts of microencapsulated porcine islets without immunosuppression. J Clin Invest. 1996;98:1417-1422. |
| 2. | Chang TM. Therapeutic applications of polymeric artificial cells. Nat Rev Drug Discov. 2005;4:221-235. |
| 3. | Orive G, Hernández RM, Gascón AR, Calafiore R, Chang TM, De Vos P, Hortelano G, Hunkeler D, Lacík I, Shapiro AM. Cell encapsulation: promise and progress. Nat Med. 2003;9:104-107. |
| 4. | Zhang FT, Fang JZ, Yu J, Wan HJ, Ye J, Long X, Yin MJ, Huang CQ. Conversion of mononuclear cells from human umbilical cord blood into hepatocyte-like cells. Junyi Daxue Xuebao. 2006;21:358-364. |
| 5. | Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168-1170. |
| 6. | Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, Krause DS. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000;31:235-240. |
| 7. | Teratani T, Yamamoto H, Aoyagi K, Sasaki H, Asari A, Quinn G, Sasaki H, Terada M, Ochiya T. Direct hepatic fate specification from mouse embryonic stem cells. Hepatology. 2005;41:836-846. |
| 8. | Sakaida I, Terai S, Nishina H, Okita K. Development of cell therapy using autologous bone marrow cells for liver cirrhosis. Med Mol Morphol. 2005;38:197-202. |
| 9. | Wang X, Ge S, McNamara G, Hao QL, Crooks GM, Nolta JA. Albumin-expressing hepatocyte-like cells develop in the livers of immune-deficient mice that received transplants of highly purified human hematopoietic stem cells. Blood. 2003;101:4201-4208. |
| 10. | Newsome PN, Johannessen I, Boyle S, Dalakas E, McAulay KA, Samuel K, Rae F, Forrester L, Turner ML, Hayes PC. Human cord blood-derived cells can differentiate into hepatocytes in the mouse liver with no evidence of cellular fusion. Gastroenterology. 2003;124:1891-1900. |
| 11. | Ishikawa F, Drake CJ, Yang S, Fleming P, Minamiguchi H, Visconti RP, Crosby CV, Argraves WS, Harada M, Key LL Jr. Transplanted human cord blood cells give rise to hepatocytes in engrafted mice. Ann N Y Acad Sci. 2003;996:174-185. |
| 12. | Danet GH, Luongo JL, Butler G, Lu MM, Tenner AJ, Simon MC, Bonnet DA. C1qRp defines a new human stem cell population with hematopoietic and hepatic potential. Proc Natl Acad Sci USA. 2002;99:10441-10445. |
| 13. | Kollet O, Shivtiel S, Chen YQ, Suriawinata J, Thung SN, Dabeva MD, Kahn J, Spiegel A, Dar A, Samira S. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112:160-169. |
| 14. | Kakinuma S, Tanaka Y, Chinzei R, Watanabe M, Shimizu-Saito K, Hara Y, Teramoto K, Arii S, Sato C, Takase K. Human umbilical cord blood as a source of transplantable hepatic progenitor cells. Stem Cells. 2003;21:217-227. |
| 15. | Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210:908-910. |
| 16. | Kim YT, Hitchcock R, Broadhead KW, Messina DJ, Tresco PA. A cell encapsulation device for studying soluble factor release from cells transplanted in the rat brain. J Control Release. 2005;102:101-111. |
| 17. | Wang YF, Xue YL, Nan X, Liang F, Luo Y, Li YL, Gao YH, Yue W, Pei XT. [Sustainment of hepatocyte function with mixed cellular co-encapsulation]. Zhonghua Yixue Zazhi. 2005;85:2481-2486. |
| 18. | Shito M, Balis UJ, Tompkins RG, Yarmush ML, Toner M. A fulminant hepatic failure model in the rat: involvement of interleukin-1beta and tumor necrosis factor-alpha. Dig Dis Sci. 2001;46:1700-1708. |