Published online Dec 7, 2011. doi: 10.3748/wjg.v17.i45.5032
Revised: June 21, 2011
Accepted: June 28, 2011
Published online: December 7, 2011
A liver-produced hormone, hepcidin, appears to be the key player in iron metabolism. The overexpression of hepcidin is the underlying cause of anemia of inflammation. The identification of compounds inhibiting hepcidin expression could ameliorate anemia associated with inflammation. In the current study, we have demonstrated for the first time that AG490 significantly abolishes hepcidin expression in mice. Our work represents a novel approach to suppress hepcidin expression for treatment of anemia of inflammation and anemias occurring under other conditions.
-
Citation: Zhang SP, Wang Z, Wang LX, Liu SJ. AG490: An inhibitor of hepcidin expression
in vivo . World J Gastroenterol 2011; 17(45): 5032-5034 - URL: https://www.wjgnet.com/1007-9327/full/v17/i45/5032.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i45.5032
Hepcidin, mainly secreted by hepatocytes, is the hormone with a central role in regulating iron homeostasis (reviewed in[1]). Hepcidin suppresses iron absorption from the duodenum and iron egress from macrophages by promoting degradation of ferroportin protein (reviewed in[1]). Thus, the hepcidin-ferroportin interaction controls iron content in serum and iron distribution in tissues, and hepcidin level plays a primary role in regulating this interaction. Hepcidin level is predominantly modulated by erythropoietic activity, iron content, and inflammatory stimuli. Both acute and chronic inflammation states lead to anemia of inflammation (AI), which represents a prevalent type of anemia worldwide (reviewed in[2]). The overexpression of hepcidin is the underlying cause of AI. Therefore, identifying selective compounds inhibiting hepcidin expression could ameliorate anemia of inflammation or anemia associated with other chronic conditions (such as tumors).
A recent study has demonstrated that AG490 compound significantly reduced hepcidin expression in vitro[3]. This study provided evidence that AG490 suppressed hepcidin transcription by inhibiting the JAK/STAT signaling pathway in mouse hepatocytes[3]. AG490 is a tyrosine kinase inhibitor which has been extensively used for inhibiting JAK2/STAT3. However, no in vivo study has been performed to investigate the inhibitory effect of AG490 on hepcidin production. Here, we are the first to show that AG490 significantly inhibits hepcidin expression in mice.
AG490 (from Calbiochem), freshly prepared in 15% ethanol, was injected intraperitoneally into 6-mo-old female BALB/c mice. For an acute treatment, AG490 was given once (15 mg/kg), and mice were then sacrificed 24 h later. For a chronic treatment, AG490 was administered every 4 d for a total of two times at the same dose, and mice were sacrificed on day 8. Control mice received an equal volume of phosphate buffer solution in 15% ethanol. At the end point of the experiments, 50 mg liver and spleen samples from each mouse were collected for tissue iron assay and another batch of 50 mg liver samples were saved for total RNA extraction. A sample of 100 μL serum for each mouse was used for serum iron examination. Iron and hepcidin quantitative real-time polymerase chain reaction assays were carried out as previously described[4,5].
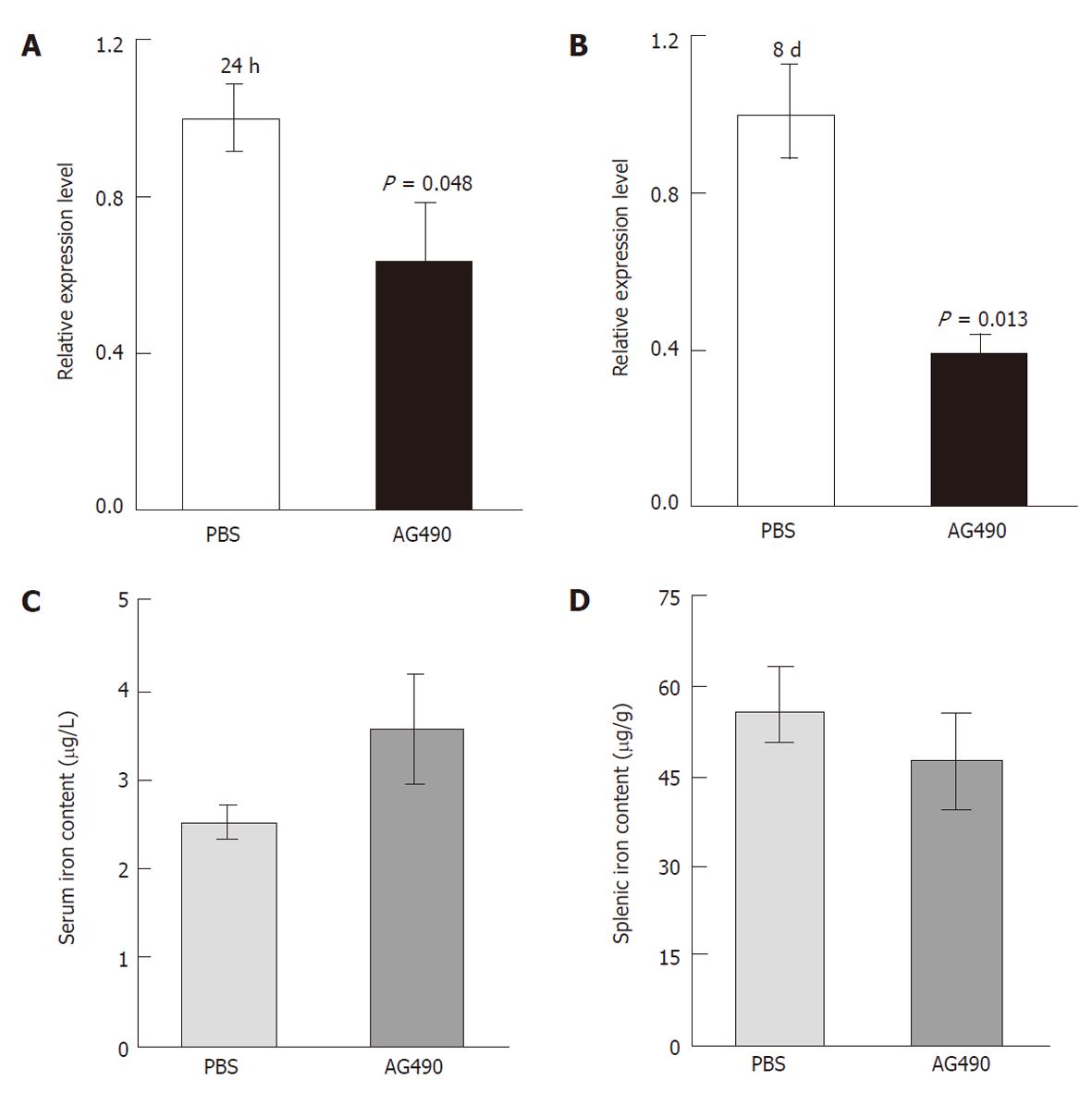
Upon acute and chronic treatment with AG490, we did not observe any abnormality with regard to mouse diet or activities, and no toxicity to various organs was demonstrated through histological examination. After 24 h of treatment with AG490, hepcidin expression from hepatocytes was reduced by 37% compared to control mice (P < 0.05, Figure 1A); however, iron content in serum and spleen was not significantly altered (data not shown). Hepcidin expression was further downregulated after two injections over a period of 8 d: the relative ex-pression level in the AG490-treated mice was reduced by 60% compared to control mice (P < 0.05, Figure 1B). As a result, serum iron was increased by about 40% in the AG490-treated mice compared to control mice (Figure 1C); there was a corresponding reduction for the splenic iron content in the AG490-treated mice compared to control mice (Figure 1D). These observations together suggested that AG490 efficiently attenuated hepcidin production from the liver to increase intestinal iron absorption and macrophagic iron egress.
Iron acquisition and distribution to tissues in mammals are strictly regulated in order to keep systemic iron homeostasis coordinated[6,7]. Iron level and its homeostasis are closely linked to inflammatory responses. Sequestration of iron presumably limits the uptake of iron by invading microbes and thus enhances resistance to infection; however, infection and inflammation increase hepcidin expression, which consequently leads to AI[8]. Thus, inhibitors such as AG490 might be beneficial to improve anemia caused by inflammation or other chronic diseases by reducing hepatic hepcidin production. Similar to our findings, a recent study indicated that heparin also has a potent inhibitory effect on hepcidin expression in vitro and in vivo[9]. Additionally, AG490 or its variants (such as WP1066) have been documented to treat cancers by diminishing active JAK2 signaling[10-12].
To summarize, AG490 represents a prospective approach to attenuate hepcidin-mediated biological actions in order to enhance iron uptake through enterocytes and iron release from macrophages in anemias, and hepcidin repression induced by AG490 in vivo reveals a promising and potentially specific therapeutic means to suppress hepcidin expression in AI or other chronic conditions such as cancers.
We thank Chang-Wen Zhang, Lei Wang and Ze-Hao Huang for assistance with experiments and reagents.
Peer reviewer: Loes van Keimpema, MSc, PhD, Department of Gastroenterology and Hepatology, Radboud University Nijmegen Medical Center, PO Box 9101, 6500 HB, Nijmegen, The Netherlands
S- Editor Yang XC L- Editor Logan S E- Editor Li JY
| 1. | Nemeth E, Ganz T. The role of hepcidin in iron metabolism. Acta Haematol. 2009;122:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 410] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 2. | Roy CN. Anemia of inflammation. Hematology Am Soc Hematol Educ Program. 2010;2010:276-280. [PubMed] |
| 3. | Fatih N, Camberlein E, Island ML, Corlu A, Abgueguen E, Détivaud L, Leroyer P, Brissot P, Loréal O. Natural and synthetic STAT3 inhibitors reduce hepcidin expression in differentiated mouse hepatocytes expressing the active phosphorylated STAT3 form. J Mol Med (Berl). 2010;88:477-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Liu S, Suragani RN, Wang F, Han A, Zhao W, Andrews NC, Chen JJ. The function of heme-regulated eIF2alpha kinase in murine iron homeostasis and macrophage maturation. J Clin Invest. 2007;117:3296-3305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Liu S, Goldstein RH, Scepansky EM, Rosenblatt M. Inhibition of rho-associated kinase signaling prevents breast cancer metastasis to human bone. Cancer Res. 2009;69:8742-8751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 208] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 6. | Andrews NC. Forging a field: the golden age of iron biology. Blood. 2008;112:219-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 422] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 7. | Liu S, Suragani RN, Han A, Zhao W, Andrews NC, Chen JJ. Deficiency of heme-regulated eIF2alpha kinase decreases hepcidin expression and splenic iron in HFE-/- mice. Haematologica. 2008;93:753-756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Wessling-Resnick M. Iron homeostasis and the inflammatory response. Annu Rev Nutr. 2010;30:105-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 353] [Article Influence: 23.5] [Reference Citation Analysis (1)] |
| 9. | Poli M, Girelli D, Campostrini N, Maccarinelli F, Finazzi D, Luscieti S, Nai A, Arosio P. Heparin: a potent inhibitor of hepcidin expression in vitro and in vivo. Blood. 2011;117:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Meydan N, Grunberger T, Dadi H, Shahar M, Arpaia E, Lapidot Z, Leeder JS, Freedman M, Cohen A, Gazit A. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature. 1996;379:645-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 732] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 11. | Ferrajoli A, Faderl S, Van Q, Koch P, Harris D, Liu Z, Hazan-Halevy I, Wang Y, Kantarjian HM, Priebe W. WP1066 disrupts Janus kinase-2 and induces caspase-dependent apoptosis in acute myelogenous leukemia cells. Cancer Res. 2007;67:11291-11299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Verstovsek S, Manshouri T, Quintás-Cardama A, Harris D, Cortes J, Giles FJ, Kantarjian H, Priebe W, Estrov Z. WP1066, a novel JAK2 inhibitor, suppresses proliferation and induces apoptosis in erythroid human cells carrying the JAK2 V617F mutation. Clin Cancer Res. 2008;14:788-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |