Published online Nov 28, 2011. doi: 10.3748/wjg.v17.i44.4905
Revised: June 21, 2011
Accepted: June 28, 2011
Published online: November 28, 2011
AIM: To investigate the association between prognosis of rectal cancer treated with chemoradiotherapy (CRT) and expression of sensitive-to-apoptosis (SAG), B-cell lymphoma-extra large (Bcl-XL) and Bcl-2 homologous antagonist/killer (Bak).
METHODS: Real-time quantitative polymerase chain reaction was used to determine the expression of proteins of interest, namely SAG, Bcl-XL, Bak and β-actin, in rectal carcinoma patients who had a follow-up period of 3 years after CRT. Biopsy specimens were excised from the rectal tumor preceding CRT.
RESULTS: SAG, Bcl-XL and Bak proteins showed significant correlations with each other. In multivariate analysis, patients with high vs low SAG expression showed a statistically significant difference in 2-year survival rates: 56% vs 73%, respectively (P = 0.056). On the other hand, there were no significant correlations between the expression levels of all three genes and metastatic rates or tumor responses to CRT. Mean overall survival in the patients with elevated SAG expression was 27.1 mo ± 3.9 mo [95% confidence interval (CI): 19.3-34.9], and in patients with reduced expression, it was 32.1 mo ± 2.5 mo (95% CI: 27.3-36.9). The corresponding values for Bcl-XL were 28.0 mo ± 4.1 mo (95% CI: 19.9-36.1) and 31.7 mo ± 2.9 mo (95% CI: 26.0-37.5), and those for Bak were 29.8 mo ± 3.7 mo (95% CI: 22.5-37.2) and 30.6 mo ± 2.4 mo (95% CI: 25.5-35.0), respectively.
CONCLUSION: Two-year survival rates significantly correlated with low SAG expression, and SAG may be a candidate gene for good prognosis, independent of therapeutic response of different individuals.
- Citation: Ozden SA, Ozyurt H, Ozgen Z, Kilinc O, Oncel M, Gul AE, Karadayi N, Serakinci N, Kan B, Orun O. Prognostic role of sensitive-to-apoptosis gene expression in rectal cancer. World J Gastroenterol 2011; 17(44): 4905-4910
- URL: https://www.wjgnet.com/1007-9327/full/v17/i44/4905.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i44.4905
Colorectal cancer is one of the most common cancers worldwide, and 30% of patients experience local recurrence, thus posing a serious problem in treatment[1,2]. Currently, preoperative radiotherapy alone or in combination with chemotherapy is widely accepted to improve local control and overall survival.
The most frequently chosen treatment methods are either short-course irradiation (25 Gy in five fractions) followed by surgery after 1 wk, or conventional fractionated chemoradiotherapy (CRT) with delayed surgery[3]. Multidrug- and/or radiation resistance is one of the main causes of treatment failure in rectal carcinoma, as in other types of cancer. Predictors of treatment response are highly valuable, and they help to reduce the occurrence of undesirable treatment side effects, improve efficacy, and reduce costs, especially in long treatment schedules. Therefore, it is very important to identify those patients who will not show a good response to CRT. The success of CRT is strongly dependent on the molecular and cellular characteristics of the cells, therefore, identification of candidate molecular markers with significant prognostic power to estimate the response to CRT is crucial. Despite accumulation of data on prognostic markers, reliable markers for satisfactory clinical outcomes remain limited in number.
Sensitive-to-apoptosis gene (SAG)/regulator of cullins (ROC) 2/RING box protein (Rbx) 2/Hrt2 is a recently identified component of Skp/Cullin/F-box containing complex (SCF) E3 ubiquitin ligase, which controls cell-cycle progression by promoting ubiquitination and degradation of cell-cycle inhibitors. It has also been reported that SAG protects cells from apoptosis induced by redox agents such as hydroxyl radicals and radiation. The prognostic value of apoptotic activity in different cancer types has been previously described[4-7]. SAG overexpression has been shown in 60% of primary colon carcinomas; furthermore, significant correlation between SAG overexpression and poor survival has been demonstrated in non-small cell lung carcinoma[8,9]. Thus, it is proposed that SAG may regulate carcinogenesis via modulating both cell proliferation and apoptosis.
Ionizing radiation causes DNA damage that can lead cell to apoptosis and thus eradication of cancer cells. Antiapoptotic proteins such as SAG may have a major impact on the progress of cancer cell formation and proliferation. The present study investigated SAG expression as a potential molecular marker of ionizing radiation effect in rectal cancer. Two members of the Bcl-2 family, B-cell lymphoma-extra large (Bcl-XL) and Bcl-2 homologous antagonist/killer (Bak), which are suggested as the most probable candidate biomarkers, were also analyzed. Our results indicate that SAG expression is a useful marker for early prognosis, regardless of local response to CRT, and that targeting SAG may have a potential role in the treatment of rectal cancer.
This prospective study included 31 patients referred to the Kartal Education and Research Hospital with a diagnosis of stage II and III rectal cancer, according to the conventional tumor, node and metastases (TNM) classification[10]. The appropriate ethics committees related to the institution approved the study and all patients provided written informed consent before undergoing diagnostic colon biopsy. Prior to the start of treatment, all patients underwent examinations, including complete blood counts, liver and renal function tests, and tumor markers. Additionally, lung X-rays were evaluated before the start of treatment. Abdominal-pelvic magnetic resonance imaging or computed tomography was used for clinical staging and supplemented with transrectal ultrasound when needed. Biopsy specimens from the tumor and adjacent normal rectal tissues were obtained during colonoscopy. Freshly removed specimens were immediately immersed in RNAlater solution (Qiagen, Germany) and stored at -20 °C for RNA extraction.
All patients received preoperative CRT. Patients were irradiated using the four-box-field technique and high-energy photon radiotherapy beams (15 mV), with a daily exposure of 1.8-2 Gy for five consecutive days. A cumulative dose of 45-50 Gy was administered during 5 wk and an additional 5.4-Gy boost was administered in three fractions. A short infusion of fluorouracil (320-400 mg/m2) and of calcium folinate (20 mg/m2) was administered on the first and last weeks, concomitantly[11,12].
Patients who were histopathologically diagnosed with rectal cancer underwent CRT prior to surgery. After a 4-6-wk interval, the patients underwent surgery. Patients were staged according to the TNM classification system[10], based on routine histopathological reports following surgery.
SAG, Bcl-XL and Bak mRNA expression levels in tumor tissues and adjacent normal tissues were quantified by real-time polymerase chain reaction (PCR) analysis. Total RNA was extracted from RNAlater-conserved tissues using the RNeasy Plus Mini Kit (Qiagen) according to the manufacturer’s guidelines. RNA samples were treated with DNaseI (MBI Fermentas, Burlington, Canada) to remove possible genomic DNA contamination. RNA was quantified by measuring A260 using a conventional spectrophotometer. cDNA was synthesized from RNA using the 1st Strand cDNA Synthesis Kit for RT-PCR according to the manufacturer’s instructions (Roche Applied Science, Mannheim, Germany). Following synthesis, 5 μg of cDNA was amplified using the appropriate primers. SAG expression was analyzed using following primers: forward (5’-CGGGATCCATGGCCGACGTGGAAG-3’) and reverse (5’-CGAAGCTTTCATTTGCCGATTCTTTGGAC-3’). Expression of two other apoptotic pathway genes (Bcl-XL and Bak) was analyzed using the following primers: Bcl-XL forward (5’-CCAGAAGGGACTGAATCG-3’) and Bcl-XL reverse (5’-CCTTGTCTACGCTTTCCAC-3’); Bak forward (5’-GACCCAGAGATGGTCACCTT-3’) and Bak reverse (5’-TCATAGCGTCGGTTGATGT-3’). β-actin gene expression was used in parallel reactions as an internal PCR control. The β-actin primers were as follows: β-actin forward (5’-CTGTGCTGTCCCTGTATGCC-3’) and β-actin reverse (5’-GTGGTGGTGAAGCTGTAGCC-3’). Amplification products were 341, 361, 103 and 203 bp, respectively. Real-time PCR was performed using a Light-Cycler 480 system (Roche) and amplification conditions were set according to the instructions supplied with the Light Cycler FastStart DNA Master SYBR Green kit (Roche). The annealing temperature for amplifications was 56 °C for β-actin, 50 °C for Bcl-XL and 60 °C for SAG and Bak proteins.
Multivariate analysis was performed using the logistic regression test. P < 0.05 was considered statistically significant. The relationship between protein regulations and disease-free survival up to 3 years was assessed by a log-rank comparison of Kaplan-Meier survival curves. All statistical analyses were conducted using SPSS 13 statistical software (SPSS, Chicago, IL, United States).
The study included 31 patients (17 males and 14 females) diagnosed with locally advanced rectal cancer. RNA was extracted from both normal and tumor tissues. Mean age of the patients was 59.9 years (range: 35-85 years). Survival, according to patient sex and age, was not significantly different (Table 1). Median follow-up was 3 years. In all, 8 of the 31 patients (34.8%) developed distant metastases. Table 1 summarizes the patients’ 1-, 2- and 3-year survival rates, the corresponding data for some known prognostic factors, and the levels of SAG, Bcl-XL and Bak protein expression.
| Survival (%) | Multivariatesignificance | ||||
| n | 12 mo | 24 mo | 36 mo | P value | |
| Age (yr) | |||||
| < 50 | 10 | 80 | 80 | 66 | 0.135 |
| > 50 | 21 | 76 | 57 | 47 | |
| Sex | |||||
| Male | 17 | 64 | 52 | 45 | 0.073 |
| Female | 14 | 92 | 78 | 62 | |
| preT | |||||
| T2 | 1 | 100 | 100 | 100 | 0.052 |
| T3 | 24 | 79 | 70 | 59 | |
| T4 | 6 | 66 | 33 | 16 | |
| preN | |||||
| N0 | 18 | 77 | 66 | 66 | 0.036 |
| N+ | 13 | 76 | 61 | 0 | |
| Grade | |||||
| Low | 4 | 100 | 75 | 75 | 0.0337 |
| Moderate | 22 | 77 | 68 | 58 | |
| High | 4 | 50 | 25 | 0 | |
| pN | |||||
| N0 | 17 | 70 | 64 | 58 | 0.044 |
| N1 | 6 | 83 | 83 | 56 | |
| N2 | 3 | 66 | 83 | 67 | |
| pT | |||||
| T0 | 6 | 83 | 66.7 | 66.7 | 0.067 |
| T1 | 2 | 100 | 100 | 100 | |
| T2 | 4 | 75 | 75 | 75 | |
| T3 | 19 | 73 | 63 | 57 | |
| Vascular invasion | |||||
| Negative | 7 | 71 | 57 | 57 | 0.019 |
| Positive | 24 | 79 | 66.7 | 52.7 | |
| Perineural invasion | |||||
| Negative | 17 | 76.5 | 64.7 | 64.7 | 0.068 |
| Positive | 13 | 76.9 | 61.5 | 35.9 | |
| Metastasis | |||||
| Negative | 23 | 78 | 73 | 63 | 0.009 |
| Positive | 8 | 75 | 50 | 25 | |
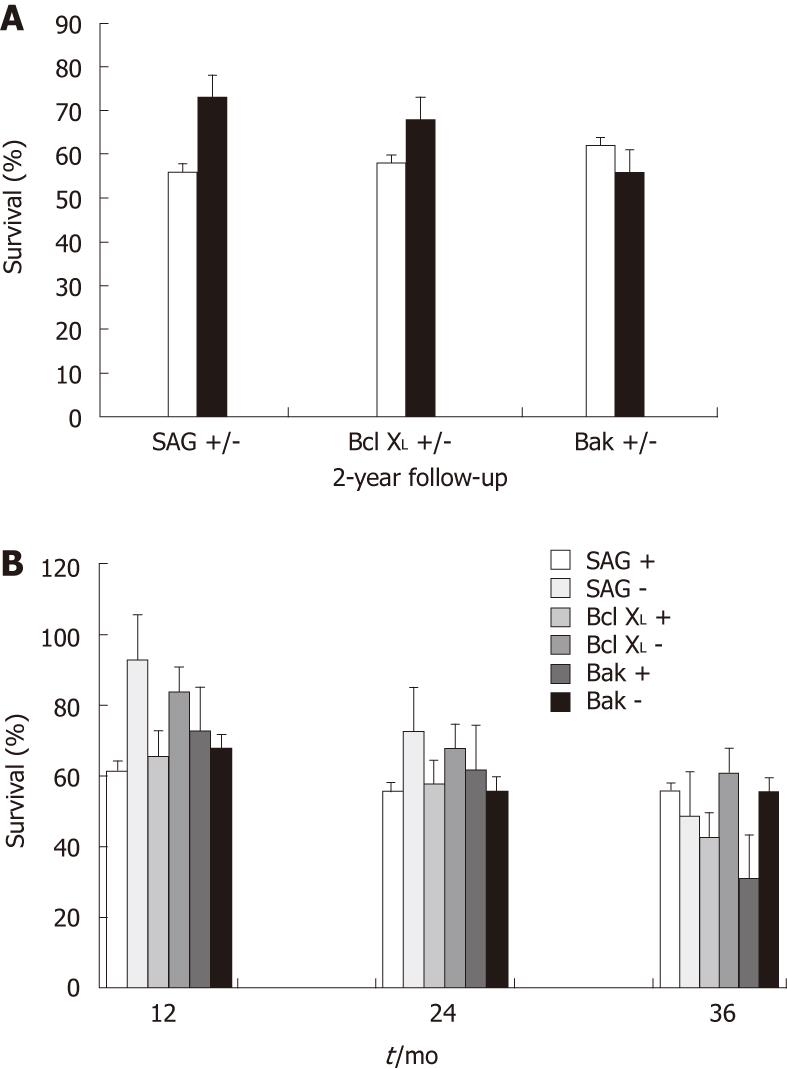
| SAG expression | |||||
| Increase | 16 | 62 | 56 | 56 | 0.056 |
| Decrease | 15 | 93 | 73 | 49 | |
| Bak expression | |||||
| Increase | 14 | 73 | 62 | 31 | 0.731 |
| Decrease | 17 | 68 | 56 | 56 | |
| Bcl-XL expression | |||||
| Increase | 12 | 66 | 58 | 43 | 0.336 |
| Decrease | 19 | 84 | 68 | 61 | |
There was an association between protein expression and survival following CRT. The 1- and 2-year survival rates were 93% and 73%, respectively, in patients with low SAG expression (fold change > 0.99), vs 62% and 56%, respectively, in patients with high SAG expression (fold change < 0.99); the correlation between SAG expression and survival was moderate (P = 0.056). A similar trend was observed in the expression of antiapoptotic protein Bcl-XL; the corresponding survival values were 84% and 68%, respectively, in patients with low Bcl-XL expression, vs 66% and 58%, respectively in patients with high Bcl-XLexpression. The levels of Bak were also in agreement with its apoptotic function. In accordance with our expectations, patients with high Bak expression had a higher 2-year survival rate (62%) than those with low Bak expression (56%). The expression patterns of SAG, Bcl-XL and Bak proteins exhibited good correlations with the 1- and 2-year survival rates, in accordance with their anti- and pro-apoptotic roles, even though statistical significance was low, possibly due to the small number of patients included in the study (Figure 1A). There was no significant association in the expression of any gene with better survival at the end of the 3-year follow-up (Figure 1B).
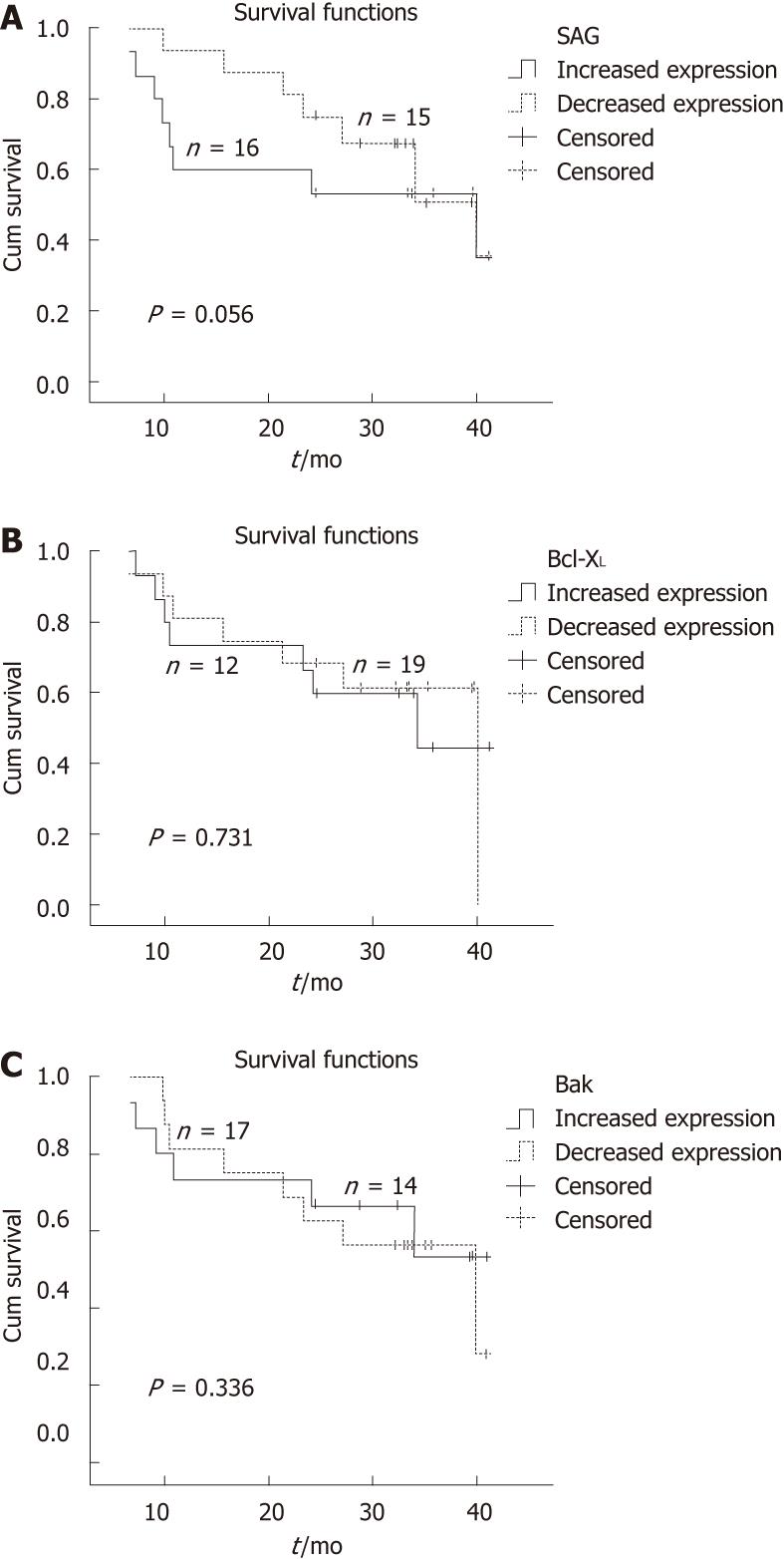
Kaplan-Meier survival curves for SAG, Bcl-XL and Bak proteins are shown in Figure 2A-C, respectively. Mean overall survival in the patients with elevated SAG expression was 27.1 mo ± 3.9 mo [95% confidence interval (CI): 19.3-34.9]; and in patients with reduced expression, it was 32.1 mo ± 2.5 mo (95% CI: 27.3-36.9). The corresponding values for Bcl-XL were 28.0 mo ± 4.1 mo (95% CI: 19.9-36.1) and 31.7 mo ± 2.9 mo (95% CI: 26.0-37.5), and those for Bak were 29.8 mo ± 3.7 mo (95% CI: 22.5-37.2) and 30.6 mo ± 2.4 mo (95% CI: 25.5-35.0), respectively.
Tumor progression caused by distant metastases occurred only in eight patients (25.8%), of whom, seven had liver metastasis and one, who was alive at the time of evaluation, had bone metastasis. Mean overall survival was 41 mo. When the levels of SAG in tumor tissue were compared to the corresponding normal tissue, half of the patients expressed higher levels of SAG, whereas the other half had lower expression. Interestingly, patients with high SAG expression had a higher mean survival (28 mo) than did patients with low expression (18 mo). These results contradict those obtained in the non-metastatic patients. Among the non-metastatic patients, those with low SAG expression showed a higher survival (36 mo) and those with high SAG expression had lower survival (26 mo) (Table 1).
Pathological complete response was observed in five patients (5/31); three of whom (60%) had lower SAG expression and two (40%) had higher SAG expression.
Almost 50% of patients with rectal carcinoma who undergo potentially curative resection die from the disease, due to high recurrence rates. There is mounting evidence indicating that local control of disease and survival rates can be significantly improved with neoadjuvant CRT; however, responses to therapy vary widely among individuals and, as such, the ability to predict the response to CRT in patients with rectal cancer significantly improves therapeutic efficacy.
One of the immediate damaging effects of ionizing irradiation is the induction of cell-cycle arrest to provide time for DNA repair or apoptosis. Spontaneous apoptosis has been reported to be an important predictor of tumor regression in rectal cancer[13]. Reactive oxygen species, such as hydroxyl radical radicals, produced by ionizing radiation are highly reactive and easily trigger apoptosis, possibly by an indirect action on redox-sensitive molecules such as p53 and p27[14,15]. Recently, SAG was identified as a redox-sensitive protein that protects cells from apoptosis, either by scavenging oxygen radicals or by acting on apoptosis-related proteins as part of the ubiquitin ligase complex SCF, thereby affecting the apoptotic sensitivity of cells. Moreover, SAG was identified as a potential regulator of p27, through inhibition of p27 accumulation[16]. SAG overexpression has also been detected in a subset of human colon cancers and non-small lung carcinomas and has been shown to be associated with poor prognosis[8,9,17].
The present results, which are in agreement with above-mentioned studies, showed that low SAG expression in tumor tissue, as compared to normal tissue, had a positive effect on survival. The patients with low SAG expression had a median disease-free survival of 31.7 mo, as compared to 28 mo in those with high SAG expression. The clinical outcomes of the 31 patients revealed a strong discrepancy at the 2-year follow-up period between the groups with high vs low SAG expression, but this profile seemed to vanish at 3 years of observation.
Previously, Bak and Bcl-XL, which are members of the Bcl-2 family, were also identified as candidate proteins for regulating chemotherapy-induced apoptosis[18]. Bcl-2 was shown to block γ-radiation-induced cell death[19,20]. Bak can form heterogenous dimers with Bcl-2 or Bcl-XL to inhibit their antiapoptotic functions. Strong interaction between endogenous Bak and Bcl-XL has been reported in hepatocytes as well as in other cells[21]. Furthermore, quantitative expression levels of Bcl-XL and Bak have also been determined to correlate with apoptotic sensitivity of tissues in different carcinomas. Both genes have previously been shown to play a major role in colorectal carcinogenesis and tumor progression. Bcl-XL, on the other hand, is suggested to be more crucial than Bcl-2 for regulation of apoptotic cell death in colon cancer[22]. Significant overexpression of Bcl-XL mRNA has been observed in the majority of colorectal carcinomas as compared to the corresponding normal tissues. Krajewska et al[23] have also reported elevated Bcl-XL and reduced Bak expression in colorectal adenocarcinoma. These studies reinforce the major roles of Bcl-XL and Bak in colorectal carcinogenesis and tumor progression; furthermore, they also imply that expression levels of Bak and Bcl-XL can be used as prognostic factors in rectal carcinoma, as pointed out by some other studies[24,25].
Thus, in addition to SAG, we also evaluated the expression of two other Bcl-2 family proteins, Bcl-XL and Bak. The expression levels of antiapoptotic Bcl-XL and proapoptotic Bak were well correlated with those of SAG, which lends further support to the role of apoptotic factors in the malignant potential of tumors. Higher survival rates for 2 years were recorded for increased expression of proapoptotic Bak, as opposed to SAG and Bcl-XL levels.
We did not observe any correlation between development of metastases and expression of apoptotic factors; however, in patients who did not develop metastases, gene regulation, especially that of SAG, was closely associated with survival. In non-metastatic tumors, tissues with low-level SAG expression (n = 12) were associated with longer mean survival (36 mo), as opposed to those with high expression levels (27.9 mo, n = 11).
In conclusion, the present study shows that the level of expression of apoptosis-related genes may be associated with the degree of resistance to radiation exposure and may significantly affect therapeutic outcome. We observed an inverse correlation between SAG expression and survival. Furthermore, the data obtained in this study imply that SAG and Bak expression may serve as predictive parameters of disease progression. On the other hand, SAG and Bak expression levels did not significantly affect overall survival. This pilot study included a rather small number of patients, therefore, prospective studies with larger cohorts that include other proteins in the molecular apoptotic pathway are required to assess the roles of individual regulators in anticancer therapy.
We thank Dr. Flemming Brand Sørensen for critical reading of the manuscript.
Apoptotic proteins have been reported to be important prognostic factors in various cancers. Sensitive-to-apoptosis gene (SAG) is a recently identified apoptotic protein, which may be a new candidate to estimate the outcome of treatment in rectal cancers.
SAG was identified as a redox-sensitive protein that protects cells from apoptosis, either by scavenging oxygen radicals or by acting on apoptosis-related proteins as part of the ubiquitin ligase Skp/Cullin/F-box containing complex (SCF), thereby affecting the radiation sensitivity of cells. This study investigated the correlation between expression levels of SAG and survival rates of patients, who have advanced rectal carcinoma. In addition to SAG, this study also examined two other proteins, B-cell lymphoma-extra large (Bcl-XL) and Bcl-2 homologous antagonist/killer (Bak) proteins, which are important members of the mitochondrial apoptotic pathway.
The present study showed that the level of expression of some apoptosis-related genes may be associated with the degree of resistance to radiation exposure and may significantly affect therapeutic outcome. The present research observed an inverse correlation between SAG expression and 2-year survival, although the overall survival rate was not affected significantly. There were no significant correlations between the expression levels of all three genes and metastatic rates or tumor responses to chemoradiotherapy (CRT).
The data obtained in this study imply that SAG and Bak expression may serve as predictive parameters of disease progression. This pilot study included a rather small number of patients, therefore, prospective studies with larger cohorts that include other proteins in the molecular apoptotic pathway are required to assess the roles of individual regulators in anticancer therapy.
SAG/regulator of cullins 2/Rbx/Hrt2 is a recently identified component of SCF E3 ubiquitin ligase, which controls cell-cycle progression by promoting ubiquitination and degradation of cell-cycle inhibitors. It has also been reported that SAG protects cells from apoptosis induced by redox agents such as hydroxyl radicals and radiation. The Bcl-2 family proteins are members of the intrinsic apoptotic pathway. Bak is a proapoptotic member, while Bcl-XL blocks apoptosis in many systems.
This was an interesting and well conducted study which should be published because it confirms the usefulness of SAG in the prognostication of rectal cancer patients.
Peer reviewer: Francis Seow-Choen, MBBS, FRCSEd, FAMS, Professor, Seow-Choen Colorectal Centre, Mt Elizabeth Medical Centre, Singapore, Singapore 238859, Singapore
S- Editor Wu X L- Editor Kerr C E- Editor Xiong L
| 1. | Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, Gunnarsson U. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol. 2005;23:5644-5650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 588] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 2. | Adell G, Sun XF, Stål O, Klintenberg C, Sjödahl R, Nordenskjöld B. p53 status: an indicator for the effect of preoperative radiotherapy of rectal cancer. Radiother Oncol. 1999;51:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Kryj M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93:1215-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 872] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 4. | de Bruin EC, van de Velde CJ, van de Pas S, Nagtegaal ID, van Krieken JH, Gosens MJ, Peltenburg LT, Medema JP, Marijnen CA. Prognostic value of apoptosis in rectal cancer patients of the dutch total mesorectal excision trial: radiotherapy is redundant in intrinsically high-apoptotic tumors. Clin Cancer Res. 2006;12:6432-6436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Adell GC, Zhang H, Evertsson S, Sun XF, Stål OH, Nordenskjöld BA. Apoptosis in rectal carcinoma: prognosis and recurrence after preoperative radiotherapy. Cancer. 2001;91:1870-1875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Schwandner O, Schiedeck TH, Bruch HP, Duchrow M, Windhoevel U, Broll R. Apoptosis in rectal cancer: prognostic significance in comparison with clinical histopathologic, and immunohistochemical variables. Dis Colon Rectum. 2000;43:1227-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Jonges LE, Nagelkerke JF, Ensink NG, van der Velde EA, Tollenaar RA, Fleuren GJ, van de Velde CJ, Morreau H, Kuppen PJ. Caspase-3 activity as a prognostic factor in colorectal carcinoma. Lab Invest. 2001;81:681-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Huang Y, Duan H, Sun Y. Elevated expression of SAG/ROC2/Rbx2/Hrt2 in human colon carcinomas: SAG does not induce neoplastic transformation, but antisense SAG transfection inhibits tumor cell growth. Mol Carcinog. 2001;30:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Sasaki H, Yukiue H, Kobayashi Y, Moriyama S, Nakashima Y, Kaji M, Fukai I, Kiriyama M, Yamakawa Y, Fujii Y. Expression of the sensitive to apoptosis gene, SAG, as a prognostic marker in nonsmall cell lung cancer. Int J Cancer. 2001;95:375-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | Fleming ID, Cooper JS, Henson DE, Hutter RVP, Kennedy BJ, Murphy GP, Kennedy BJ, Murphy GP, O’Sullivan BO, Sobin LH. American Joint Committee on Cancer (AJCC). 5th ed. Philadelphia: J.B. Lippincott 1997; . |
| 11. | Nozue M, Isaka N, Maruyama T, Kawamoto T, Seino KI, Tanagichi H, Fukao K. Treatment of advanced colorectal adenocarcinoma with weekly high-dose l-leucovorin and 5-fluorouracil. Oncol Rep. 2002;9:93-96. [PubMed] |
| 12. | Ng E, Maroun J, Berthelot J-M, Dahrouge S. Chemotherapy practice patterns for colorectal cancer patients in 1998: A survey of Canadian oncologist. Current Oncology. 2001;8:150-158. |
| 13. | Rödel C, Grabenbauer GG, Papadopoulos T, Bigalke M, Günther K, Schick C, Peters A, Sauer R, Rödel F. Apoptosis as a cellular predictor for histopathologic response to neoadjuvant radiochemotherapy in patients with rectal cancer. Int J Radiat Oncol Biol Phys. 2002;52:294-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Esposito G, Pucciarelli S, Alaggio R, Giacomelli L, Marchiori E, Iaderosa GA, Friso ML, Toppan P, Chieco-Bianchi L, Lise M. P27kip1 expression is associated with tumor response to preoperative chemoradiotherapy in rectal cancer. Ann Surg Oncol. 2001;8:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Pawlik TM, Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59:928-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 669] [Cited by in RCA: 789] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 16. | Duan H, Tsvetkov LM, Liu Y, Song Y, Swaroop M, Wen R, Kung HF, Zhang H, Sun Y. Promotion of S-phase entry and cell growth under serum starvation by SAG/ROC2/Rbx2/Hrt2, an E3 ubiquitin ligase component: association with inhibition of p27 accumulation. Mol Carcinog. 2001;30:37-46. [PubMed] |
| 17. | Gu Q, Bowden GT, Normolle D, Sun Y. SAG/ROC2 E3 ligase regulates skin carcinogenesis by stage-dependent targeting of c-Jun/AP1 and IkappaB-alpha/NF-kappaB. J Cell Biol. 2007;178:1009-1023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Simonian PL, Grillot DA, Merino R, Nuñez G. Bax can antagonize Bcl-XL during etoposide and cisplatin-induced cell death independently of its heterodimerization with Bcl-XL. J Biol Chem. 1996;271:22764-22772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Sentman CL, Shutter JR, Hockenbery D, Kanagawa O, Korsmeyer SJ. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991;67:879-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 881] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 20. | Strasser A, Harris AW, Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991;67:889-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 765] [Cited by in RCA: 830] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 21. | Petros AM, Olejniczak ET, Fesik SW. Structural biology of the Bcl-2 family of proteins. Biochim Biophys Acta. 2004;1644:83-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 546] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 22. | Maurer CA, Friess H, Bühler SS, Wahl BR, Graber H, Zimmermann A, Büchler MW. Apoptosis inhibiting factor Bcl-xL might be the crucial member of the Bcl-2 gene family in colorectal cancer. Dig Dis Sci. 1998;43:2641-2648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Krajewska M, Moss SF, Krajewski S, Song K, Holt PR, Reed JC. Elevated expression of Bcl-X and reduced Bak in primary colorectal adenocarcinomas. Cancer Res. 1996;56:2422-2427. [PubMed] |
| 24. | Wolf HK, Stöber C, Hohenfellner R, Leissner J. Prognostic value of p53, p21/WAF1, Bcl-2, Bax, Bak and Ki-67 immunoreactivity in pT1 G3 urothelial bladder carcinomas. Tumour Biol. 2001;22:328-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Nadler HL. Current status of treatment in storage disorders. Birth Defects Orig Artic Ser. 1976;12:177-188. [PubMed] |