Published online Nov 28, 2011. doi: 10.3748/wjg.v17.i44.4875
Revised: October 12, 2011
Accepted: November 4, 2011
Published online: November 28, 2011
AIM: To study the inhibition of tumor angiogenesis by 5,2,4´-trihydroxy-6,7,5´-trimethoxyflavone (TTF1) isolated from an extract of herbal medicine Sorbaria sorbifolia.
METHODS: Angiogenic activity was assayed using the chick embryo chorioallantoic membrane (CAM) method. Microvessel density (MVD) was determined by staining tissue sections immunohistochemically for CD34 using the Weidner capillary counting method. The mRNA and protein levels of vascular endothelial growth factor (VEGF), vascular endothelialgrowth factor receptor 2 (VEGFR2, Flk-1/KDR), basic fibroblast growth factor (bFGF), cyclo-oxygenase (COX)-2 and hypoxia-inducible factor (HIF)-1α were detected by quantitative real-time polymerase chain reaction and Western blotting analysis.
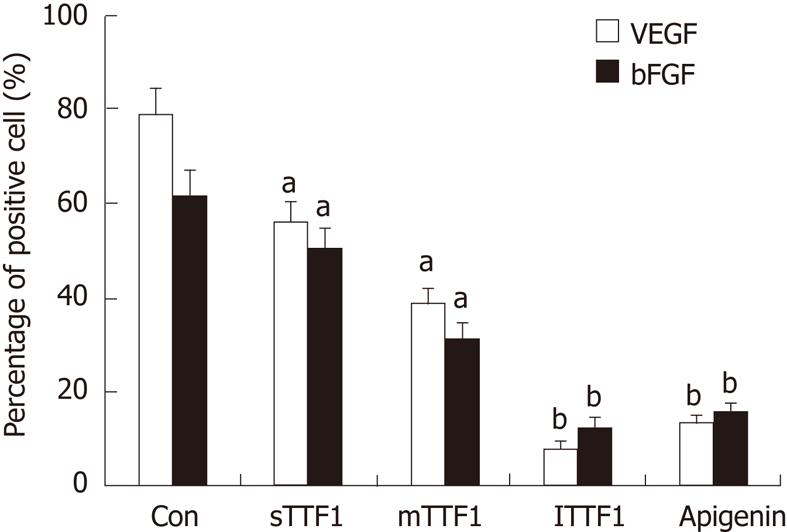
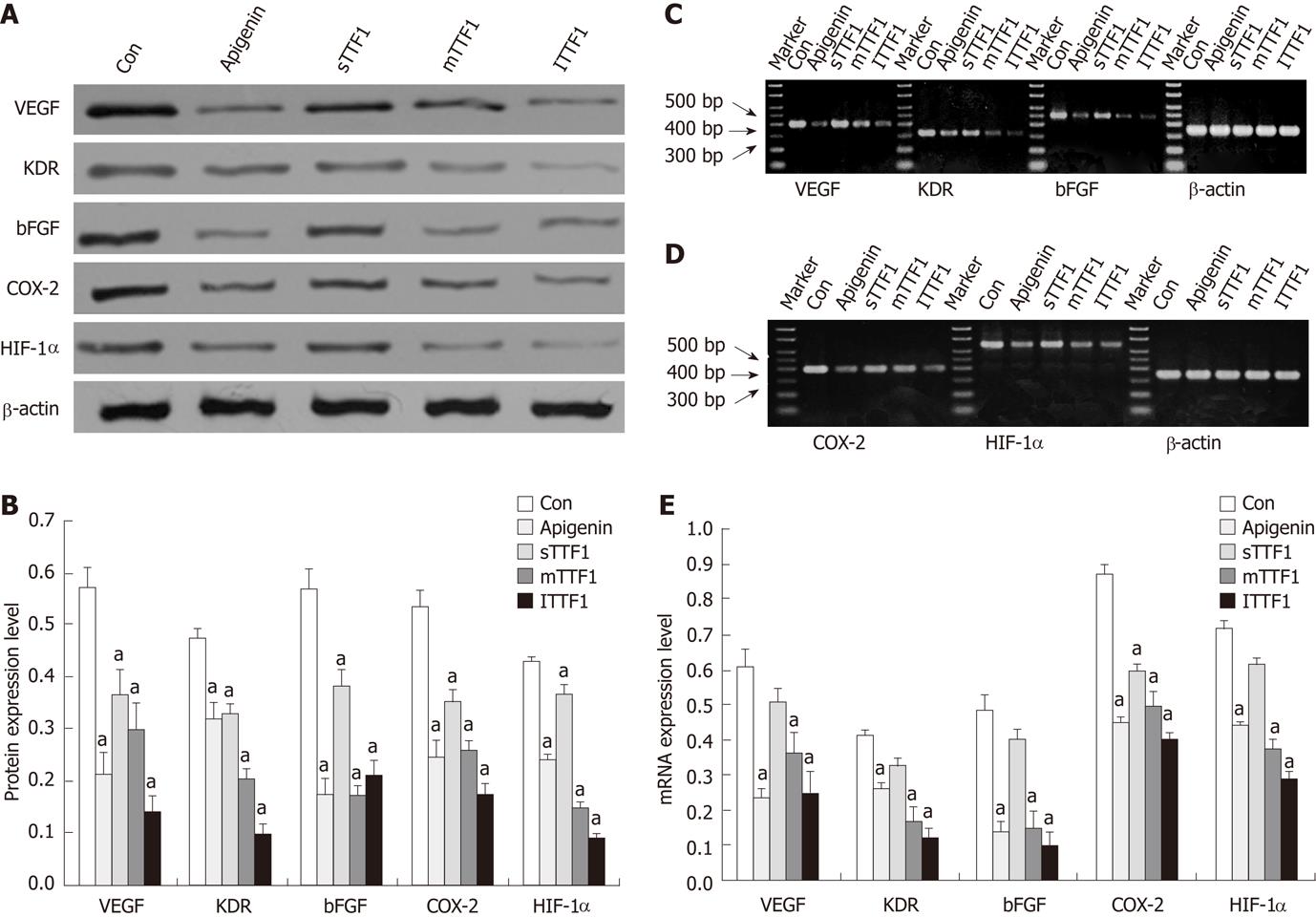
RESULTS: The TTF1 inhibition rates for CAM were 30.8%, 38.2% and 47.5% with treatment concentrations of 25, 50 and 100 μg/embryo × 5 d, respectively. The inhibitory rates for tumor size were 43.8%, 49.4% and 59.6% at TTF1 treatment concentrations of 5, 10, and 20 μmol/kg, respectively. The average MVD was 14.2, 11.2 and 8.5 at treatment concentrations of 5 μmol/kg, 10 μmol/kg and 20 μmol/kg TTF1, respectively. The mRNA and protein levels of VEGF, KDR, bFGF, COX-2 and HIF-1α in mice treated with TTF1 were significantly decreased.
CONCLUSION: TTF1 can inhibit tumor angiogenesis, and the mechanism may be associated with the down-regulation of VEGF, KDR, bFGF, HIF-1α and COX-2.
- Citation: Liu C, Li XW, Cui LM, Li LC, Chen LY, Zhang XW. Inhibition of tumor angiogenesis by TTF1 from extract of herbal medicine. World J Gastroenterol 2011; 17(44): 4875-4882
- URL: https://www.wjgnet.com/1007-9327/full/v17/i44/4875.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i44.4875
Angiogenesis is the process by which a new blood-vascular system grows from the existing vascular bed through the interaction of cytokines, the cellular matrix and proteolylic enzymes. Tumor angiogenesis is closely associated with tumor growth, metastasis, recurrence and overall prognosis. For this reason, tumor angiogenesis is a desirable target for tumor treatment[1]. Anti-angiogenesis is an important strategy for tumor therapy[2]. Many studies have demonstrated that tumor angiogenesis can be inhibited by the flavones present in Chinese herbal medicines, including apigenin, silibinin, quercetin, wogonin, genistein and luteolin[3-9]. Previously, we have reported that acetic ether extracts of the medicinal plant Sorbaria sorbifolia (S. sorbifolia) inhibits the growth of HepG-2 cells[10] and mouse S180 sarcoma, down-regulates the levels of tumor necrosis factor (TNF)-α and interleukin (IL)-2, and reduced the cellular activity of natural killer cells[11]. In addition, extracts inhibit the placental glutathione S transferase formation of positive foci in hepatoma precancerous rats and down-regulated the expression of p53 and Bcl-2. They increase the activity of superoxide dismutase and glutathione peroxidase and decrease the nitrogen monoxide (NO) synthase activity and malondiadehyde and NO concentrations[12,13]. Six compounds have been identified in the S. sorbifolia acetic ether extracts, including 5,2´,4´-trihydroxy-6,7,5´-trimethoxyflavone (TTF1), 5,7- dihydroxy-8-methoxyflavone, rutin, quercetin, daucosterol, benzoate and p-hydroxybenzoic acid, and TTF1 was the first active flavonoid compound identified[11]. After testing the six compounds, we found that TTF1 inhibited vascular endothelial growth factor (VEGF) expression in HepG-2 cells and VEGF165-induced human umbilical vein endothelial cells proliferation and vascular endothelial growth factor receptor 2 (VEGFR2, Flk-1/KDR) protein expression[10]. This study focused on the effect of TTF1 specifically on the inhibition of tumor angiogenesis.
TTF1 was separated using the water extraction and alcohol precipitation method from 10 kg S. sorbifolia (collected from Jilin Province) as previously described[11].
The HepG-2 cell line was purchased from KeyGEN Co., Ltd. (Nanjing, China). Cells were grown in RPMI1640 supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 mg/L streptomycin. Cells were cultured at 37 °C in a humidified incubator containing 5% CO2. Cells in the logarithmic growth phase were used for tests.
Angiogenic activity was assayed using a chick embryo chorioallantoic membrane (CAM) as described previously[14]. HepG-2 cell resuspensions (1 × 106) were inoculated into the chick embryo CAM. Using 4-d-old chick embryos in shells, 50 μL of different concentrations of TTF1, apigenin (KeyGEN), and normal saline were added to the chick chorioallantoic membrane once per day for 5 d. Each experimental group included five eggs, and experiments were repeated five times. Chorioallantoid membranes were collected for microscopy and photographic documentation. Five visual fields were randomly chosen for analyzing the angiogenesis inducing rate and inhibitory rate using the SmartScape microscope photography analysis system.
Inducing rate (%) = (vascular branchpoint number after inoculating tumor cells minus the vascular branchpoint number in non-inoculated tumor cells the vascular branchpoint number in non-inoculated tumor cells) × 100%
Inhibitory rate (%) = (vascular branchpoint number after inoculating tumor cells minus the vascular branchpoint number with drug treament/the vascular branchpoint number after inoculating tumor cells) × 100%
BALB/c nude mice were obtained from the Laboratory Animal Center of the Academy of Military Medical Sciences (Jilin, China). All studies were in compliance with guidelines of the Institutional Animal Care and Use Committee. 0.1 mL HepG-2 cell resuspensions (1× 106) were transplanted into the armpits of test mice subcutaneously as an experimental model. Ten days after HepG-2 cell transplantation, 40 mice bearing tumors were selected and divided into five groups, and orally administered 5, 10 or 20 μmol/kg of TTF1 or 10 μmol/kg of apigenin once a day for 10 d. The control group was treated with normal saline. Mice were sacrificed and the tumors were collected and weighed. The tumor inhibition rate was calculated as follows: inhibition rate (%) = (1- the tumor weight in treatment group/the tumor weight in control group) × 100%. Samples were fixed in a 10% formaldehyde solution to prepare the slides for hematoxylin and eosin staining and microscopy.
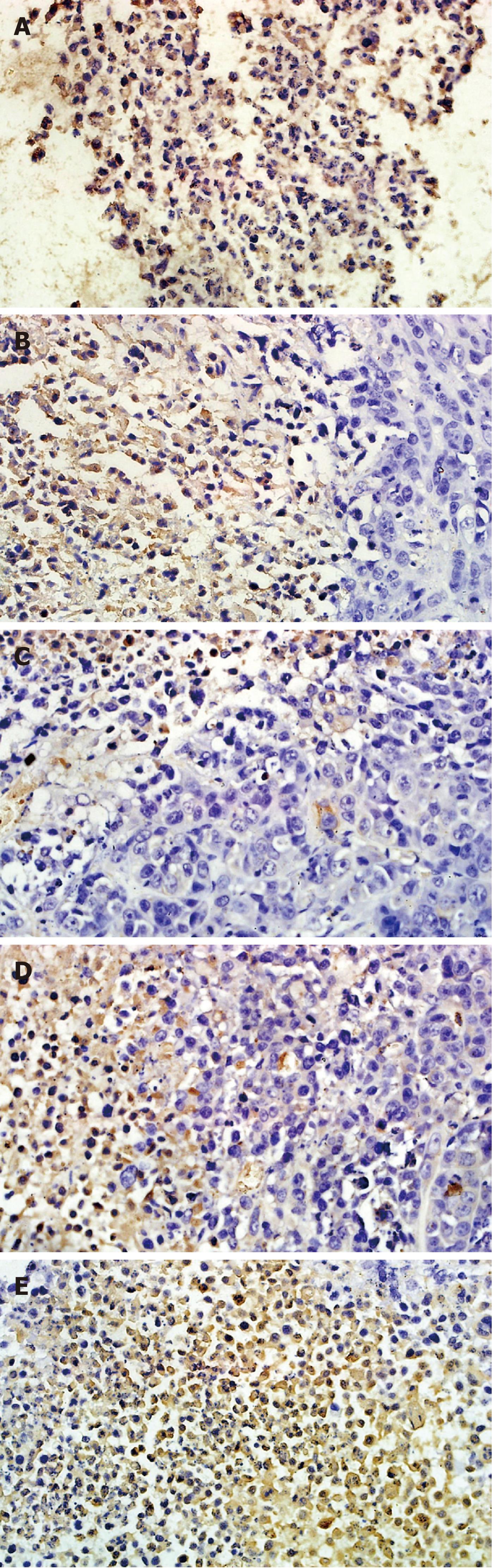
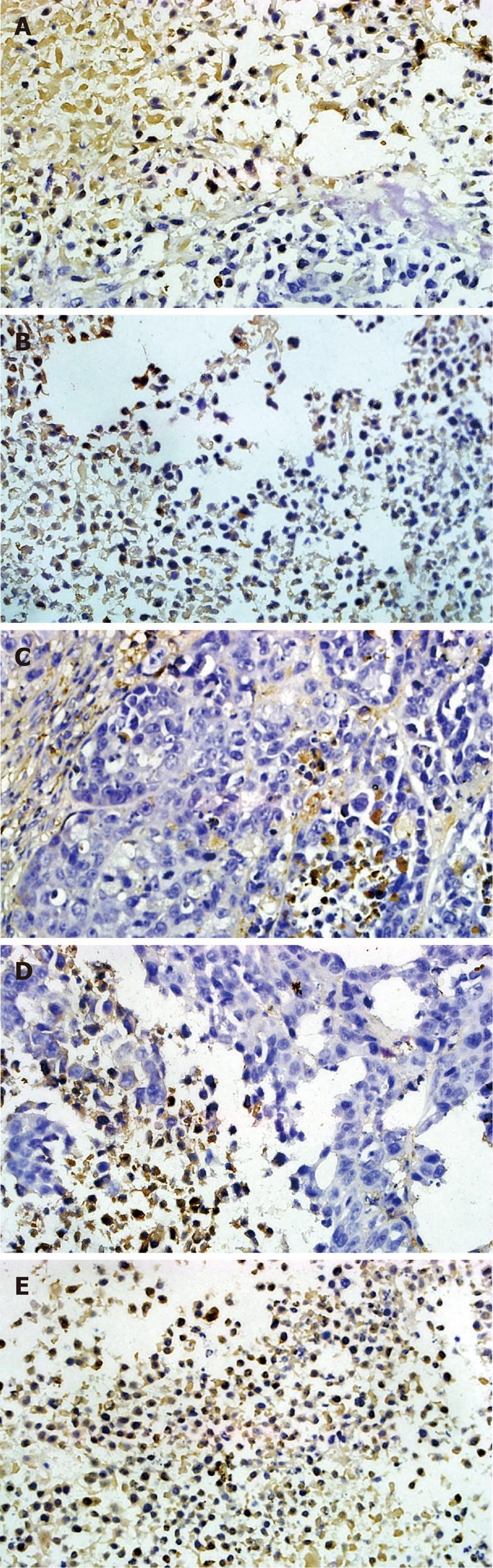
Tissues were fixed in 10% buffered formalin and embedded in paraffin. Immunodetection of blood vessels in mouse tumor sections was performed with an anti-CD34 Ab (Boshide Biotechnology Company, Wuhan, China). Sections were incubated with a biotinylated anti-rat Ab (CD34) and then with peroxidase-conjugated streptavidin (Boshide Biotechnology Company, Wuhan, China). To quantify angiogenesis, microvessel density (MVD) was determined by staining tissue sections immunohistochemically for CD34 using the Weidner capillary counting method[15]. Entire sections were scanned under low magnification, and vascularization was subjectively graded. Three highly vascularized areas per tumor were then evaluated at low magnification (× 200). Any brown-staining CD34 distinct from adjacent microvessels, tumor cells, or other stromal cells was considered a single countable microvessel. The total number of microvessels was determined from five vessels in each area, and the average number was recorded for each tumor. To test TTF1 treatment effect on VEGF and basic fibroblast growth factor (bFGF) expression in tumor, the slides were prepared by following the protocol of S-P Kit. Using the double-blind method, the pictures from at least five representative high-power fields were observed in each slice, and no less than 100 cells in each field were counted for analysis.
Tumors were lysed in lysis buffer (Pierce Roche, United States) and then centrifuged at 12 000 g for 15 min. Protein concentration was determined using the BCA kit (Pierce Rockford, United States) following the manufacturer’s instructions. Seventy μg of protein was separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to a polyvinylidene fluoride membrane (Pall Corporation, Port Washington, NY, United States). After blocking for 1 h with 5% milk in tris-buffuered saline and tween 20, the primary antibody (anti-VEGF, KDR, bFGF, COX-2 or HIF-1α; 1:400) (Boshide Biotechnology Company) was added and incubated at 4 °C overnight. After incubation with secondary antibodies (1:5000), membranes were visualized by chemiluminescence. The intensity of protein bands was quantitatively determined using a ultraviolet crosslinkers (Bio-Rad, United States) and normalized with the intensity of Actin band in each gel.
Total RNA was extracted from tumors using the RNeasy Plus Mini Kit (KeyGEN) following the manufacturer’s instructions. cDNA was generated with the iScript Select cDNA Synthesis Kit (KeyGEN) and analyzed by quantitative real-time polymerase chain reaction (PCR) using SyberGreen qPCR primer assays (KeyGEN) and the iCycler iQ multicolor real time PCR detection system (KeyGEN). Relative expression levels were normalized against β-actin expression run concurrently as a reference control. The primers used were as follows: VEGF (forward, 5’-TACGTTGGTGCCCGCTGCTG-3’; reverse, 5’-GCCCTCCGGACCCAAAGTGC-3’; amplicon size of 400 bp), KDR (forward, 5’-AGCGTGTGGCACCCACGATC-3’; reverse, 5’-GGCAATCACCGCCGTGCCTA-3’; amplicon length of 338 bp); COX-2 (forward, 5’-TTGCCCGACTCCCTTGGGTGT-3’; reverse, 5’-CTCCTGCCCCACAGCAAACCG-3’; amplicon length of 397 bp); HIF1-α (forward, 5’-ACAGCAGCCAGACGATCATGCAG-3’; reverse, 5’-TGGCTACCACGTACTGCTGGCA-3’; amplicon length of 724 bp); β-actin (forward, 5’-GCTCGTCGTCGACAACGGCTC-3’; reverse, 5’-CAAACATGATCTGGGTCA TCTTCTC-3’; amplicon length of 353 bp).
Data in all experiments are shown as mean ± SD. Statistical difference was evaluated using a one-way ANOVA and independent t test of sample pairs with SPSS 13.0 software.
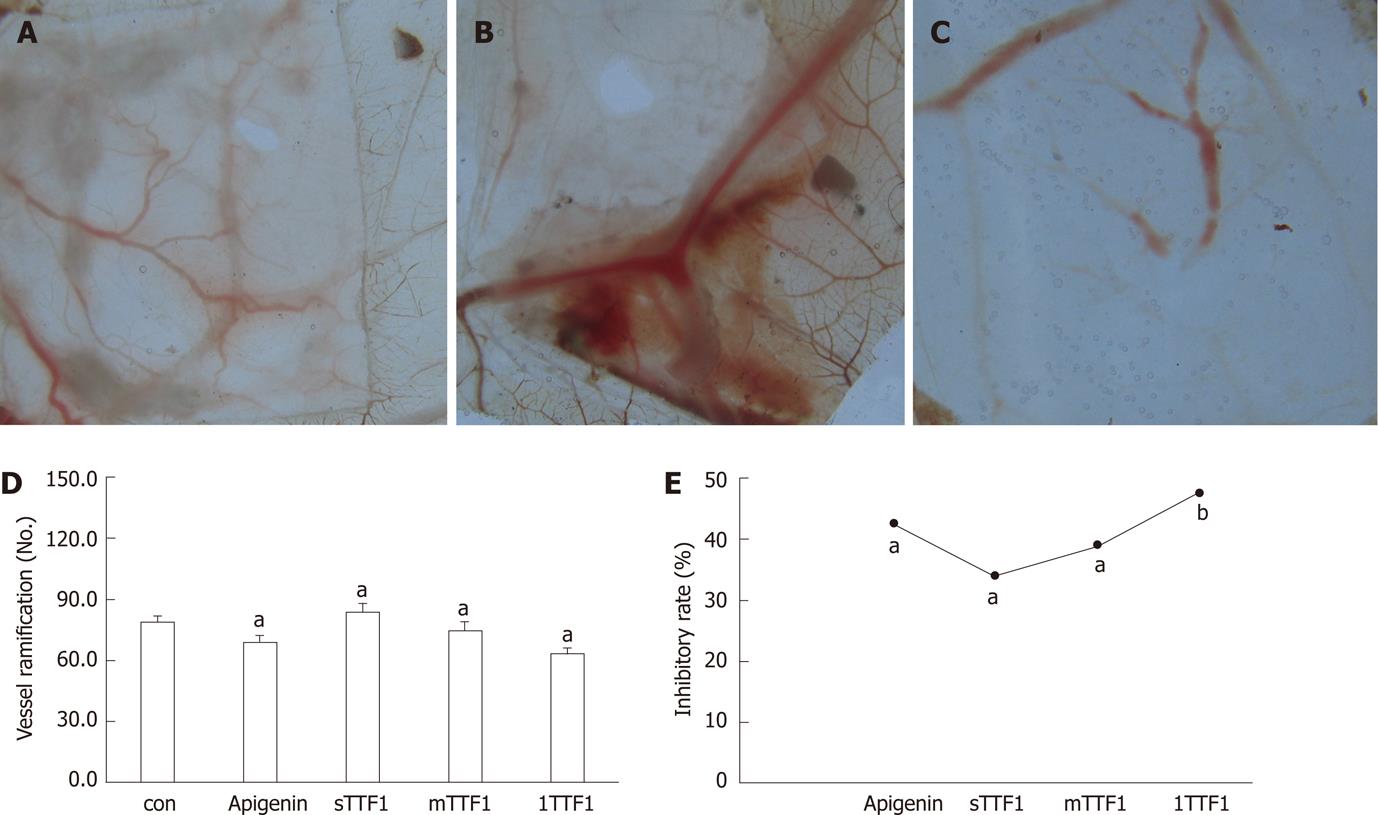
The antiangiogenic activities of TTF1 (Figure 1) were tested using the CAM assay. HepG-2 cells induced CAM angiogenesis (Figure 2B). Capillary vessels were intensively spread in HepG-2 cell-inoculated regions, vessel branching significantly increased (P < 0.05) (Figure 2A, B and D), and the inhibitory rate was 53.9%. TTF1 inhibited angiogenesis: the number of capillary vessels significantly decreased (P < 0.05) in the TTF1 treatment group (Figure 2C and D), with inhibitory rates of 30.8%, 38.2% and 47.5% with TTF1 treatment concentrations of 25, 50 and 100 μg/embryo × 5 d, respectively (Figure 2D and E). The inhibitory effect on angiogenesis in vivo by TTF1 was dose-dependent. These results indicate that TTF1 inhibited angiogenesis induced by HepG-2 cells in CAM.
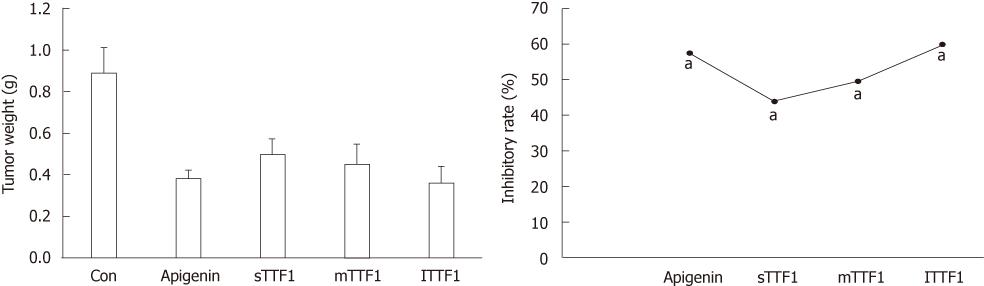
To test whether TTF1 inhibited tumor growth, we measured tumor weight after TTF1 treatment. Compared to the control group, the tumor weights in the TTF1-treated group were significantly lower (P < 0.01), with inhibitory rates of 43.8%, 49.4% and 59.6% at treatment concentrations of 5, 10 and 20 µmoL/kg, respectively (Figure 3). These results suggest that TTF1 administration blocked the growth of HepG-2 cell-induced tumors in mice and that the inhibitory rate of TTF1 was dose-dependent.
Compared to the tumors in the control group, the tumors in the TTF1 and apigenin-treated groups were smaller in size with gray surfaces. Their texture was hard, and necrosis was present in the central area but few capillary hemorrhages were observed (data not shown). Microscopy of tumors from the TTF1 and apigenin-treated groups revealed that they had fewer tumor cells, increased cell gaps with clearly visible cell boundaries, and few capillaries in the central area (data not shown).
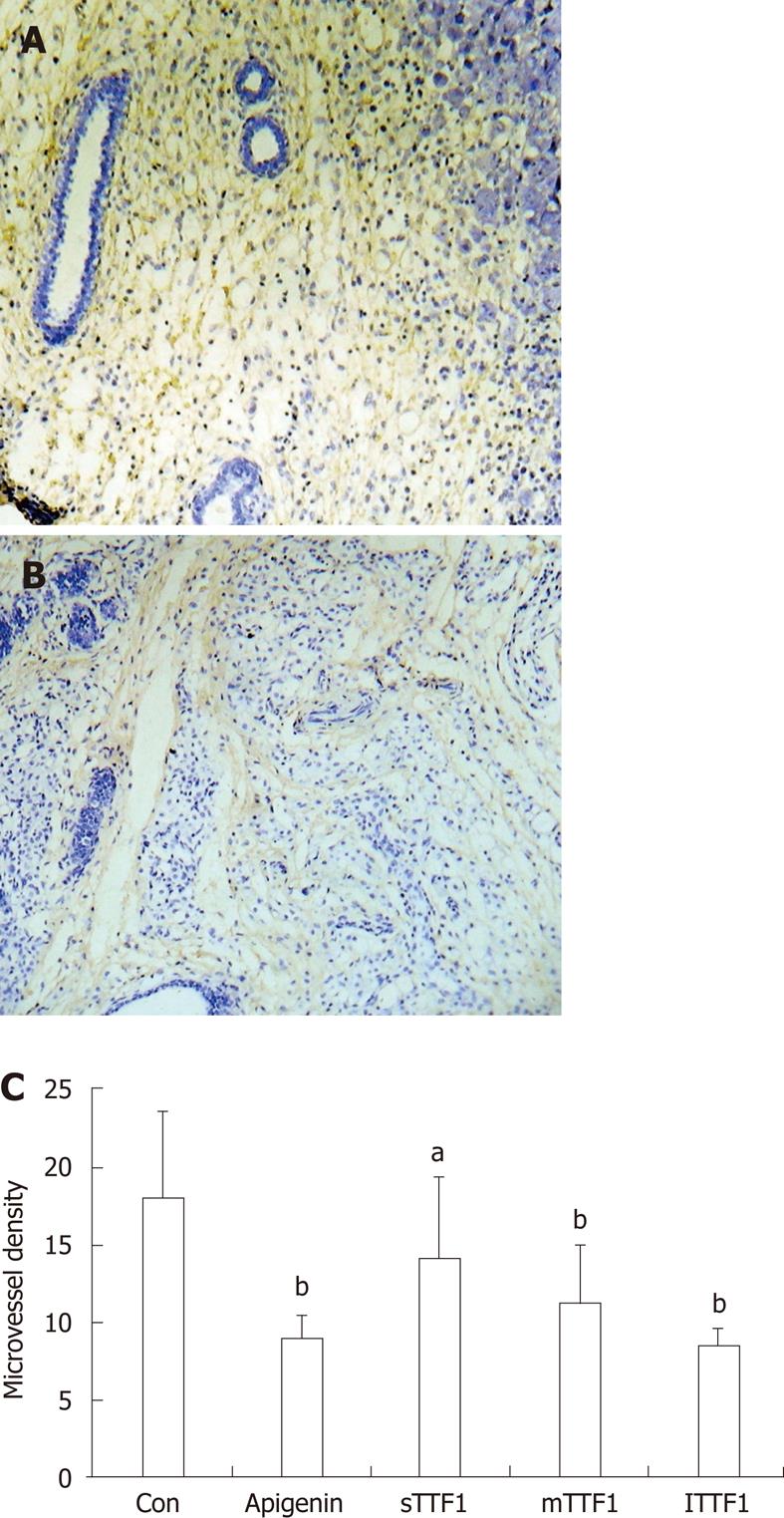
To quantify the HepG-2 cell-induced angiogenesis in mouse tumors, MVD was determined by staining tissue sections immunohistochemically for CD34. The positive staining of CD34 was brown and mainly located in the vascular endothelium of the cytomembrane and the cytoplast of capillary vessels, venules and arterioles (Figure 4A). The results showed that the number of capillary vessels greatly increased in tumor tissues in the control group, while they significantly decreased in the TTF1 treatment group (Figure 4B and C). The average MVD was 14.2, 11.2 and 8.5 at the treatment concentrations of 5 μmol/kg, 10 μmol/kg and 20 μmol/kg TTF1, respectively, and it decreased in a dose-dependent manner (Figure 4C). These results indicated that TTF1 inhibited HepG-2 cell-induced angiogenesis in mouse tumors.
To test whether TTF1 affects the expression of the angiogenesis regulation factors including VEGF, KDR, bFGF, COX-2 and HIF-1α, we analyzed the protein levels of these factors in HepG-2 cell-induced tumors in mice. Immunohistochemistry results (as shown in Figures 5 and 6) showed the effect of TTF1 on the expression of VEGF and bFGF. In the control group, expression of VEGF and bFGF was demonstrated by brown staining of the cytoplasm and membrane of cancer cells, with a focal or diffuse distribution (Figures 5E and 6E). In the TTF1 treatment group, the brown-stained VEGF and bFGF cancer cells were significantly reduced, and most of the cells were stained blue (negative), as shown in Figures 5C and 6C. Combining these results showed that treatment with TTF1 resulted in significant down-regulation of VEGF and bFGF expression in tumors (Figure 7). Western blotting indicated that the protein levels of VEGF, KDR, bFGF, COX-2 and HIF-1α were lower in tumors that were treated with TTF1 than in control tumors (Figure 8A and B). We found that the decrease in protein levels occurred in a dose-dependent manner and showed significant differences at the 10 μmol/kg and 20 μmol/kg doses (as shown in Figure 8A and B) when compared to the controls. To explore whether TTF1 inhibits gene transcription to decrease the expression of these angiogenesis regulation factors, quantitative real-time PCR (qRT-PCR) was performed to determine the mRNA levels of VEGF, KDR, bFGF, COX-2 and HIF-1α in mice treated with TTF1. Representative qRT-PCR graphs for these genes is shown in Figure 8C-E. The effect of TTF1 on the mRNA levels of VEGF, KDR, bFGF, COX-2 and HIF-1α was consistent with the effect TTF1 on their protein levels. Our results indicate that TTF1 inhibits tumor angiogenesis by decreasing the RNA and protein levels of angiogenesis regulation factors (VEGF, KDR, bFGF, COX-2 and HIF-1α).
S. sorbifolia is a Chinese medicinal plant that grows on Changbai Mountain. Our group began systematic research on its medicinal properties in 2002. An earlier study showed that an acetic ether extract of S. sorbifolia has anti-tumor, liver protective and anti-inflammatory effects. Six chemicals were identified in the acetic ether extract, and the novel monomeric compound TTF1 was separated for the first time.
Angiogenesis mainly occurs during embryo development as well as in some pathological conditions, such as damage repair, inflammation, and in particular, tumor growth and metastasis[16]. CAM is the ideal in vivo model to study angiogenesis and anti-vascular formation. Our research demonstrated that TTF1 inhibited HepG-2-induced CAM angiogenesis. We also found that TTF1 inhibited tumor growth in HepG-2-transplanted nude mice with an inhibition rate similar to that of apigenin, a flavone extracted from another Chinese medicinal plant that is currently in clinical use.
MVD is a marker to assess the level of tumor angiogenesis. An increase in MVD in tumor tissue suggests a fast-growing and potentially more metastatic tumor. After treatment with TTF1 on the transplanted tumors of nude mice, MVD decreased, suggesting that it inhibited tumor angiogenesis. VEGF is the most important inducing factor for angiogenesis, which specifically stimulates the proliferation of vascular endothelial cells and angiogenesis. VEGF proteins function in association with VEGF receptor (VEGFR) proteins. The five types of VEGFR include VEGFR-1 (Flt-1), VEGFR-2 (KDR), VEGFR-3 (Flt-4), NP-1 and NP-2. VEGF primarily functions through dimerization with KDR, and its intracellular tyrosine residues autophosphorylate after VEGF and KDR bind together. bFGF is another important inducing factor for angiogenesis. Tumor cells produce bFGF, and induce the vascular endothelial cells to produce bFGF, at the same time, increasing angiogenesis[17,18]. The expression levels of VEGF, VEGFR and bFGF were down-regulated after treatment with TTF1, suggesting that TTF1 may inhibit tumor growth through decreasing angiogenesis-inducing factors in HepG-2-transplanted nude mice.
An insufficient blood supply in fast-growing tumor tissues may cause hypoxia. HIF-1α is the transcription factor that regulates gene transcription during tissue hypoxia. TTF1 may inhibit expression of HIF-1α by suppressing its association with the regulatory sequences of VEGF and bFGF, and therefore resulting in decreased transcription. The expression of VEGF and bFGF may further decrease the expression of KDR through negative feedback. Recent studies have shown that COX-2 is associated with tumor formation, development, and angiogenesis[19]. COX-2 was down-regulated after TTF1 treatment in tumor tissues, in accordance with the down-regulation of the other angiogenesis-inducing factors VEGF, bFGFand VEGFR.
Identification of the compounds responsible for the anti-tumor angiogenesis effects of Chinese herbal medicines is a research hotspot. Our study used the anti tumor drug apigenin as a positive control and comparison for TTF1 treament. Its mechanism of anti tumor activity includes inhibition of tumor angiogenesis, induction of tumor cell apoptosis, disturbing cellular signal pathways, and anti oxidation. Our experiments showed that the inhibitory effect of TTF1 on tumor angiogenesis surpassed that of apigenin.
S. sorbifolia is a rosaceous plant that grows extensively in Changbai Mountain, in Yunnan, Guizhou, Sichuan, Hubei, Gansu and Ningxia Provinces. It is traditionally used in activating blood, dissolving stasis, reducing swelling, easing pain, and healing fractures and injuries from falls[20]. It is a perennial herbaceous plant that has low toxicity and is liver-protective. Our study explored the inhibitory effect of TTF1 on tumor growth and angiogenesis. Further study needs to focus on the different regulatory factors and their interaction using molecular biological techniques after TTF1 inhibition of tumor angiogenesis. The relationship of the chemical structure of TTF1 to its activity should be studied, so that further structural modification may lead to new inhibitors of tumor angiogenesis with better curative effect and easier production. Moreover, further study is also needed to determine whether there are other pathways (such as inducing apoptosis, regulation of nuclear factor-κB or mitogen-activated protein kinase pathways) through which TTF1 inhibits tumor growth.
Anti-angiogenesis is an important strategy for tumor therapy. Many studies have demonstrated that tumor angiogenesis can be inhibited by the flavones present in Chinese herbal medicine. Previously, the authors reported that acetic ether extracts of the medicinal plant Sorbaria sorbifolia (S. sorbifolia) inhibites the growth of HepG-2 cells and mouse S180 sarcoma, down-regulates the levels of tumor necrosis factor-α and interleukin-2, and reduces the activity of natural killer cells. 5,2´,4´-trihydroxy-6,7,5´-trimethoxyflavone (TTF1) was the first active flavonoid compound identified in S. sorbifolia. This study focused on the effect of TTF1 specifically on the inhibition of tumor angiogenesis.
Identification of the compounds responsible for the anti-tumor angiogenesis effects of Chinese herbal medicine is a research hotspot. The study used the anti-tumor drug apigenin, which is currently used clinically, as a positive control and comparison for TTF1 treatment. Its mechanism of anti-tumor activity includes inhibition of tumor angiogenesis, induction of tumor cell apoptosis, disturbing cellular signal pathways, and anti-oxidation. The experiments showed that TTF1 had an inhibitory effect on tumor angiogenesis, as did apigenin.
Six compounds were identified in acetic ether extracts of S. sorbifolia, including TTF1,5,7- dihydroxy-8-methoxyflavone, rutin, quercetin, daucosterol, benzoate, and p-hydroxybenzoic acid and TTF1 was the first active flavonoid compound identified in S. sorbifolia. After testing the six compounds, the authors found that TTF1 inhibited vascular endothelial growth factor (VEGF) expression in HepG-2 cells and VEGF165-induced human umbilical vein endothelial cell proliferation and vascular endothelial growth factor receptor 2 (VEGFR2, Flk-1/KDR) protein expression. The study explored the inhibitory effect of TTF1 on tumor growth and tumor angiogenesis.
The study results suggest that the TTF1 extracts of the medicinal plant S. sorbifolia is a potential therapeutic compound that could be used for tumor inhibition.
Sorbaria sorbifolia (S. sorbifolia) is a Chinese medicinal plant that grows on Changbai Mountain. An earlier study has shown that an acetic ether extract of S. sorbifolia has anti-tumor, liver protective and anti-inflammatory effects. Chick embryo chorioallantoic membrane, is the ideal in vivo model for studying angiogenesis and anti-vascular formation. Microvessel density (MVD), is a marker to assess the level of tumor angiogenesis. An increase in MVD in tumor tissue suggests a fast-growing and potentially more metastatic tumor.
The present paper examining the effects of extracts of the Chinese herb S. sorbifolia (TTF1) on tumor growth is work that extends and builds upon previously published work by this research group. The paper will gather a lot of interest amongst practicing gastroenterologists and oncologists.
Peer reviewer: Dr. Jeff Butterworth, MB, FRCP, Department of Gastroenterology, Shrewsbury and Telford Hospital NHS Trust, Mytton Oak Road, Shrewsbury, Shropshire SY3 8XQ, United Kingdom
S- Editor Tian L L- Editor Kerr C E- Editor Xiong L
| 1. | Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1925] [Cited by in RCA: 1998] [Article Influence: 105.2] [Reference Citation Analysis (0)] |
| 2. | Folkman J. Antiangiogenesis in cancer therapy--endostatin and its mechanisms of action. Exp Cell Res. 2006;312:594-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 467] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 3. | Bagli E, Stefaniotou M, Morbidelli L, Ziche M, Psillas K, Murphy C, Fotsis T. Luteolin inhibits vascular endothelial growth factor-induced angiogenesis; inhibition of endothelial cell survival and proliferation by targeting phosphatidylinositol 3'-kinase activity. Cancer Res. 2004;64:7936-7946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 144] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Gamble JR, Xia P, Hahn CN, Drew JJ, Drogemuller CJ, Brown D, Vadas MA. Phenoxodiol, an experimental anticancer drug, shows potent antiangiogenic properties in addition to its antitumour effects. Int J Cancer. 2006;118:2412-2420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Mirzoeva S, Kim ND, Chiu K, Franzen CA, Bergan RC, Pelling JC. Inhibition of HIF-1 alpha and VEGF expression by the chemopreventive bioflavonoid apigenin is accompanied by Akt inhibition in human prostate carcinoma PC3-M cells. Mol Carcinog. 2008;47:686-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Chen Y, Li XX, Xing NZ, Cao XG. Quercetin inhibits choroidal and retinal angiogenesis in vitro. Graefes Arch Clin Exp Ophthalmol. 2008;246:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Singh RP, Gu M, Agarwal R. Silibinin inhibits colorectal cancer growth by inhibiting tumor cell proliferation and angiogenesis. Cancer Res. 2008;68:2043-2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | Lu N, Gao Y, Ling Y, Chen Y, Yang Y, Gu HY, Qi Q, Liu W, Wang XT, You QD. Wogonin suppresses tumor growth in vivo and VEGF-induced angiogenesis through inhibiting tyrosine phosphorylation of VEGFR2. Life Sci. 2008;82:956-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Zhong Y, Krisanapun C, Lee SH, Nualsanit T, Sams C, Peungvicha P, Baek SJ. Molecular targets of apigenin in colorectal cancer cells: involvement of p21, NAG-1 and p53. Eur J Cancer. 2010;46:3365-3374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 94] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Zhang X, Zhang Y, Guan L, Quan Y, Sun Q. [Study on extraction and isolation of active constituents from Sorbaria sorbifolia and antitumor effect of the constituents in vivo]. Zhong Yao Cai. 2004;27:36-38. [PubMed] |
| 11. | Zhang XW, Cui CX, Chen LY. [Inhibition of Sorbaria sorbifolia on proliferarion of hepatoma HepG-2 cell line]. Zhong Yao Cai. 2007;30:681-684. [PubMed] |
| 12. | Zhang XW, Sun Q, Jin M, Piao CM, Li LH. [Effect of Sorbaria Sorbifolia extract on anti-oxidative activities in rats with precancerosis induced by diethylnitrosamine]. Zhong Xi Yi Jie He Xue Bao. 2003;1:47-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Zhang XW, Zhang XB, Quan JS, Shen MH, Jin HL. Inhibitory effects of sorbaria sorbifolia on den-induced precancerous hepatic foci and its antioxidative activities in rats. China J Cancer Prev Treat. 2003;10:1137-1140. |
| 14. | Wu JM, Lu Y, Gao M, Zhang WW. The advances of angiogenesis assay models. Chin Pharmacol Bull. 2008;24:11-14. |
| 15. | Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat. 1995;36:169-180. [PubMed] |
| 16. | Pandya NM, Dhalla NS, Santani DD. Angiogenesis--a new target for future therapy. Vascul Pharmacol. 2006;44:265-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 232] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 17. | Chen QJ, Zhang MZ, Wang LX. Gensenoside Rg3 inhibits hypoxia-induced VEGF expression in human cancer cells. Cell Physiol Biochem. 2010;26:849-858. [PubMed] |
| 18. | Talar-Wojnarowska R, Gasiorowska A, Olakowski M, Lekstan A, Lampe P, Smolarz B, Romanowicz-Makowska H, Kulig A, Malecka-Panas E. Vascular endothelial growth factor (VEGF) genotype and serum concentration in patients with pancreatic adenocarcinoma and chronic pancreatitis. J Physiol Pharmacol. 2010;61:711-716. [PubMed] |
| 19. | Han YD, Hong YK, Kang JG, Choi YJ, Park CH. Relation of the expression of cyclooxygenase-2 in colorectal adenomas and adenocarcinomas to angiogenesis and prognosis. J Korean Soc Coloproctol. 2010;26:339-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Zhang X, Ma C, Guan L, Quan Y. [Experimental study on Sorbaria sorbifolia extract against chronic liver damage in rats]. Zhong Yao Cai. 2004;27:751-753. [PubMed] |