INTRODUCTION
Juvenile polyposis syndrome (JPS) is a rare autosomal dominant hereditary disorder characterized by multiple distinct juvenile polyps in the gastrointestinal tract and an increased risk of colorectal cancer. Sporadic solitary colorectal juvenile polyps occur in approximately 2% of the paediatric population but these polyps are not associated with an increased risk of gastrointestinal cancer[1,2]. Juvenile polyposis syndrome is defined by the presence of five or more juvenile polyps in the colorectum, juvenile polyps throughout the gastrointestinal tract or any number of juvenile polyps, and a positive family history of juvenile polyposis[1,3]. About 50%-60% of JPS patients have a germline mutation in the SMAD4 or BMPR1A gene[4-6].
HISTOLOGY
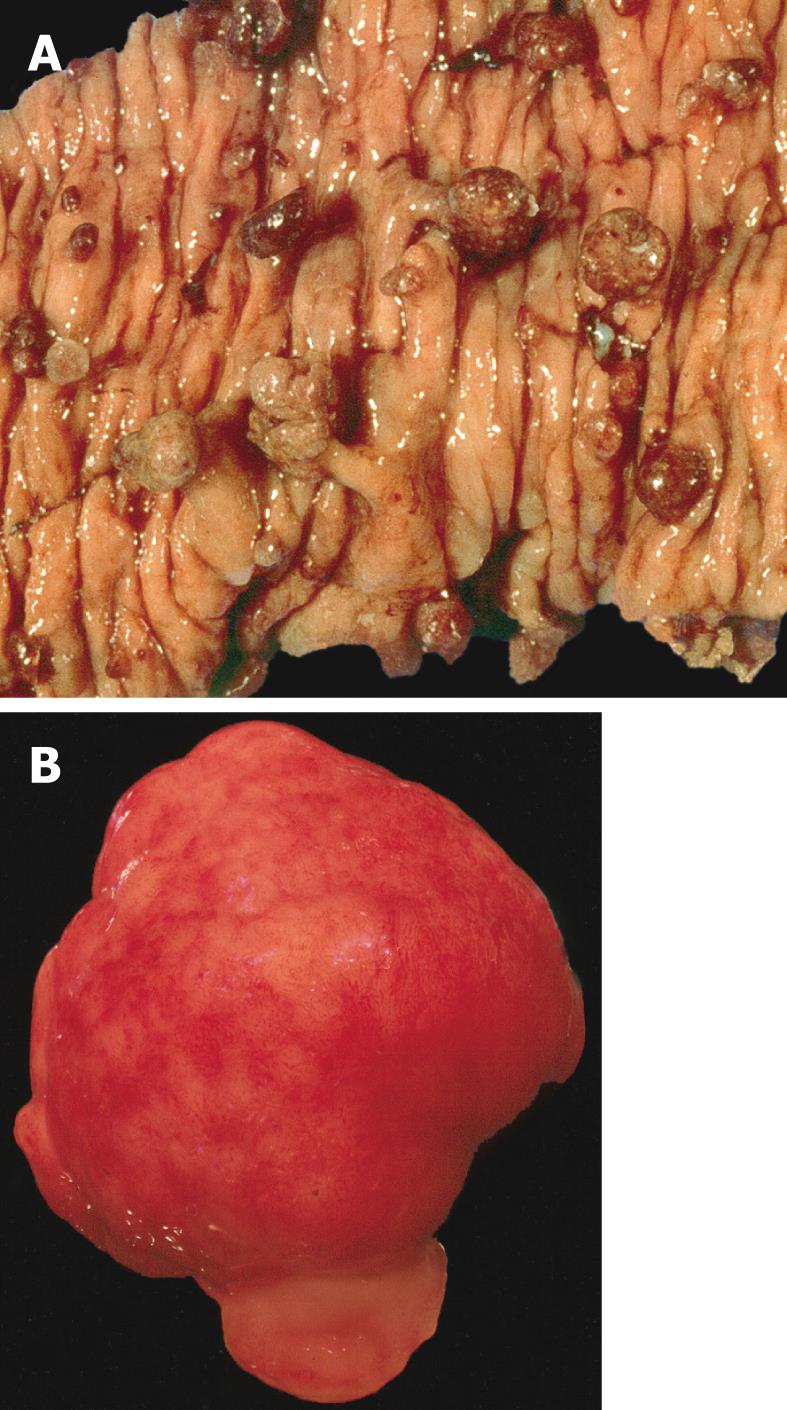
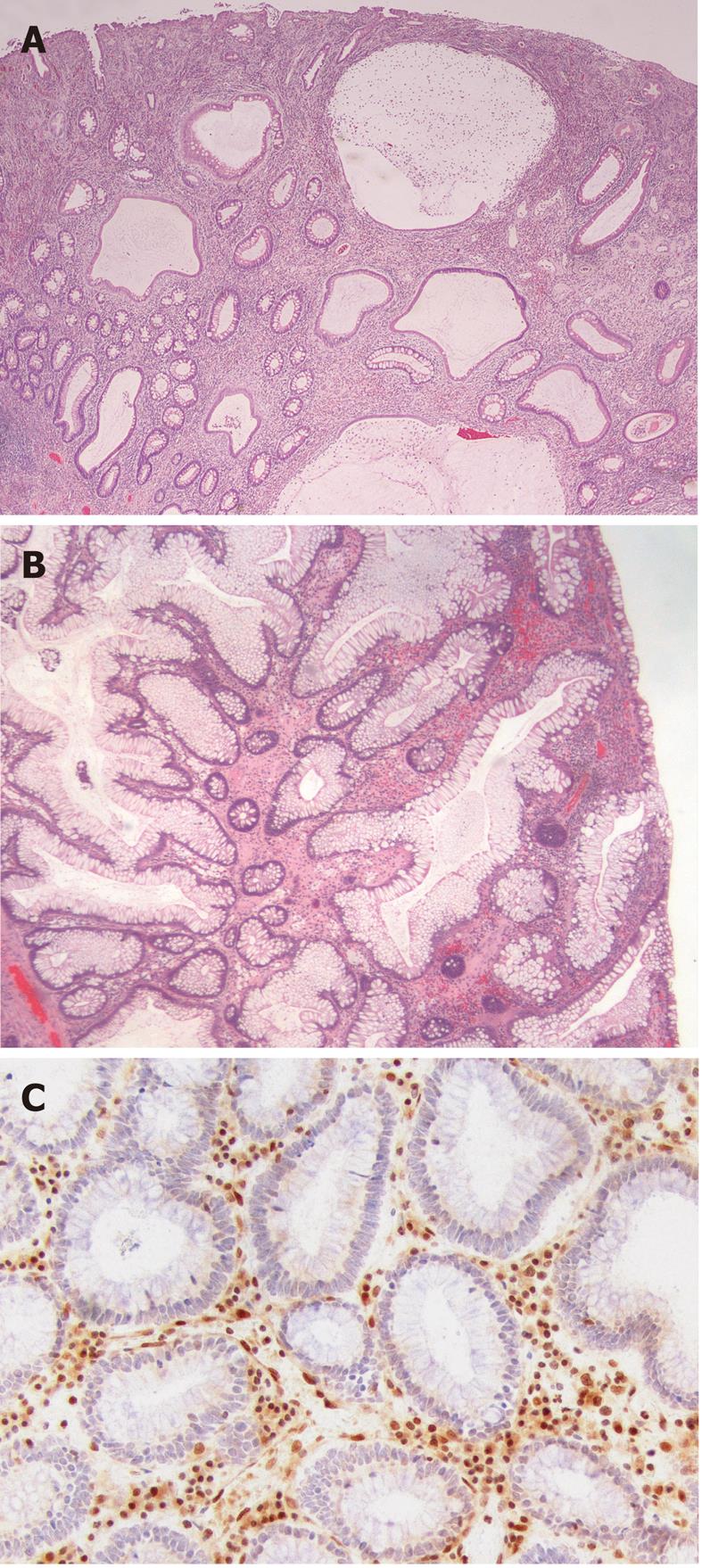
The juvenile polyp is a histopathological entity first reported by Diamond[7] in 1939 and later described in more detail by Helwig[8]. Macroscopically, juvenile polyps vary in size from 5 mm to 50 mm, and typically have a spherical, lobulated and pedunculated appearance with surface erosion (Figure 1A and B). Microscopically, a juvenile polyp is characterized by an abundance of edematous lamina propria with inflammatory cells and cystically dilated glands lined by cuboidal to columnar epithelium with reactive changes (Figure 2A and B). The distinction between an inflammatory and a juvenile polyp is often difficult. In essence, juvenile polyps in juvenile polyposis syndrome appear similar to sporadic solitary juvenile polyps, although syndromic polyps often have a frond-like growth pattern with fewer stroma, fewer dilated glands and more proliferative smaller glands[9]. In addition, polyps in juvenile polyposis syndrome frequently show neoplastic changes to the epithelium not found in sporadic solitary juvenile polyps. Colorectal polyps from individuals with a SMAD4 germline mutation often have a more proliferative epithelial phenotype and fewer stroma compared to those from patients with a BMPR1A germline mutation (Figure 2A and B)[10]. In addition, absence of the SMAD4 protein on immunohistochemistry of a juvenile polyp indicates that the patient carries a germline SMAD4 mutation (Figure 2C)[11].
Figure 1 Macroscopic appearance of juvenile polyposis.
A: Bowel resection of a patient with juvenile polyposis syndrome showing multiple spherical pedunculated polyps with a smooth surfaces; B: Gross appearance of a juvenile polyp from a patient with juvenile polyposis syndrome. Note the smooth surface, in contrast with a Peutz-Jeghers polyp.
Figure 2 Histological appearance of juvenile polyposis.
A: Histological section of a juvenile polyp from a juvenile polyposis patient with a germline mutation of BMPR1A. Typically, juvenile polyps are characterized by prominent lamina propria with edema and inflammatory cells, and cystically dilated glands lined by cuboidal to columnar epithelium with reactive changes; B: Histological section of a juvenile polyp from a juvenile polyposis patient with a germline mutation of SMAD4. This polyp shows relatively fewer stroma, fewer dilated glands and more proliferative smaller glands; C: SMAD4 immunohistochemistry on a juvenile polyp showing absent SMAD4 expression in the epithelium, indicating that this patient carries a germline SMAD4 mutation.
Small intestinal polyps in JPS have been classified as juvenile[12,13], hyperplastic and/or inflammatory polyps[14-16], and as lymphoid hyperplasia[15,17]. The larger small intestinal polyps resemble juvenile polyps in the colon[17]. In addition, juvenile/hamartomatous polyps with dysplastic changes and adenomas have been found in the duodenum, jejunum, and ileum of patients with JPS[12,14,16]. Moreover, we have seen a Brunner gland hamartoma in the duodenum of a juvenile polyposis patient with a SMAD4 germline mutation. Most gastric polyps in JPS patients have been diagnosed as hyperplasic polyps[14] and are indistinguishable from gastric hyperplastic polyps[18].
GENETICS
A germline mutation in the SMAD4 or BMPR1A gene is found in about 50%-60% of JPS patients[4-6]. Both genes are involved in the BMP/TGF-beta signalling pathway. Most germline defects are point mutations or small base pair deletions in the coding regions of SMAD4 or BMPR1A that can be identified by conventional sequence analysis. About 15% of the germline genetic defects are deletions of one or more exons, or the entire SMAD4 or BMPR1A coding sequence, which necessitates identification by techniques that analyze large genomic deletions, such as multiplex ligation-dependent probe amplification (MLPA)[4,6]. Recently, previously unknown mutations in the BMPR1A promoter region were found in about 10% of JPS patients[19].
About 30%-40% of JPS patients have no germline mutation; therefore, a number of candidate genes, mostly involved in the transforming growth factor β (TGF-β)/bone morphogenetic proteins (BMP) pathway, have been investigated for a role in JPS pathogenesis. Although not confirmed, and questioned by others, germline mutations of the TGB-β co-receptor Endoglin has been reported in two JPS patients[4,20]. In addition, SMAD1, SMAD2, SMAD3, SMAD5, SMAD7, BMPR2, BMPR1B, ACVRL1, TGFBRII and CDX2 have been analyzed; however, no germline mutations have been found in these genes[20]. In addition, PTEN, the gene linked to Cowden (CS) and Bannayan-Riley-Ruvalcaba syndrome (BRRS), has been suggested as a JPS gene. However, PTEN mutations in patients with juvenile polyps likely represent CS or BRRS patients that have not (yet) developed extraintestinal clinical features specific to these conditions[21]. A recent study involving a large number of PTEN germline-mutation positive Cowden syndrome patients substantiated this notion by showing that both upper- and lower gastrointestinal polyps are a common manifestation of this syndrome[22]. Patients afflicted by Cowden syndrome may develop colorectal juvenile polyps indistinguishable from those in juvenile polyposis syndrome. Therefore, although the exact gastrointestinal manifestations of Cowden syndrome remain to be clarified, particularly with respect to the upper gastrointestinal tract, Cowden syndrome should be part of the differential diagnosis in a patient presenting with a juvenile polyp.
CLINICAL PRESENTATION
Clinically, juvenile polyposis can present in two forms. The first is called juvenile polyposis of infancy. This is a generalized form occurring in infants with polyps in the stomach, small bowel and colon. The polyps vary in size from 1 to 30 mm and may be sessile or pedunculated. These infants suffer from diarrhoea, haemorrhage, malnutrition and intussusception. Death usually occurs at an early age. In addition, many of these patients have congenital abnormalities, including macrocephaly and generalized hypotonia[23]. Some investigators suggest that this rare form of juvenile polyposis is caused by continuous deletion of BMPR1A and PTEN genes located on chromosome 10q23.2 and 10q23.3 respectively, although others disagree[24,25].
In addition, generalized juvenile polyposis and juvenile polyposis coli (juvenile polyps restricted to the colorectum) have been defined[26]. However, these forms appear to be variable expressions of the same disease, because patients of both forms have been reported to segregate according to a dominant mode in the same family[24,27]. These forms may be sporadic, i.e., ‘de novo’, or inherited, and usually present later in childhood or in adult life. They are characterized by the presence of gastrointestinal juvenile polyposis and an increased risk of gastrointestinal cancer[28]. A variety of extra-intestinal manifestations have been reported in these patients[23]. In approximately 50% of juvenile polyposis coli or generalized JPS cases, a heterozygous germline mutation in the SMAD4 or BMPR1A gene is identified[24]. Several differences in phenotypic expression between carriers of a SMAD4 and BMPR1A mutations have been noted. SMAD4 mutations are associated with a more aggressive gastrointestinal phenotype, involving higher incidence of colonic adenomas and carcinomas and more frequent upper gastrointestinal polyps and gastric cancer than patients with a BMPR1A mutation[6,29,30]. Also, the combined syndrome of JPS and hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu syndrome) is associated with germline mutations in SMAD4[31].
POLYP DISTRIBUTION
Polyps in JPS predominantly occur in the colorectum, varying in number from five to several hundred. In addition, polyps can be found in the stomach, duodenum, jejunum, and ileum, although the incidence of upper gastrointestinal tract polyps in JPS is less well studied. Rarely, profuse gastric juvenile polyposis is found in the absence colonic polyps[32]. As noted above, upper gastrointestinal polyposis and gastric cancer has been associated with SMAD4 germline mutation[6,29,30].
Few studies systematically examined upper gastrointestinal tract involvement in juvenile polyposis[12,14,33]. One investigation found gastric polyps in 10 out of 12 (83%) patients, mostly located in the antrum, but throughout the stomach in six individuals. Rarely, profuse gastric juvenile polyposis is found in the absence of colonic polyps. Duodenal polyps were found in four out of 12 (33%) JPS patients, with two having multiple polyps ranging in size from 0.5 to 1.5 cm. and two others with minute polyps[14]. Using capsule endoscopy, small-bowel polyps beyond the range of standard gastroscopy were found in 2 of 10 (20%) patients and duodenal polyps in 4 others (40%)[33]. Another study reported small bowel polyps in 8 of 56 JPS patients (14%)[12]. Moreover, a number of case reports of duodenal, jejunal, and ileal polyps in JPS patients exist[13,15-17,34], In addition, juvenile polyps are frequently found in the ileal pouch of juvenile polyposis patients who have undergone proctocolectomy[35,36].
CANCER RISK
Juvenile polyposis is associated with an increased risk of gastrointestinal cancer. A recent cancer risk analysis calculated a cumulative life-time risk for colorectal cancer in JPS of 39% and a relative risk of colorectal cancer of 34[37]. However, this may be a conservative estimate, because some patients in this study had already undergone prophylactic colectomy. Jass reported a 68% cumulative risk of colorectal cancer in patients from the St Mark’s Registry, but details were not provided[38]. In addition, several cases of stomach, duodenal, and pancreatic cancer in JPS have been described in the literature, but no formal risk analysis for these malignancies exists[28]. One study found small bowel carcinoma in six out of 56 (11%) JPS patients, but four of these cancers occurred in one family[12]. Evaluation of literature reports suggests that gastric and small bowel carcinoma, together, occur at about one-fifth the frequency of colorectal cancers in this patient group[37].
CANCER PATHOGENESIS
Cancer pathogenesis in juvenile polyposis has not yet been unravelled and may develop through the so-called “landscaper mechanism”. The landscaper model was proposed after the observation that the genetic alterations at chromosome 10q22 (BMPR1A locus) occurred predominantly in the stroma of juvenile polyps. This paradigm postulates that cancer develops as a result of an abnormal stromal environment, which leads to neoplastic transformation of the adjacent epithelium[39]. Support for a “landscaper” defect triggering juvenile polyposis came from a study in which disrupted BMP signalling, through expression of a natural pathway inhibitor, resulted in development a juvenile polyposis-like phenotype in mice[40]. BMP-4 expression is normally limited to the mesenchymal compartment of the murine intestine, suggesting that disruption of this mesenchymal signal is involved in mediating juvenile polyposis.
Notwithstanding the concept that faulty transmission or receipt of mesenchymal signals by the epithelium may trigger juvenile changes, others have found that homozygous SMAD4 deletions are limited to the epithelium of juvenile polyps from JPS patients with germline SMAD4 mutations and in Smad4 knockout mice[11,41]. Although further studies are needed, this suggests that SMAD4 may act as a “gatekeeper”, instead of a “landscaper” in JPS pathogenesis, consistent with the role of SMAD4 in other cancer types[42].
MANAGEMENT
Management of JPS is mainly based on expert opinion[23,43-45]. Patients at risk or with a high suspicion of JPS should have endoscopic screening of the colon and upper gastrointestinal tract at age 15 or at the time of first symptoms[44]. At diagnosis of JPS, the entire gastrointestinal tract should be examined for the presence of polyps[23]. Genetic testing can be useful for at-risk members from families, where germline mutations have been identified. If no germline mutation is found in at-risk persons, then they do not have JPS and can be followed according to the guidelines for screening programs for the general population[44].
Endoscopic examination of the colon and upper gastrointestinal tract is recommended every two to three years in patients with JPS. In patients with polyps, endoscopic screening should be performed yearly, until the patient is deemed polyp-free. Patients with mild polyposis can be managed by frequent endoscopic examinations and polypectomy[23,36,44]. Intraoperative enteroscopy to evaluate small intestinal polyps can be considered at the time of colorectal surgery[16]. Endoscopic treatment of gastric polyps can be difficult, and patients with symptomatic gastric polyposis (e.g., severe anaemia) may need subtotal or total gastrectomy.
Prophylactic surgery is considered in patients with colorectal polyposis unmanageable by endoscopy (> 50-100 polyps), those with severe gastrointestinal bleeding or diarrhoea, juvenile polyps with dysplasia, and patients with a strong family history of colorectal cancer[35-37]. Surgical options include subtotal colectomy with ileorectal anastomosis, or total proctocolectomy with pouch[35,36]. Analogous to familial adenomatous polyposis, surgical type may depend on the extent of rectal polyposis. Recurrence of rectal polyps in patients with subtotal colectomy is frequent, and about half of these individuals require subsequent proctectomy[35,36]. Therefore, total proctocolectomy has been advocated as the initial surgery for patients with massive juvenile polyposis, who are unable to be managed endoscopically[36]. Although the surgery of choice in JPS remains debatable, patients need frequent post-operative endoscopic surveillance because of the high recurrence rates of polyps in the remnant rectum and the pouch[35].
In JPS patients with a germline SMAD4 mutation, screening should be considered for signs of hereditary hemorrhagic teleangiectasia, including chest radiography for arteriovenous malformations, magnetic resonance imaging of the brain, and liver sonography[31]. Digital clubbing and pulmonary osteoarthropathy are frequently described in combination with arteriovenous malformations[17].
COX-2 expression is higher in JPS polyps than in sporadic juvenile polyps and correlates with polyp size and dysplasia[46]. This observation suggests that chemoprevention using selective or non-selective COX-2 inhibitors could be beneficial in JPS. Currently, nonsteroidal anti-inflammatory drugs (NSAID) chemoprevention in JPS has not been systematically studied; however, two JPS patients who had undergone proctocolectomy with pouch reconstruction and subsequent polypectomy from the pouch had no further polyp development in the pouch while on sulindac[35]. However, the value of NSAID chemoprevention in JPS requires further investigation.
CONCLUSION
JPS is a rare hamartomatous polyposis syndrome characterized by the presence of multiple distinct juvenile polyps in the gastrointestinal tract. The primary defect in JPS may be stromal rather than epithelial. This so-called ‘landscaper’ defect may ultimately lead to neoplastic transformation in the overlying epithelium, although the polyps are not neoplastic per se. On the contrary, juvenile polyps may be considered true hamartomas, i.e., anomalies in the developmental patterning of the gut. Juvenile polyposis syndrome is, therefore, a unique model for studying carcinogenesis in the gastrointestinal tract.
Although rare, recognition of this condition is important in view of the consequences for patients and their families. Each clinician confronted with the diagnosis of a juvenile polyp should consider the possibility of juvenile polyposis syndrome. The number of juvenile polyps should be documented, along with the family history of gastrointestinal polyps and cancer. If a patient fulfils the clinical criteria of JPS, further diagnostic evaluation is indicated.
Future studies on the molecular and clinical aspects of JPS will result in a better understanding of gastrointestinal carcinogenesis and improved management of patients afflicted by this disorder.
Peer reviewer: Zoran V Krivokapic, Professor, First Surgical Clinic, Third Department, Clinical Center of Serbia, 6, Dr Koste Todorovica, Belgrade, 11000 Serbia, Republic of Ireland
S- Editor Wu X L- Editor Stewart GJ E- Editor Xiong L