Published online Nov 14, 2011. doi: 10.3748/wjg.v17.i42.4729
Revised: January 24, 2011
Accepted: January 31, 2011
Published online: November 14, 2011
Composite colorectal carcinomas are rare. There are a modest number of cases in the medical literature, with even fewer cases describing composite carcinoma with neuroendocrine and squamous components. There are to our knowledge no reports of composite carcinoma molecular alterations. We present a case of composite carcinoma of the splenic flexure in a 33 year-old Caucasian male to investigate the presence and prognostic significance of molecular alterations in rare colonic carcinoma subtypes. Formalin-fixed paraffin-embedded (FFPE) tissue was hematoxylin and eosin- and mucicarmine-stained according to protocol, and immuno-stained with cytokeratin (CK)7, CK20, CDX2, AE1/AE3, chromogranin-A and synaptophysin. DNA was extracted from FFPE tissues and molecular analyses were performed according to lab-developed methods, followed by capillary electrophoresis. Hematoxylin and eosin staining showed admixed neuroendocrine and keratinized squamous cells. Positive nuclear CDX2 expression confirmed intestinal derivation. CK7 and CK20 were negative. Neuroendocrine cells stained positively for synaptophysin and AE1/AE3 and negatively for chromogranin and mucicarmine. Hepatic metastases showed a similar immunohistochemical profile. Molecular analysis revealed a G13D KRAS mutation. BRAF mutational testing was negative and microsatellite instability was not detected. The patient had rapid disease progression on chemotherapy and died 60 d after presentation. Although the G13D KRAS mutation normally predicts an intermediate outcome, the aggressive tumor behavior suggests other modifying factors in rare types of colonic carcinomas.
- Citation: Wentz SC, Vnencak-Jones C, Chopp WV. Neuroendocrine and squamous colonic composite carcinoma: Case report with molecular analysis. World J Gastroenterol 2011; 17(42): 4729-4733
- URL: https://www.wjgnet.com/1007-9327/full/v17/i42/4729.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i42.4729
Composite carcinomas are composed of at least two different intermingled malignant cell types. Such tumors have been documented in numerous gastrointestinal organs[1-4]. The usual composite carcinoma contains adenocarcinoma and neuroendocrine components; however, spindle cell, osteoid metaplasia, squamous, and pleomorphic patterns have been identified[5]. Although up to 20% of colonic adenocarcinomas contain neuroendocrine cells, these have not been shown to influence prognosis[5]. When rendering a diagnosis of composite carcinoma, it is recommended that tumors contain a neuroendocrine component comprising at least one-third to one-half of the tumor[5].
The behavior of composite carcinomas is variable. In some cases, tumor behavior is similar to adenocarcinoma[5]; however, some case reports show progression to death within months[6]. The follow-up interval is not known in all of these cases. In some composite carcinomas, metastatic disease is solely from the neuroendocrine component[7], while others have shown composite metastases[8].
Primary squamous carcinoma of the colon occurs in one out of every 2000-4000 colorectal carcinomas[9]. Diagnostic criteria include the exclusion of metastases from other organs, the absence of a squamous fistula tract, the absence of concurrent anal squamous carcinoma, and the presence of squamous features on histologic examination[10]. The most frequent sites of primary squamous carcinoma of the colon are the proximal colon and rectum. Patients usually present during the fifth decade of life and more than 15% have distant metastases at presentation[11]. The average survival after diagnosis is 30 mo and the 5-year survival is approximately 30%[11]. Surgical resection remains the mainstay of therapy, and cisplatin and 5-fluorouracil are commonly used chemotherapeutic agents[12]. Squamous carcinomas with small cell or undifferentiated features have a very poor prognosis[13].
Tumor microsatellite instability (MSI) and KRAS and BRAF mutations have prognostic and predictive significance in colorectal adenocarcinomas, but little is known regarding these findings in the rarer subtypes of colorectal carcinomas such as high grade neuroendocrine carcinoma or squamous cell carcinoma. Microsatellite instability is found in 10-15% of sporadic colorectal carcinomas. MSI-H patients typically have proximal tumors with an overall more favorable outcome compared to tumors with microsatellite-instability. BRAF mutations, of which greater than 90% are V600E, occur in 70% of sporadic MSI cases and in 10%-15% of microsatellite-stable cases[14]. KRAS mutations are found in 30%-50% of colorectal carcinomas. The G12D, G12V and G13D mutations are the most common KRAS mutations, in order of decreasing frequency[15]. In particular, the G12V mutation is an independent risk factor for a 30% increase in relapse or death[16], while the G13D mutation predicts an intermediate outcome between two broad groups of cases; those with BRAF, G12D and G12V mutations and those with MSI-H with concurrent G13D mutations[15]. To our knowledge molecular alterations have not been characterized in composite carcinomas of the colon with neuroendocrine and squamous components. This case report describes an aggressive and fatal tumor in an unusual gastrointestinal location in an unusual age demographic, with a G13D KRAS mutation.
A 33 year-old Caucasian male veteran presented to the Nashville Veterans Affairs Medical Center with a 3-wk history of anorexia, dry heaves, bloating, mid-epigastric pain, and night sweats. The patient’s past medical history is unremarkable except for an esophageal stricture treated with endoscopic dilatation, and gastroesophageal reflux disease treated with proton pump inhibitors. On examination, the abdomen was mildly distended with no palpable mass. Laboratory studies revealed a leukocytosis (white blood cell 18 600/μL) and a creatinine of 1.19 mg/dL (to convert to millimoles per liter, multiply by 0.0555), indicating renal impairment due to spontaneous tumor lysis. Alkaline phosphatase was 929 U/L, and aspartate aminotransferase and alanine aminotransferase were 221 and 93 U/L. A carcinoembryonic antigen level was not obtained. Right upper quadrant ultrasound revealed a multinodular liver, and non-contrast computed tomography of the chest, abdomen and pelvis showed descending colon wall thickening with enlarged mesenteric lymph nodes, and an enlarged liver with innumerable coalescing lesions measuring 2-4 cm. No other masses were identified.
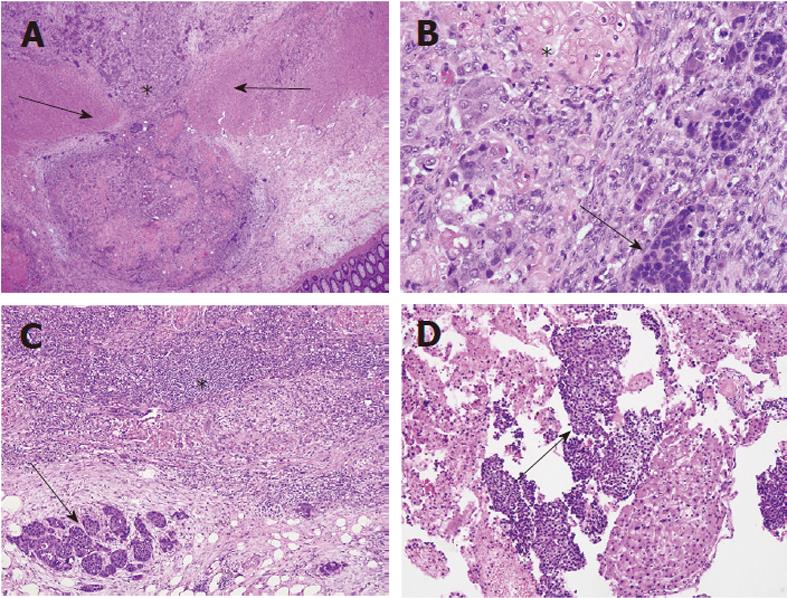
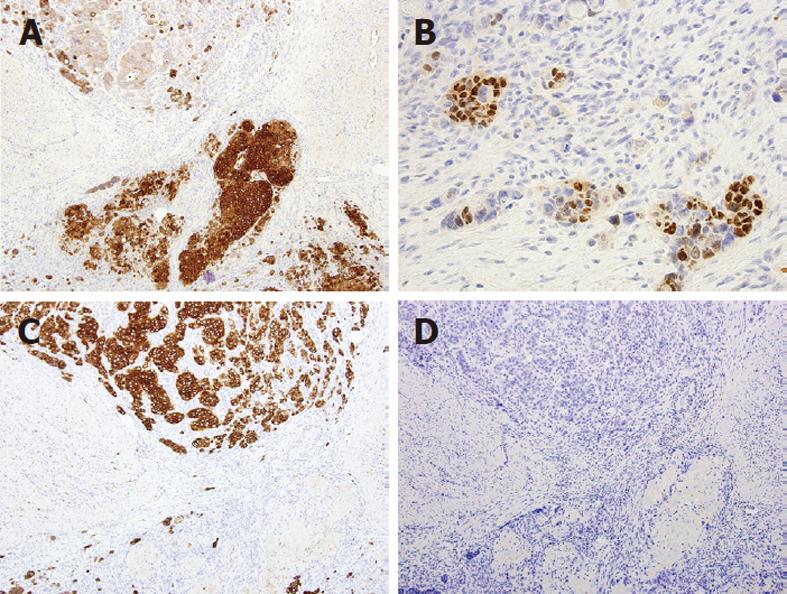
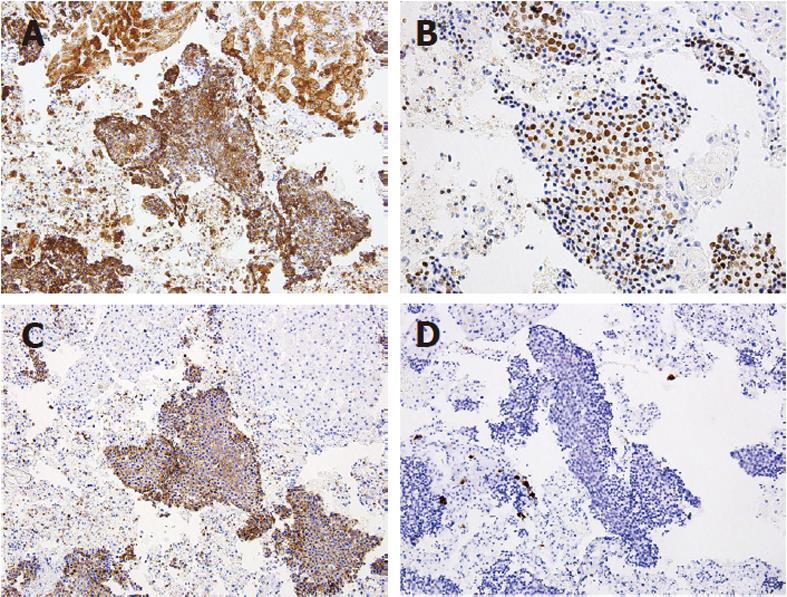
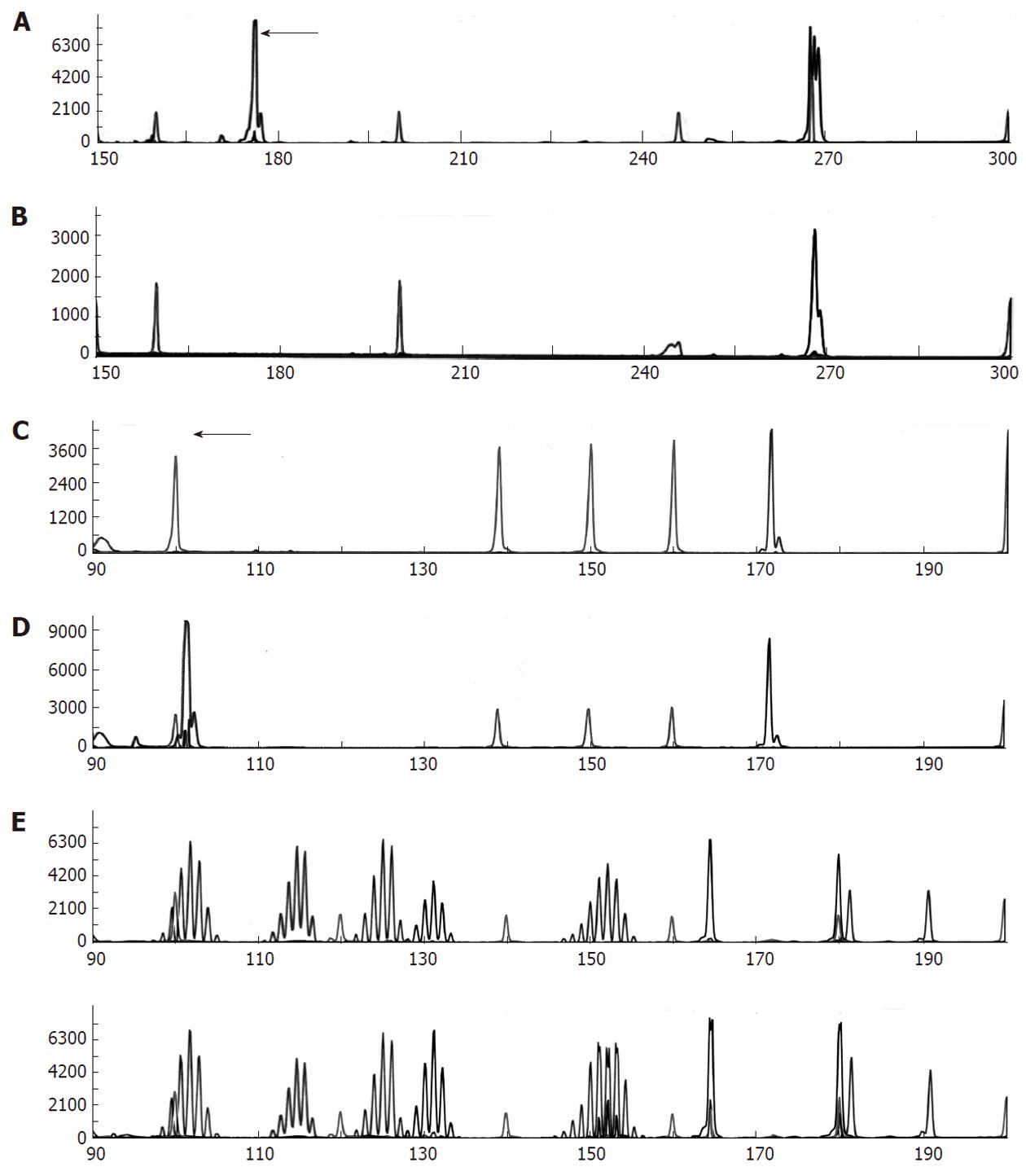
The patient underwent percutaneous liver biopsy and endoscopic biopsy of the circumferential and nearly obstructing colonic lesion. Initial Hematoxylin and Eosin (HE) sections of the colonic and hepatic biopsies showed a metastatic, predominantly neuroendocrine tumor which positively stained for CDX2, AE1/AE3 and synaptophysin and negatively stained for cytokeratin (CK)7, CK20 and chromogranin-A. There was extensive necrosis and mitotic activity. The patient began cisplatin and etoposide chemotherapy; however interval computed tomography showed enlarging hepatic metastases. The patient underwent emergent sleeve resection with colonic diversion for obstruction. Final histopathologic staging showed a ypT3ypN2ypM1 composite carcinoma, which was grossly white-tan with slight lobulations. HE sections showed submucosal keratinizing squamous cells admixed with neuroendocrine cells (Figure 1). There was extensive necrosis and mitotic activity. The immunohistochemical profile was similar to the initial biopsies (Figures 2 and 3). Mucicarmine staining in several sections confirmed the absence of mucin production. Lymphovascular invasion was composed of neuroendocrine cells, and four of seven lymph nodes were involved by both tumor components. There was no associated squamous metaplasia or fistula. The patient’s functional and clinical status declined, and therapy was shifted to palliative. The patient died at home, 60 d after initial presentation. An autopsy was not performed. Post-mortem molecular analyses on the surgical resection specimen demonstrated the tumor was microsatellite-stable, was negative for the V600E BRAF mutation, and harbored a G13D KRAS mutation (Figure 4).
Biopsy and surgical sections were formalin-fixed, paraffin-embedded, and HE stained according to standard protocol. CK7, CK20, CDX2, AE1/AE3, chromogranin-A and synaptophysin immunostains were run on Leica BondMax instruments (Bannockburn, IL) as follows: primary antibody incubation for 15 min, post-primary (proprietary, Leica) for 8 min, polymer (proprietary, Leica) for 8 min, peroxidase block for 5 min, DAB chromogen for 10 min, and hematoxylin counterstain for 10 min. CK7 additionally uses Epitope Retrieval Solution 1 (proprietary, Leica) for 10 min. Mucicarmine staining was performed according to standard protocol. For molecular analyses, DNA was extracted from three 10-micron thick tissue sections representing tumor or normal tissue, respectively, using the QIAquick PCR Purification Kit (QIAGEN, Valencia, CA) following prior tissue lysis and treatment with proteinase K.
Five ng of normal and tumor DNA were amplified using multiplex polymerase chain reaction (PCR) containing fluorescently labeled primers for 5 mononucleotide markers corresponding to NR-21, BAT-26, BAT-25, NR-24, MONO-27 and 2 pentanucleotide markers for identity confirmation (Promega, Madison WI). The assay was performed according the instructions listed by the manufacturer, amplicons were subjected to capillary electrophoresis using an ABI 3130xl, and the results were analyzed using GeneMapper v3.7 software.
Five ng of tumor DNA was analyzed for the common BRAF mutation V600E using a lab developed allele-specific (AS) PCR assay for this mutation. The AS PCR assay uses a common forward primer (5’-TGTTTTCCTTTACTTACTACACCTCAGA-3’) and two reverse primers (5’-CAGTGGAAAAATAGCCTCAATTCT-3’ and 5’-ACCCACTCCATCGAGATTTCT-3’), each tagged with a different fluorochrome to produce an internal control amplicon and, if present, a V600E (c.1799T > A) mutation-specific amplicon. Amplicons were separated using capillary electrophoresis on an ABI 3130xl and the results were analyzed using GeneMapper v3.7 software.
Ten ng of tumor DNA was analyzed for common KRAS mutations using allele specific PCR and previously described primers for the detection of mutations G12A, G12C, G12D, G12R, G12S, G12V, G13C and G13D[17]. In addition to the fluorescent tagged mutation specific primer, each of the eight PCR reactions also contained a previously described common wild type reverse primer to produce a mutation-specific amplicon of 176, 179 or 180 base pairs in length depending on the location and mutation present[18]. To confirm the amplification efficiency of the DNA, a third, lab-developed wild-type forward primer (5’-GTGTATTAACCTTATGTGTGACATG-3’) was included to yield a wild type amplicon of 267 base pairs in length. Amplification conditions included a 5 min denaturation at 95 °C followed by 40 cycles of 95 °C for 30 s, 60 °C for 2 min and 72 °C for 2 min. The PCR assay concluded with a final extension phase of 10 min at 72 °C. PCR products were separated using capillary electrophoresis on an ABI 3130xl, and the results were analyzed using GeneMapper v3.7 software.
This case report highlights a rare subtype of colorectal carcinoma with unusual features, including early age of onset and location at the splenic flexure. Additionally, this case documents molecular alterations which have not previously been documented in composite carcinomas with squamous and neuroendocrine features.
Most composite carcinomas are neuroendocrine and adenomatous, while this case showed squamous, rather than adenomatous, differentiation. While squamous carcinomas typically arise in the proximal colon and rectum when patients are in the fifth decade of life[11], this case describes a 33 year-old with a lesion at the splenic flexure. Furthermore, the tumor was entirely submucosal and intramural, with no mucosal involvement, raising the possibility of a distant metastasis from an unidentified primary tumor. A computerized tomography scan of the chest, abdomen, and pelvis failed to demonstrate any other mass which could have potentially metastasized. The tumor was CDX2 positive, confirming lower gastrointestinal origin.
Due to the young age of the patient, aggressive nature of the tumor, and unusual histology, we performed molecular analyses of well-known genetic alterations in colorectal carcinoma which have prognostic and predictive significance. We anticipated that the tumor was microsatellite-stable, as it did not have mucinous or me-dullary histology or prominent tumor-infiltrating lymphocytes. As BRAF mutations are the least common of the alterations analyzed, we also anticipated this would be negative. Due to the patient’s rapid progression, it was possible that the tumor harbored a KRAS mutation. The G13D mutation we identified would have excluded the patient from treatment with cetuximab or panitumumab. Current literature suggests this mutation results in an intermediate prognosis; however, this patient’s clinical course was representative of a mutation with a very poor outcome. Given that the majority of colorectal car-cinomas are adenocarcinomas, it is possible that rarer colorectal carcinoma histologic subtypes with similar genetic alterations may not have similar clinical courses as adenocarcinomas. The presence of other genetic alterations or modifier genes in these histologic subtypes remains to be determined. This study demonstrates prognostic and predictive value of molecular alterations is not uniform across tumor subtypes and further information is needed before such results drive therapeutic decision-making in cases of rare tumors.
In summary, we present a rare case of metastatic composite colorectal carcinoma containing squamous and neuroendocrine components. The young age of presentation and location within the colon are unusual. The tumor harbored a G13D KRAS mutation, with more aggressive behavior than expected from such a mutation.
Peer reviewer: Dr. Ashok Kumar, MD, Department of Surgical Gastroenterology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow 226014, India
S- Editor Sun H L- Editor Rutherford A E- Editor Li JY
| 1. | Gould VE, Jao W, Chejfec G, Banner BF, Bonomi P. Neuroendocrine carcinomas of the gastrointestinal tract. Semin Diagn Pathol. 1984;1:13-18. [PubMed] |
| 2. | Kubo T, Watanabe H. Neoplastic argentaffin cells in gastric and intestinal carcinomas. Cancer. 1971;27:447-454. [PubMed] |
| 3. | Soga J, Tazawa K. Pathologic analysis of carcinoids. Histologic reevaluation of 62 cases. Cancer. 1971;28:990-998. [PubMed] |
| 4. | Wisniewski M, Toker C. Composite tumor of the gallbladder exhibiting both carcinomatous and carcinoidal patterns. Am J Gastroenterol. 1972;58:633-637. [PubMed] |
| 5. | Lewin K. Carcinoid tumors and the mixed (composite) glandular-endocrine cell carcinomas. Am J Surg Pathol. 1987;11 Suppl 1:71-86. [PubMed] |
| 6. | Levendoglu H, Cox CA, Nadimpalli V. Composite (adenocarcinoid) tumors of the gastrointestinal tract. Dig Dis Sci. 1990;35:519-525. [PubMed] |
| 7. | Chaturvedi KU, Kaur H. Composite carcinoid carcinoma of the colon--a case report. Indian J Cancer. 1994;31:203-206. [PubMed] |
| 8. | Ulich TR, Kollin M, Lewin KJ. Composite gastric carcinoma. Report of a tumor of the carcinoma-carcinoid spectrum. Arch Pathol Lab Med. 1988;112:91-93. [PubMed] |
| 9. | Comer TP, Beahrs OH, Dockerty MB. Primary squamous cell carcinoma and adenocanthoma of the colon. Cancer. 1971;28:1111-1117. [PubMed] |
| 10. | Williams GT, Blackshaw AJ, Morson BC. Squamous carcinoma of the colorectum and its genesis. J Pathol. 1979;129:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 156] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Berardi RS, Chen HP, Lee SS. Squamous cell carcinoma of the colon and rectum. Surg Gynecol Obstet. 1986;163:493-496. [PubMed] |
| 12. | Vezeridis MP, Herrera LO, Lopez GE, Ledesma EJ, Mittleman A. Squamous-cell carcinoma of the colon and rectum. Dis Colon Rectum. 1983;26:188-191. [PubMed] |
| 13. | Juturi JV, Francis B, Koontz PW, Wilkes JD. Squamous-cell carcinoma of the colon responsive to combination chemotherapy: report of two cases and review of the literature. Dis Colon Rectum. 1999;42:102-109. [PubMed] |
| 14. | French AJ, Sargent DJ, Burgart LJ, Foster NR, Kabat BF, Goldberg R, Shepherd L, Windschitl HE, Thibodeau SN. Prognostic significance of defective mismatch repair and BRAF V600E in patients with colon cancer. Clin Cancer Res. 2008;14:3408-3415. [PubMed] |
| 15. | Zlobec I, Kovac M, Erzberger P, Molinari F, Bihl MP, Rufle A, Foerster A, Frattini M, Terracciano L, Heinimann K. Combined analysis of specific KRAS mutation, BRAF and microsatellite instability identifies prognostic subgroups of sporadic and hereditary colorectal cancer. Int J Cancer. 2010;127:2569-2575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Andreyev HJ, Norman AR, Cunningham D, Oates J, Dix BR, Iacopetta BJ, Young J, Walsh T, Ward R, Hawkins N. Kirsten ras mutations in patients with colorectal cancer: the 'RASCAL II' study. Br J Cancer. 2001;85:692-696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 632] [Cited by in RCA: 666] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 17. | Lecomte T, Berger A, Zinzindohoué F, Micard S, Landi B, Blons H, Beaune P, Cugnenc PH, Laurent-Puig P. Detection of free-circulating tumor-associated DNA in plasma of colorectal cancer patients and its association with prognosis. Int J Cancer. 2002;100:542-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 231] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Takeda S, Ichii S, Nakamura Y. Detection of K-ras mutation in sputum by mutant-allele-specific amplification (MASA). Hum Mutat. 1993;2:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 89] [Article Influence: 2.8] [Reference Citation Analysis (0)] |