Published online Nov 7, 2011. doi: 10.3748/wjg.v17.i41.4596
Revised: June 2, 2011
Accepted: June 9, 2011
Published online: November 7, 2011
AIM: To compare the reliability of gastritis staging systems in ranking gastritis-associated cancer risk in a large series of consecutive patients.
METHODS: Gastric mucosal atrophy is the precancerous condition in which intestinal-type gastric cancer (GC) most frequently develops. The operative link for gastritis assessment (OLGA) staging system ranks the GC risk according to both the topography and the severity of gastric atrophy (as assessed histologically on the basis of the Sydney protocol for gastric mucosal biopsy). Both cross-sectional and long-term follow-up trials have consistently associated OLGA stages III-IV with a higher risk of GC. A recently-proposed modification of the OLGA staging system (OLGIM) basically incorporates the OLGA frame, but replaces the atrophy score with an assessment of intestinal metaplasia (IM) alone. A series of 4552 consecutive biopsy sets (2007-2009) was retrieved and reassessed according to both the OLGA and the OLGIM staging systems. A set of at least 5 biopsy samples was available for all the cases considered.
RESULTS: In 4460 of 4552 cases (98.0%), both the high-risk stages (III + IV) and the low-risk stages (0 +I + II) were assessed applying the OLGA and OLGIM criteria. Among the 243 OLGA high-risk stages, 14 (5.8%) were down-staged to a low risk using OLGIM. The 67 (1.5%) incidentally-found neoplastic lesions (intraepithelial or invasive) were consistently associated with high-risk stages, as assessed by both OLGA and OLGIM (P < 0.001 for both). Two of 34 intestinal-type GCs coexisting with a high-risk OLGA stage (stage III) were associated with a low-risk OLGIM stage (stage II).
CONCLUSION: Gastritis staging systems (both OLGA and OLGIM) convey prognostically important information on the gastritis-associated cancer risk. Because of its clinical impact, the stage of gastritis should be included as a conclusive message in the gastritis histology report. Since it focuses on IM alone, OLGIM staging is less sensitive than OLGA staging in the identification of patients at high risk of gastric cancer.
- Citation: Rugge M, Fassan M, Pizzi M, Farinati F, Sturniolo GC, Plebani M, Graham DY. Operative link for gastritis assessment vs operative link on intestinal metaplasia assessment. World J Gastroenterol 2011; 17(41): 4596-4601
- URL: https://www.wjgnet.com/1007-9327/full/v17/i41/4596.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i41.4596
Gastric mucosal atrophy is by far the greatest risk factor for non-hereditary, intestinal-type distal gastric cancer (GC)[1]. The gold standard for atrophy assessment is histology, but non-invasive tests (mainly pepsinogen serology) are also applied for this purpose[1-3].
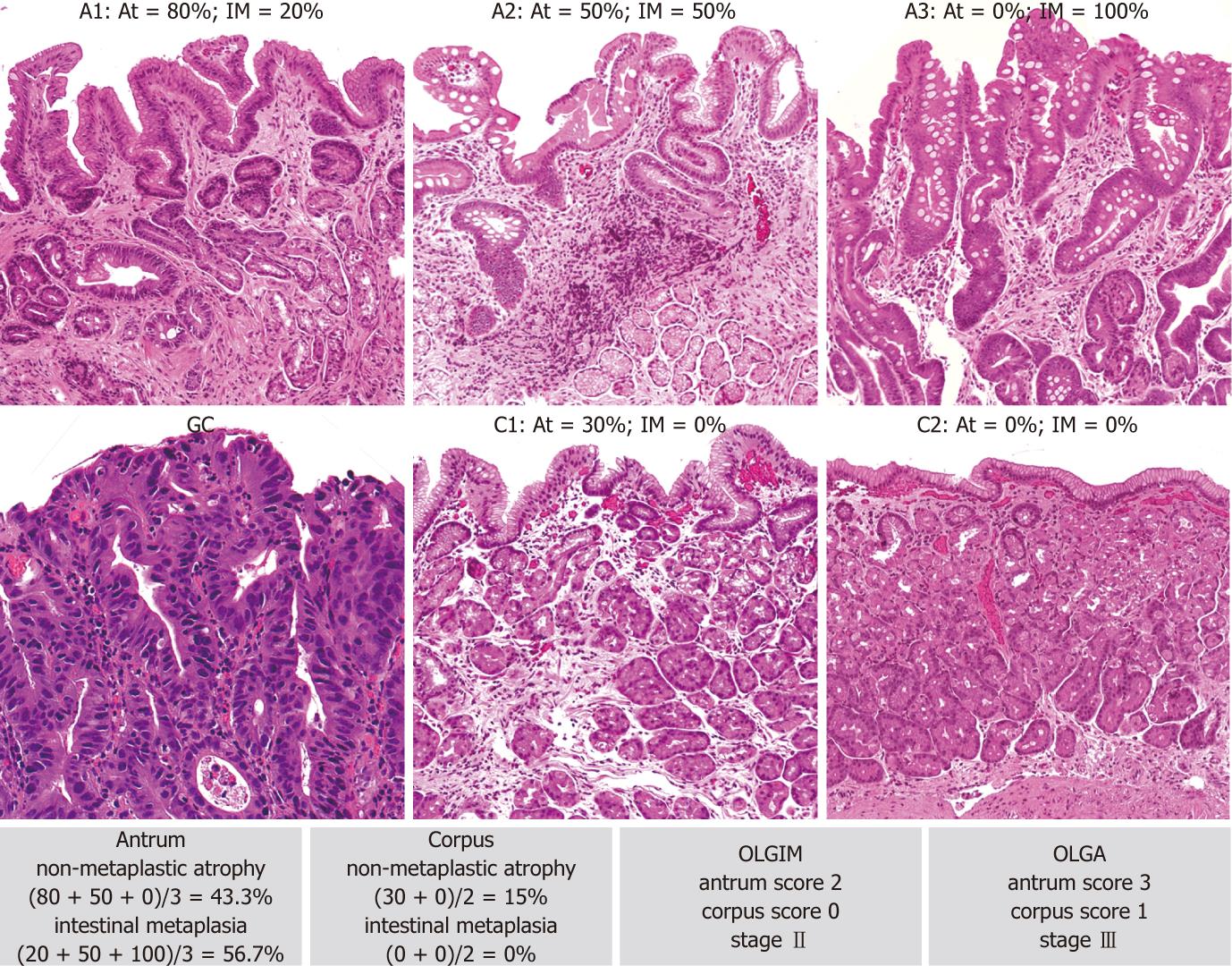
According to the current international literature, atrophy is defined as the “loss of appropriate glands”. This definition covers both the “loss” of native glands (replaced by fibrosis) and the metaplastic replacement of the appropriate (native) glands due to antral intestinalization, corpus antralization [i.e., spasmolytic polypeptide-expressing metaplasia (SPEM)] and/or intestinalization[2].
Consistent evidence correlates the extent/topography of atrophy with the risk of GC, and it is on these grounds that a system for staging gastritis [the operative link for gastritis assessment (OLGA) staging system] was proposed[4]. Gastritis stages (0 to IV) express increasing extents of atrophy, as assessed histologically on antral and corpus biopsies. In different epidemiological settings, both cross-sectional and long-term follow-up studies have consistently allocated a small minority of gastritis patients to stages III-IV, associating only this population with a significantly higher GC risk (high-risk OLGA stages)[3,5-10]. OLGA stages III-IV have also been consistently associated with molecular tissue markers of high-risk gastritis[11,12]. These correlations potentially support the advisability of endoscopic follow-up for such high-stage patients.
A significant correlation has been demonstrated between high-risk OLGA stages and pepsinogen serology; this correlation between organic and functional disease supports the rationale for implementing serology in GC secondary prevention programs[5].
A recently-proposed modification of the OLGA staging system (OLGIM)[10] basically incorporates the same staging frame, but replaces the “global” score for atrophy (in its different phenotypic variants) with the histological assessment of intestinal metaplasia (IM) alone. The rationale behind the OLGIM proposal lies in the fact that IM is easier to assess histologically than the “global” spectrum of the atrophic lesions (as in the OLGA approach).
This study compares the OLGA and OLGIM staging systems in the assessment of gastritis-associated gastric cancer risk (i.e., stages 0-I-II = low-risk stages vs Stages III-IV = high-risk stages).
All gastric biopsy sets recorded between January 2007 and December 2009 were retrieved from the archives of the Surgical Pathology and Cytopathology Unit at the Department of Diagnostic Medical Sciences and Special Therapies of Padova University. Case recruitment did not distinguish between initial or follow-up endoscopies; all the patients considered were natives of the Veneto region and underwent endoscopy at the same institution (Padova Teaching Hospital). The institute’s ethical regulations on research conducted on human tissues were followed.
For all the cases considered, a set of at least 5 biopsy samples was available (2 samples from the antral mucosa, 1 from the mucosa of the incisura angularis, and 2 from the anterior and posterior walls of the oxyntic stomach). In accordance with the biopsy sampling protocol, additional specimens had been obtained from any focal lesions. Details were always available regarding the site of the biopsy.
For the purposes of the study, pediatric patients (under 18 years old), patients with a history of autoimmune gastritis, and those who had undergone esophageal or gastric surgery, esophagogastric endomucosal resection or sub-mucosal dissection were excluded.
Original slides or serial sections (4-6 microns thick) obtained from archival paraffin-embedded tissue samples [hematoxylin and eosin, Alcian- Blue and Periodic Acid Schiff stain and Giemsa for Helicobacter pylori (H. pylori)] were histologically re-considered.
Three trained gastroenterology pathologists (Fassan M, Pizzi M and Rugge M), blinded to any endoscopic or clinical information, jointly examined all the histology specimens and reached a consensus on the score for each of the histological variables considered. For OLGA staging purposes, atrophy was defined as the loss of appropriate glands with or without epithelial metaplasia (i.e., IM in antral and/or oxyntic biopsy samples; pseudo-pyloric metaplasia in oxyntic biopsy samples)[2]. Glandular atrophy was scored according to the recommendations in the OLGA staging tutorial[4,13]. For OLGIM staging purposes, only IM was considered and scored according to the recommendations of the OLGIM proposers[10]. The inter-observer consistency in assessing the two staging systems was tested by means of K statistics in a randomly selected series of 100 cases and was ranked as “excellent” (k coefficient = 0.75 and 0.77 for OLGA and OLGIM, respectively).
Any incidentally-found neoplastic lesions were histologically assessed according to internationally validated criteria[14,15]. Within the spectrum of gastric intra-epithelial neoplasia (IEN), the categories considered were: low-grade IEN (LG-IEN) and high-grade IEN (HG-IEN). The inter-observer consistency in assessing IEN lesions was tested by means of K statistics in a randomly selected series of 35 IEN/gastric cancer cases and was ranked as “fair
to good” (k coefficient = 0.66). Gastric cancer was diagnosed in the presence of neoplastic epithelia infiltrating the lamina propria.
The strength of the association between the stage of gastritis and the demographic and pathological features was calculated using Wilcoxon’s signed rank test (W), and the modified Kruskal-Wallis nonparametric test for trend (KW), as appropriate. The inter-observer consistency in classifying atrophic and IEN lesions was tested in two series of 100 and 35 randomly-selected biopsy sets, respectively, calculated as the overall proportion of agreement (the number of total paired observations in which the same result was obtained), and tested using Fleiss’s kappa statistic[16]. Stata software (Stata Corporation, College Station, TX) was used for all the calculations. A P value < 0.05 was considered significant.
Overall, 4552 consecutive biopsy sets were considered. The male/female ratio was 1/1.18, and the patients’ mean age was 55.1 years (median 57.0, range 20-89). For the males, the mean age was 55.0 years (range 20-88), while for the females it was 55.1 years (range 20-89).
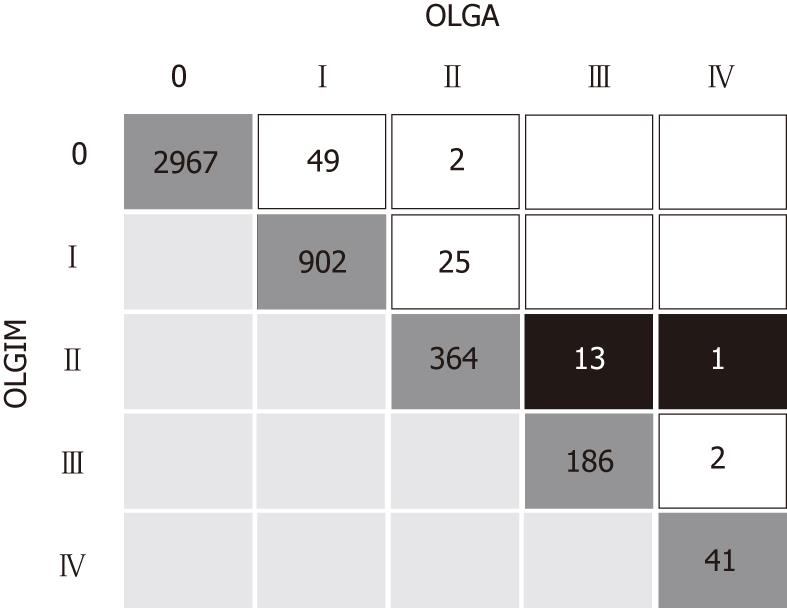
Overall, 2967 biopsy sets (65.2%) showed no atrophic changes (i.e., stage 0 gastritis according to both OLGA & OLGIM) and the prevalence of the low-risk stages was 94.7% and 95.0% according to OLGA and OLGIM, respectively (Table 1).
| Total | Stage 0 | Stage I | Stage II | Stage III | Stage IV | ||||||
| OLGA | OLGIM | OLGA | OLGIM | OLGA | OLGIM | OLGA | OLGIM | OLGA | OLGIM | ||
| Cases | 4552 | 2967 | 3018 | 951 | 927 | 391 | 378 | 199 | 188 | 44 | 41 |
| Age (yr) mean ± SD (median) | 55.1 ± 15.8 (57.0) | 51.0 ± 15.9 (50.5) | 51.1 ± 15.9 (50.6) | 60.7 ± 13.1 (63.1) | 61.1 ± 12.8 (63.3) | 64.4 ± 10.9 (65.4) | 64.7 ± 10.8 (66.3) | 67.1 ± 9.6 (67.7) | 67.0 ± 9.7 (67.7) | 67.5 ± 13.1 (67.6) | 67.4 ± 13.3 (68.4) |
| Sex (M/F) | 2085/2467 | 1344/1623 | 1364/1 654 | 447/504 | 438/489 | 164/227 | 158/220 | 98/101 | 94/94 | 32/12 | 31/10 |
| Hp + ve (%) | 1698 (37.30) | 1188 (40.00) | 1198 (39.70) | 322 (33.90) | 318 (34.30) | 130 (33.20) | 126 (33.30) | 47 (23.60) | 45 (23.90) | 11 (25.00) | 11 (26.80) |
| Neoplastic lesions1 (No. of cases) | 67 | 3 | 3 | 2 | 3 | 3 | 4 | 39 | 38 | 20 | 19 |
In all, there were 67/4552 (1.5%) incidentally-found neoplastic lesions (either intraepithelial or invasive), including: 21 cases of LG-IEN; 6 cases of HG-IEN; 40 cases of GC (6 cases of diffuse-type proximal GC; 34 of intestinal-type distal GC). The M/F ratio among the neoplastic patients was 1.03/1 and their mean age (67.8 years; median 68; range 45-86) was significantly higher than that of the non-neoplastic patients (mean 54.8 years; median 57 years; range 20-89) (W; P < 0.001).
H. pylori was assessed histologically in 1698 patients (37.3%). Its prevalence among the neoplastic cases was 26 (38.8%), disregarding any previous eradication therapies. However, this study only focuses on the neoplastic risk associated with the stage of gastritis, without any specific reference to, or speculation about, the impact of the etiology on the morphogenesis of the gastric disease.
For 4460 out of 4552 cases (97.98%), low-risk stages (0 +I+ II) and high-risk stages (III + IV) were staged consistently using either OLGA or OLGIM criteria (Figure 1). For the 92 (2.0%) cases staged inconsistently, 14 were considered as low-risk using the OLGIM criteria and as high-risk according to OLGA. No cases staged as high-risk by OLGIM were down-staged when the OLGA criteria were applied.
The number of patients with high-risk stages (III-IV) was 243 according to OLGA and 229 according to OLGIM, i.e., among the 243 OLGA high-risk stages, 16 (6.6%) were down-staged by OLGIM; in particular, 14 OLGA stages III and IV were classified as stage II according to OLGIM.
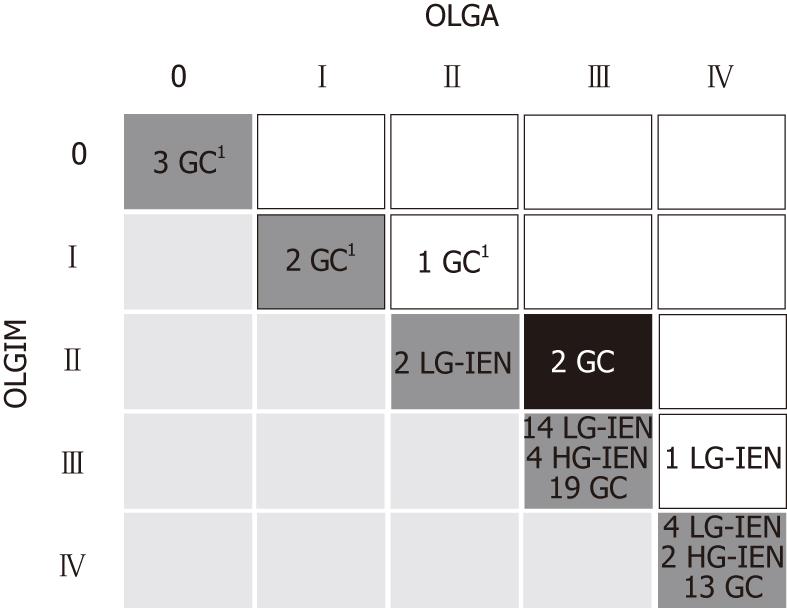
In all, 67 intraepithelial (i.e., non-invasive) or invasive neoplastic lesions were detected. All the 27 intraepithelial neoplasia coexisted with intestinalized glands. Among the 40 cases of invasive adenocarcinoma, 6 (15%) were located in the cranial stomach and histologically featured a solid/diffuse-type GC; the other 34 (85%) were cases of intestinal-type GC. After distinguishing between low- and high-risk stages, a significant association emerged between stages III-IV and both intraepithelial and invasive neoplasia according to both the staging systems [W; P < 0.001 for both (Table 1)].
Fifty-nine of 67 (88.1%) and 57/67 (85.1%) intraepithelial or invasive neoplastic lesions were associated with high-risk OLGA and OLGIM stages, respectively. Six gastric cancers were detected in cases classified as OLGA/OLGIM low-risk gastritis: all 6 were diffuse-type gastric cancers (Figure 2). Two intestinal-type GCs coexisting with OLGA stage III were associated with OLGIM stage II gastritis (Figures 2 and 3).
Gastric cancer is still a health priority in Western Europe and it represents an epidemiological emergency in Eastern Europe, Central and Eastern Asia, and some South American regions[17-26].
Gastric mucosal atrophy is generally considered the “cancerization field” in which GC develops. Based on such a rationale, and incorporating the experience gained with the Sydney system[27], the OLGA staging system ranks the gastritis-associated cancer risk according to both the topography and the extent of gastric mucosa atrophy[2,4-6,26-28].
As regards topography, extensive biopsy sampling protocols (such as the one applied in the Houston experience) potentially increase the prognostic reliability of any staging system and they should be theoretically preferred. In line with the Sydney system[27], however, both OLGA and OLGIM systems require a (minimum) set of 5 biopsy samples for gastritis staging, which should strike a good compromise between the priority of obtaining a representative biopsy set and the operative limits of daily clinical practice[27].
The OLGIM proposal replaces the “global” atrophy score with a semiquantitative assessment of intestinal metaplasia (extent and site); according to its proposers, such a strategy should considerably increase the inter-observer agreement - an undeniable advantage[29,30].
In the present series of more than four thousand consecutive cases, 98% of stage III-IV gastritis were consistently staged by applying either OLGA or OLGIM. The finding that patients’ ages increase with higher stages further supports the clinico-biological plausibility of both systems.
It is worth noting that two intestinal-type GCs (both coexisting with OLGA stage III gastritis) were found associated with OLGIM-II gastritis (i.e., low-risk atrophic gastritis). In fact, by focusing on IM alone, OLGIM is less sensitive in identifying high-risk gastritis, and this may result in the down-staging of patients who should be offered follow-up[29,30]. Comparative studies involving non-GI (i.e., specialist) pathologists are needed to test which system (OLGA or OLGIM) provides more accurate results in relation to the time and effort spent on the histology assessment.
In his seminal work on gastric carcinogenesis, Pelayo Correa described mucosal atrophy as a cardinal step in the biological pathway that may eventually progress to gastric adenocarcinoma[31]. The current definition of gastric mucosal atrophy includes two different phenotypes: (1) loss (shrinkage or disappearance) of glands, which are replaced by fibrotic expansion of the lamina propria; and (2) metaplastic replacement of native glands by intestinalized and/or pseudopyloric glands (corpus antralization or SPEM). Focusing on IM alone excludes pseudopyloric metaplasia (i.e., SPEM) from the spectrum of atrophy, although it has recently been found increasingly important in gastric carcinogenesis (through transdifferentiation from mature chief cells following parietal cell loss)[32-34].
Lastly, considering IM alone carries the risk of us losing the correlation between gastric atrophy (as assessed by gastric serology, and PgI in particular) and its organic counterpart (resulting from the concurrence of the different phenotypes of gastric atrophy)[35-38].
In conclusion, gastritis staging effectively conveys an unequivocal message regarding the gastritis-associated cancer risk and may point to follow-up strategies tailored to a patient-specific clinico-pathological situation. This priority supports the inclusion of staging in gastritis histology reports and the demand for further efforts to improve the reproducibility of any staging criteria - bearing in mind that “easier” does not necessarily mean “better”!
Despite its declining incidence, gastric cancer is still the second cause of cancer-related death worldwide. Gastric atrophy is by far the main risk factor for non-hereditary, intestinal-type distal gastric cancer. Consistent evidence relates the extent and topography of atrophy to the gastric cancer risk.
Building on current knowledge of the biology of gastritis, an international group of gastroenterologists and pathologists has proposed a system for reporting gastritis in terms of stage [the operative link for gastritis assessment (OLGA) staging system]. The OLGA staging system basically ranks gastric cancer risk according to the extent/topography of gastric atrophy. A recently proposed modification of the OLGA, called the OLGIM staging system, basically incorporates the OLGA frame, but considers intestinal metaplasia alone, instead of the global atrophy score.
In a large series of consecutive retrospective cases, the present study compares the reliability of the OLGA vs the operative link on intestinal metaplasia assessment (OLGIM) systems in ranking gastritis-associated cancer risk. Both staging systems stratify gastritis patients in different cancer risk classes, providing a clinico-biological rationale for a patient-tailored clinico-endoscopic follow-up. OLGIM staging, however, includes cases of gastritis at high risk of gastric neoplasia in its low-risk stage II.
Gastritis staging conveys information on the clinico-pathological outcome of gastritis that is relevant to patient management, leading to an adequate patient stratification according to different cancer risks.
OLGA is the acronym for “operative link for gastritis assessment”, an innovative gastritis staging system proposed by an international group of gastroenterologists and pathologists that basically ranks the gastric cancer risk according to the extent and severity of gastric atrophy. OLGIM is the acronym for “operative link on intestinal metaplasia assessment”, a modification of the OLGA staging system, which basically adopts the OLGA staging frame, but replaces the global atrophy score with an assessment of intestinal metaplasia alone.
The authors compared the reliability of histological gastritis staging systems, OLGA and OLGIM, in ranking gastritis-associated cancer risk in a large series of consecutive cases. They concluded that both staging systems provide prognostically useful information on the gastritis-associated cancer risk even if OLGIM is less sensitive.
Peer reviewers: Mitsunori Yamakawa, Professor, Department of Pathological Diagnostics, Yamagata University, Faculty of Medicine, 2-2-2 Iida-Nishi, Yamagata 990-9585, Japan; Chakshu Gupta, MD, FCAP, Pathology and Laboratory Medicine, Heartland Regional Medical Center, 5325 Faraon Street, St. Joseph, MO 64506, United States
S- Editor Tian L L- Editor Logan S E- Editor Xiong L
| 1. | Correa P. The biological model of gastric carcinogenesis. IARC Sci Publ. 2004;301-310. [PubMed] |
| 2. | Rugge M, Correa P, Dixon MF, Fiocca R, Hattori T, Lechago J, Leandro G, Price AB, Sipponen P, Solcia E. Gastric mucosal atrophy: interobserver consistency using new criteria for classification and grading. Aliment Pharmacol Ther. 2002;16:1249-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 265] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 3. | Graham DY, Rugge M. Clinical practice: diagnosis and evaluation of dyspepsia. J Clin Gastroenterol. 2010;44:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Rugge M, Correa P, Di Mario F, El-Omar E, Fiocca R, Geboes K, Genta RM, Graham DY, Hattori T, Malfertheiner P. OLGA staging for gastritis: a tutorial. Dig Liver Dis. 2008;40:650-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 211] [Article Influence: 12.4] [Reference Citation Analysis (1)] |
| 5. | Rugge M, de Boni M, Pennelli G, de Bona M, Giacomelli L, Fassan M, Basso D, Plebani M, Graham DY. Gastritis OLGA-staging and gastric cancer risk: a twelve-year clinico-pathological follow-up study. Aliment Pharmacol Ther. 2010;31:1104-1111. [PubMed] |
| 6. | Rugge M, Kim JG, Mahachai V, Miehlke S, Pennelli G, Russo VM, Perng CL, Chang FY, Tandon RK, Singal DK. OLGA gastritis staging in young adults and country-specific gastric cancer risk. Int J Surg Pathol. 2008;16:150-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Satoh K, Osawa H, Yoshizawa M, Nakano H, Hirasawa T, Kihira K, Sugano K. Assessment of atrophic gastritis using the OLGA system. Helicobacter. 2008;13:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Rugge M, Meggio A, Pennelli G, Piscioli F, Giacomelli L, De Pretis G, Graham DY. Gastritis staging in clinical practice: the OLGA staging system. Gut. 2007;56:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 349] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 9. | Ramírez-Mendoza P, González-Angulo J, Angeles-Garay U, Segovia-Cueva GA. [Evaluation of Gastric Atrophy. Comparison between Sidney and OLGA Systems]. Rev Med Inst Mex Seguro Soc. 2008;46:135-139. [PubMed] |
| 10. | Capelle LG, de Vries AC, Haringsma J, Ter Borg F, de Vries RA, Bruno MJ, van Dekken H, Meijer J, van Grieken NC, Kuipers EJ. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest Endosc. 2010;71:1150-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 375] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 11. | Carrasco G, Diaz J, Valbuena JR, Ibanez P, Rodriguez P, Araya G, Rodriguez C, Torres J, Duarte I, Aravena E. Overexpression of p73 as a tissue marker for high-risk gastritis. Clin Cancer Res. 2010;16:3253-3259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Rugge M, Fassan M, Pizzi M, Pennelli G, Nitti D, Farinati F. Operative Link for Gastritis Assessment gastritis staging incorporates intestinal metaplasia subtyping. Hum Pathol. 2011;42:1539-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Rugge M, Pennelli G, Pilozzi E, Fassan M, Ingravallo G, Russo VM, Di Mario F. Gastritis: the histology report. Dig Liver Dis. 2011;43 Suppl 4:S373-S384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 14. | Rugge M, Correa P, Dixon MF, Hattori T, Leandro G, Lewin K, Riddell RH, Sipponen P, Watanabe H. Gastric dysplasia: the Padova international classification. Am J Surg Pathol. 2000;24:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 249] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 15. | Fenoglio-Preiser CM, Isaacson PG, Lantz PE, Noffsinger AE, Stemmermann GN. Gastrointestinal Pathology: an Atlas and Text. New York: Lippincott Williams & Wilkins 2007; . |
| 16. | Fleiss JI. Statistical methods for rates and proportions. New York: Wiley 1981; . |
| 17. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3181] [Article Influence: 132.5] [Reference Citation Analysis (0)] |
| 18. | Lauwers GY, Riddell RH. Gastric epithelial dysplasia. Gut. 1999;45:784-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 126] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Cassaro M, Rugge M, Gutierrez O, Leandro G, Graham DY, Genta RM. Topographic patterns of intestinal metaplasia and gastric cancer. Am J Gastroenterol. 2000;95:1431-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 182] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Kuipers EJ. Review article: Relationship between Helicobacter pylori, atrophic gastritis and gastric cancer. Aliment Pharmacol Ther. 1998;12 Suppl 1:25-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | den Hoed CM, van Eijck BC, Capelle LG, van Dekken H, Biermann K, Siersema PD, Kuipers EJ. The prevalence of premalignant gastric lesions in asymptomatic patients: predicting the future incidence of gastric cancer. Eur J Cancer. 2011;47:1211-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Tsukanov VV, Butorin NN, Maady AS, Shtygasheva OV, Amelchugova OS, Tonkikh JL, Fassan M, Rugge M. Helicobacter pylori Infection, Intestinal Metaplasia, and Gastric Cancer Risk in Eastern Siberia. Helicobacter. 2011;16:107-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Vannella L, Lahner E, Osborn J, Bordi C, Miglione M, Delle Fave G, Annibale B. Risk factors for progression to gastric neoplastic lesions in patients with atrophic gastritis. Aliment Pharmacol Ther. 2010;31:1042-1050. [PubMed] |
| 24. | Capelle LG, Van Grieken NC, Lingsma HF, Steyerberg EW, Klokman WJ, Bruno MJ, Vasen HF, Kuipers EJ. Risk and epidemiological time trends of gastric cancer in Lynch syndrome carriers in the Netherlands. Gastroenterology. 2010;138:487-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 25. | de Vries AC, van Grieken NC, Looman CW, Casparie MK, de Vries E, Meijer GA, Kuipers EJ. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008;134:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 585] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 26. | Rugge M, Genta RM. Staging gastritis: an international proposal. Gastroenterology. 2005;129:1807-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3550] [Article Influence: 122.4] [Reference Citation Analysis (3)] |
| 28. | El-Zimaity HM. Gastric atrophy, diagnosing and staging. World J Gastroenterol. 2006;12:5757-5762. [PubMed] |
| 29. | Rugge M, Fassan M, Farinati F, Genta RM. The war of the worlds: metaplastic versus nonmetaplastic atrophic gastritis. Gastrointest Endosc. 2011;73:411-42; author reply 411-42;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Capelle LG, Kuipers EJ. Response to “The war of the worlds: metaplastic versus non-metaplastic atrophic gastritis.”. Gastrointest Endosc. 2011;73:412-413. |
| 31. | Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735-6740. [PubMed] |
| 32. | Gutiérrez-González L, Wright NA. Biology of intestinal metaplasia in 2008: more than a simple phenotypic alteration. Dig Liver Dis. 2008;40:510-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Weis VG, Goldenring JR. Current understanding of SPEM and its standing in the preneoplastic process. Gastric Cancer. 2009;12:189-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 34. | Nam KT, Lee HJ, Sousa JF, Weis VG, O'Neal RL, Finke PE, Romero-Gallo J, Shi G, Mills JC, Peek RM. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology. 2010;139:2028-2037.e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 229] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 35. | Graham DY, Nurgalieva ZZ, El-Zimaity HM, Opekun AR, Campos A, Guerrero L, Chavez A, Cardenas V. Noninvasive versus histologic detection of gastric atrophy in a Hispanic population in North America. Clin Gastroenterol Hepatol. 2006;4:306-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Iijima K, Abe Y, Kikuchi R, Koike T, Ohara S, Sipponen P, Shimosegawa T. Serum biomarker tests are useful in delineating between patients with gastric atrophy and normal, healthy stomach. World J Gastroenterol. 2009;15:853-859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Graham DY, Kato M, Asaka M. Gastric endoscopy in the 21st century: appropriate use of an invasive procedure in the era of non-invasive testing. Dig Liver Dis. 2008;40:497-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Sipponen P, Graham DY. Importance of atrophic gastritis in diagnostics and prevention of gastric cancer: application of plasma biomarkers. Scand J Gastroenterol. 2007;42:2-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |