Published online Nov 7, 2011. doi: 10.3748/wjg.v17.i41.4572
Revised: June 16, 2011
Accepted: June 23, 2011
Published online: November 7, 2011
AIM: To study the relationship between the cyclooxygenase (COX)-2 gene and the proliferation and apoptosis of esophageal squamous carcinoma EC109 cells.
METHODS: The techniques of RNA interference (RNAi) and cell transfection, as well as the levels of oncogenicity in nude mice, were used to study the role of COX-2 in the esophageal squamous carcinoma cell (ESCC) line EC109. Following RNAi and transfection, Western blotting analysis was used to determine the expression of the COX-2 protein. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) reduction assay was used to evaluate cell growth, and flow cytometry was used to detect cell apoptosis.
RESULTS: Western blotting analysis demonstrated that COX-2 expression was significantly reduced in EC109 cells treated with COX-2-specific short interfering RNA (siRNA) but was increased in EC109 cells transfected with COX-2. Furthermore, COX-2 siRNA treatment inhibited cell proliferation (P < 0.01) and induced apoptosis in EC109 cells, as determined by an MTT assay and by flow cytometry, respectively. In contrast, transfected COX-2 led to increased cell proliferation (P < 0.05) and decreased apoptosis in EC109 cells. In addition, combination treatment of cells with COX-2 siRNA and aspirin had a synergistic effect (P < 0.01). For experiments measuring tumorigenicity, xenograft tumors of a greater volume and weight were found in the COX-2 group compared with other groups (P < 0.05). A large dose of aspirin inhibited tumor growth in nude mice effectively (P < 0.05), and the rate of tumor suppression was 51.8% in the high-dose aspirin group.
CONCLUSION: COX-2 plays a very critical role in ESCC carcinogenesis, and COX-2 siRNA combined with aspirin has the potential to be an anticancer therapy for the treatment of ESCC.
- Citation: Zhang L, Wu YD, Li P, Tu J, Niu YL, Xu CM, Zhang ST. Effects of cyclooxygenase-2 on human esophageal squamous cell carcinoma. World J Gastroenterol 2011; 17(41): 4572-4580
- URL: https://www.wjgnet.com/1007-9327/full/v17/i41/4572.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i41.4572
Esophageal carcinoma is a common malignant neoplasm worldwide with high fatality rates[1]. This condition can be classified as esophageal squamous cell carcinoma (ESCC) or esophageal adenocarcinoma. Cancers arising from esophageal tissue, especially ESCC, have high incidence rates in China. Despite surgical treatment and chemotherapy, the prognosis of ESCC had until recently been very poor. Thus, early identification and effective treatment are highly desirable for an improved prognosis.
Cyclooxygenase is an enzyme responsible for the synthesis of prostaglandins, and it has two isoforms, COX-1 and COX-2. COX-1 is a constitutive enzyme and is found in most mammalian cells. COX-2, on the other hand, is undetectable in most normal tissues but can be induced by tumor-promoting agents, growth factors and inflammation.
To date, many studies have shown COX-2 to play a very important role in carcinogenesis. COX-2 has been reported to be over-expressed in many malignant tumors, such as those in breast, lung, stomach, colon and pancreatic cancer[2-6], and levels of COX-2 expression are associated with poor prognosis of some cancers[7]. Non-steroidal anti-inflammatory drugs (NSAIDs) and COX-2 inhibitors have been shown to effectively suppress tumor development[8]. For instance, recent studies have indicated that the regular use of aspirin can reduce the risk of esophageal cancer by as much as 90%[9,10]. Due to the inhibitory effect of aspirin on COX activity, we hypothesized that COX-2 is involved in the development of esophageal cancer.
In fact, the association between COX-2 and ESCC has previously been examined. In these studies, the common findings were that COX-2 was over-expressed in ESCC and that it contributed to carcinogenesis. However, the molecular mechanism by which COX-2 promotes carcinogenesis in squamous cells remained unclear.
Previous research has shown that the mechanisms behind COX-2 gene expression differed by cell type and the cell growth conditions. The pleiotropic effects of COX-2 on carcinogenesis include increased cellular proliferation, inhibition of apoptosis, increased angiogenesis, impaired cell adhesion and increased invasion of malignant cells[11-13].
In the present study, we have delineated the effects of increased or decreased levels of COX-2 on human ESCC proliferation and apoptosis. Specifically, we have investigated the effect of COX-2 overexpression on ESCC cell proliferation and apoptosis. We have also analyzed the effects of aspirin, a nonspecific COX-2 inhibitor and the specific depletion of COX-2 by short interfering RNA (siRNA) in ESCC. The results showed that COX-2 overexpression induced antiapoptotic activity and promoted tumorigenesis, while the inhibition of COX-2 effectively suppressed the proliferation of cancer cells and tumorigenesis in nude mice.
A recent study by Yang GZ et al[14] found that COX-2 expression was upregulated during an early stage of ESCC, especially in more fully differentiated carcinomas. Therefore, the inhibition of COX-2 by RNAi or aspirin treatment could be an effective strategy for the prevention and treatment of early stages of ESCC.
EC109 is a cell line that was derived from a patient with a well-differentiated ESCC, and the line was obtained from the Cancer Institute at the Chinese Academy of Medical Sciences. EC109 cells were maintained in RPMI 1640 culture medium (Invitrogen, United States) supplemented with 10% fetal calf serum, 1% penicillin/streptomycin and 2% L-glutamine. The cells were grown in a humidified 37 °C incubator with 5% CO2. They were fed every 3 d with complete medium and were subcultured when confluent.
The modified pOSML-PGHS-2 plasmid, kindly provided by Dr. Smith WL (University of Michigan, United States), contains the full-length hCOX-2 gene. After the sequence of full-length hCOX-2 cDNA had been confirmed by sequence analysis, COX-2 cDNA (approximately 1.9 kb) was cloned into the pcDNA3.1/V5HisA expression vector. One day before transfection, EC109 cells were seeded at 2.5 × 105/plate in 6 cm dishes in RPMI 1640 antibiotic-free medium containing 10% fetal bovine serum (FBS) until they were 80%-90% confluent. After 24 h, 800 μL of RPMI 1640 medium without FBS or antibiotics was added to each well, and the cells were transfected with either the COX-2 expression plasmid (pcDNA3.1V5HisA/hCOX-2) or with an empty control vector (pcDNA3.1V5HisA) using Lipofectamine™2000 (Invitrogene, Carlsbad, CA) according to the manufacturer΄s protocol. In brief, 5 μg of COX-2 plasmid DNA was diluted in serum- and antibiotic-free RPMI 1640 medium to a total volume of 100 μL. In addition, 5 μL of Lipofectamine™2000 was diluted with serum- and antibiotic-free RPMI 1640 medium to a total volume of 100 μL. The diluted plasmid DNA was combined with the diluted Lipofectamine™2000. Following incubation for 20 min at room temperature, 200 μL of the mixture was added to each well, and the cells were incubated at 37 °C in a CO2 incubator for 5 h. The RPMI 1640 medium containing 10% FBS was changed after 5 h, and the cells were incubated for an additional 24 h at 37°C in a CO2 incubator. For the combined treatment of COX-2 transfection and aspirin, 8 mmol/L aspirin was added 24 h after the COX-2 plasmid (pcDNA3.1V5HisA/hCOX-2) was transfected into the EC109 cells, and the cells were treated with aspirin for 48 h.
COX-2 siRNA was synthesized by GeneChem (Shanghai, China). The COX-2-specific siRNA was designed to target positions 293-311 (5′-CUGCUCAACACCGGAAUUUtt-3′) of the COX-2 transcript (GenBank Accession No: NM_000963). We also used a scrambled siRNA as a negative control. This negative control had no significant homology to any known human, mouse or rat gene sequence (non-silencing-FITC: 5′-UUCUCCGAACGUGUCACGUtt-3′). EC109 cells were plated in 6-well plates at 2 × 105 cells per well, and RPMI 1640 medium without antibiotics was added to each well. After 24 h, the cells were grown to reach 40%-50% confluence and were transfected with COX-2 siRNA using the Lipofectamine™2000 reagent according to the manufacturer’s instructions. The original stock of siRNA was resuspended in siRNA suspension buffer provided by the manufacturer. From this stock (20 μmol/L), 15 μL was diluted with 250 μL of Opti-MEM, and 5 μL Lipofectamine™2000 was diluted with 250 μL of Opti-MEM. After 5 min at room temperature, these were combined and incubated for 20 min. The siRNA-Lipofectamine complexes were then added to the plated cells and mixed gently. The cells were incubated at 37 °C in a humidified atmosphere of 5% CO2 for 84 h. For combination treatment of COX-2 siRNA with aspirin (Sigma, United States), 8 mmol/L aspirin was added 48 h after the COX-2 siRNA was transfected into the EC109 cells, and the cells were treated with aspirin for 36 h.
Cells were harvested and lysed in lysis buffer, and Western blotting analysis was performed using conventional protocols. Briefly, the protein concentration of the extracts was determined using a bicinchoninic acid kit (Pierce, United States) with bovine serum albumin as the standard. Total protein samples (30 μg) were loaded and separated by 10% SDS-PAGE gels and then transferred to protran nitrocellulose membranes (Whatman, United Kingdom). The membranes were incubated with an anti-COX-2 antibody (1:200; Santa Cruz, United States) and, after extensive washing, a horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (1:2000; Santa Cruz) for 1 h at room temperature. The signal was detected by chemiluminescence using an ECL Detection Kit (Amersham, United States). The membranes that were probed for COX-2 were re-probed for β-actin (1:10 000; Santa Cruz, United States) to normalize for loading and/or quantification errors and to allow for the direct comparison of protein expression. The bands were quantified using a Gel EDAS analysis system (Cold Spring United States Corporation) and Gel-Pro Analyzer 3.1 software (Media Cybernetics, United States).
To evaluate the effects of COX-2 overexpression and aspirin treatment on the proliferation of the EC109 cells, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay was used. In brief, EC109 cells were seeded in 96-well plates at a density of 2 × 105 cells/well and cultured for 24 h. The cells were then transfected with the COX-2 expression plasmid (pcDNA3.1V5HisA/hCOX-2) or the empty control vector (pcDNA3.1V5HisA) for 72 h. At 24 h post-transfection, the co-treated cells were treated with aspirin (8 mmol/L) for an additional 48 h. Then, 20 µL of 5 mg/mL MTT solution (Gibco, Grand Island, United States) was added to 200 µL of the media in each well. After an additional incubation for 4 h, the media was discarded, and 150 µL of DMSO was added to each well to dissolve the formazan crystals. The optical density at 492 nm was read using an automated microplate reader. The experiments were conducted in triplicate, and the results are shown as the mean ± standard deviation (SD) of three independent experiments.
To determine the effects of COX-2 overexpression and aspirin treatment on apoptosis, EC109 cells were transfected with either an empty control vector, hCOX-2 cDNA alone or with hCOX-2 cDNA plus aspirin treatment. The cells were collected by centrifugation at 200 g for 5 min and stained with propidium iodide (PI; R&D Company, Minneapolis, United States). The pellets were washed twice with ice-cold phosphate-buffered saline (PBS), fixed overnight at 4 °C in 70% ethanol and stored at -20 °C. The pellets were washed twice with PBS, and the cells were incubated with 5 μg/mL PI and 50 μg/mL RNase A in PBS for 1 h at room temperature in the dark. Flow cytometry was conducted using a FACSCalibur flow cytometer (BectonDickson, Mountain View, CA). Ten thousand events were collected per sample, and the cells were analyzed for the rate of apoptosis using CELLQUEST software. This experiment was performed in triplicate.
To generate stably transfected cell lines, cells transfected with pcDNA3.1V5HisA/hCOX-2 as well as cells transfected with the empty control vector (pcDNA3.1V5HisA) were selected with 500 μg/mL G418. After 30 d of selection with G418, a G418-resistant stably transfected cell line was created. EC109 parent cells and stably transfected cells were trypsinized and collected. The cell viability was > 95% as determined by trypan blue staining. Cells (4 × 106) in 0.1 mL PBS were inoculated subcutaneously onto the backs of 4- to 6-wk-old male BALB/c nude mice (five per group). Male BALB/c nu/nu mice weighing (15.24 ± 0.57) g were purchased from the Experimental Animal Center at the Chinese Academy of Medical Sciences and Peking Union Medical College. The mice were housed under sterile conditions. For mice given the combination of COX-2 transfection and aspirin treatment, a high dose (0.315 mg/dL-1) or a low dose (0.016 mg/dL-1) of aspirin was orally administrated once per day starting one day before the inoculation of COX-2-transfected EC109 cells. The aspirin administered to experimental animals was provided by the Bayer Animal Health Company. The tumor size was monitored weekly using calipers, and the tumor volume was calculated using the following formula: volume = 0.5 × length × width[2]. At the end of 4 wk, all the mice were sacrificed and their weight was recorded. The tumors were excised and kept in 4% formalin for immunohistochemical analysis. The percentage of cells expressing nuclear Ki67 was used for the assessment of cellular proliferation rate. The nuclear Ki67 expression and the nuclear COX-2 protein expression was detected by immunohistochemistry. The Ki67 and COX-2 antibodies for immunohistochemistry were purchased from the Fuzhou Mai Xin Company (Fuzhou, China). PV6001 Kits were purchased from Zhong Shan Golden Bridge Biological Technology (Beijing, China). All procedures using animal experimentation were approved by the local animal research authorities, and animal care was conducted in accordance with institutional guidelines.
Statistical analysis was performed using SPSS statistical software (SPSS 11.0, United States). The differences between the groups were assessed using the analysis of variance test. The results were considered statistically significant when the P value was < 0.05 or < 0.01.
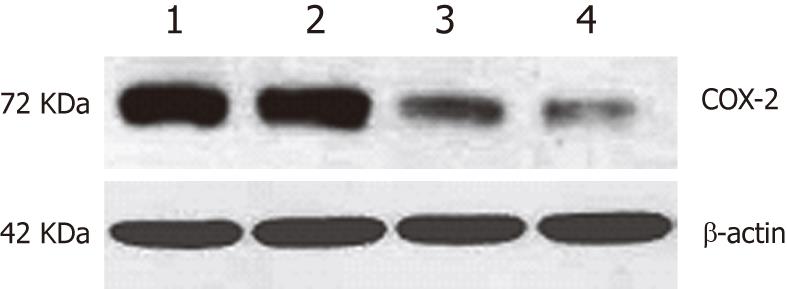
The expression of COX-2 was evaluated in EC109 cells transfected with either the pcDNA3.1V5HisA/hCOX-2 or pcDNA3.1V5HisA vector. For the combined treatment group, 8 mmol/L aspirin was added to the cell culture 24 h after transfection with the COX-2 plasmid (pcDNA3.1V5HisA/hCOX-2) for a period of 48 h. COX-2 protein expression was evaluated by Western blotting analysis. In comparison to the parent cell line or the control vector transfectants, a slight increase in COX-2 expression was observed in the COX-2-transfected EC109 cells. However, the expression of COX-2 was suppressed after aspirin was added to the COX-2-transfected EC109 cells. For Western blotting analysis, the COX-2 expression was normalized to β-actin expression by band intensity (Figure 1).
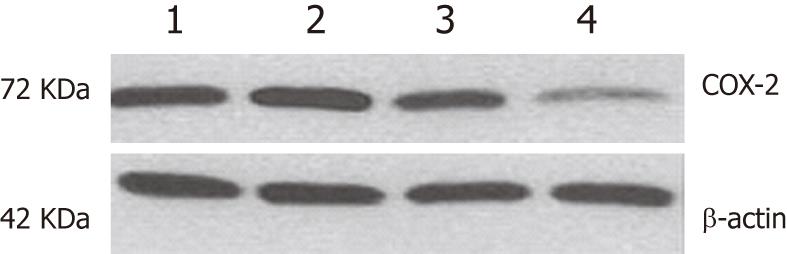
Given the importance of COX-2 in ESCC carcinogenesis, we used an in vitro experimental model that used ESCC cell lines to determine whether COX-2-specific siRNA was able to downregulate COX-2 expression. From a panel of ESCC cell lines, we choose a well-differentiated line (EC109) that had a moderate level of COX-2 expression. The effect of COX-2 siRNA treatment on protein expression was assessed by Western blotting analysis. As shown in Figure 2, COX-2 expression was reduced in EC109 cells 84 h after transfection with the COX-2 siRNA (100 nmol/L), while the negative control siRNA had no effect on the expression of COX-2. COX-2 expression was normalized to β-actin expression by band intensity. COX-2 expression was significantly further decreased in the EC109 cells transfected with COX-2 siRNA for 36 h and also in the EC109 cells treated with aspirin for 48 h (Figure 2). These results suggest that the co-administration of aspirin and siRNA had a synergistic effect on the inhibition of COX-2 gene expression.
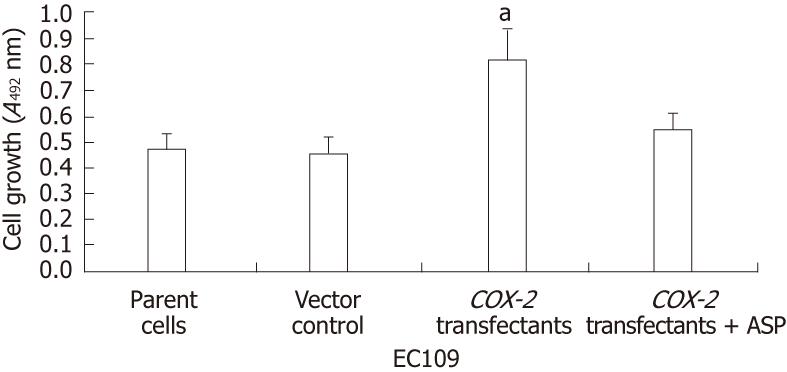
The MTT assay was conducted to elucidate the effects of COX-2 transfection and aspirin treatment on EC109 cell proliferation. The values are expressed as the mean ± SD of three independent experiments. The results from this assay indicated that COX-2 transfection led to a marked increase in cell proliferation at 72 h (Figure 3). Statistical analysis found that cell proliferation was significantly increased in COX-2-transfected EC109 cells (P < 0.05). However, there was no significant increase in the proliferation of cells transfected with the empty vector, as compared to parent cells. Furthermore, cell proliferation was not significantly affected by the administration of aspirin to COX-2-transfected cells as compared with control cells (Figure 3). These data demonstrate that cell proliferation was increased following COX-2-transfection in EC109 cells and decreased upon co-administration of aspirin. Therefore, the expression of COX-2 was important for proliferation in EC109 cells. Moreover, COX-2 overexpression by transfection promoted cell proliferation, and the inhibition of COX-2 expression by aspirin treatment inhibited cell proliferation in EC109 cells.
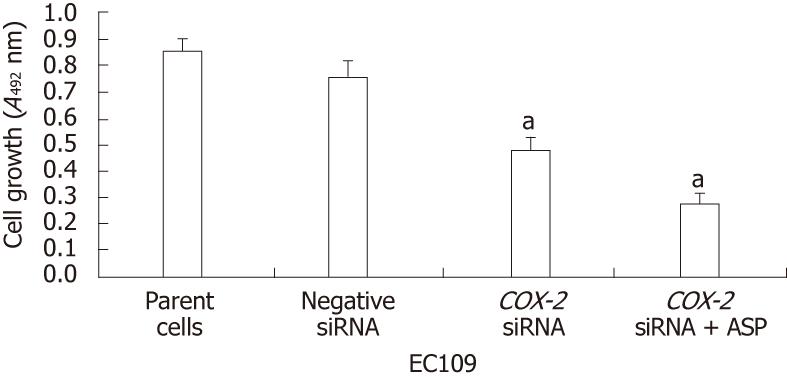
To elucidate the effects of the siRNA and aspirin treatments on EC109 cell proliferation, the MTT assay was used, and cell proliferation was determined by counting the numbers of viable cells. The values are expressed as the mean ± SD of three independent experiments. There was no significant reduction in the number of viable EC109 cells at 24 h post-treatment. However, at 48 h, we detected a significant decrease in the number of viable cells. Furthermore, COX-2 siRNA treatment resulted in a significant inhibition of cell proliferation in the EC109 cells (P < 0.01) at 84 h post-transfection (Figure 4). The proliferation was not affected by treatment with the control siRNA. Additionally, the treatment of siRNA-transfected cells with aspirin further increased the inhibition of cell proliferation (P < 0.01, Figure 4). These data demonstrate that COX-2 expression is important for proliferation in EC109 cells and that the inhibition of COX-2 by siRNA alone or combined with aspirin treatment can inhibit the proliferation of EC109 cells.
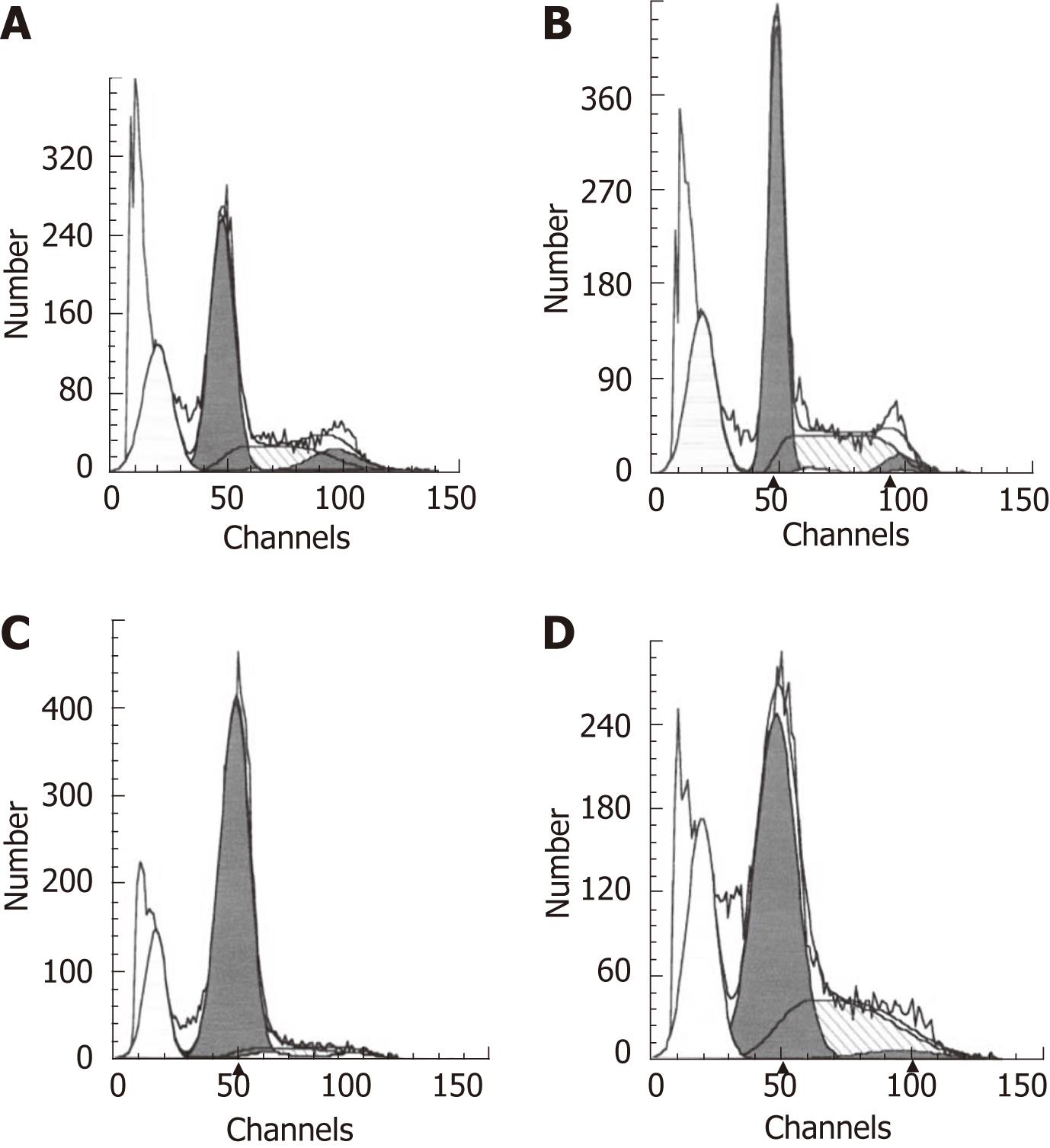
To determine the effect of COX-2 transfection and aspirin treatment on apoptosis in esophageal cancer EC109 cells, flow cytometry was performed on PI-stained cells. The results showed that EC109 cells transfected with hCOX-2 were resistant to apoptosis. Comparing to the rate of apoptosis that was 28.7% in parent cells group and 26.96% in empty vector transfected cells group, the rate of apoptosis was decreased to 17.75% in COX-2-transfected EC109 cells (Figure 5). Apoptosis was not significantly influenced by transfection with the empty vector control. However, the rate of apoptosis increased to 24.97% when the transfected EC109 cells were treated with aspirin (Figure 5). These data indicate that apoptosis in EC109 cells was affected by COX-2 transfection and aspirin treatment.
To determine the effect of siRNA and aspirin treatment on the rate of apoptosis in EC109 cells, treated cells were labeled with PI and analyzed by flow cytometry. The inhibition of COX-2 by siRNA alone or with aspirin treatment resulted in a significant decrease in the growth of the EC109 cells due to the induction of apoptosis. Apoptosis was not significantly influenced by treatment with the negative control siRNA. Comparing to the rate of apoptosis that was 1.35% in parent cells group and 1.72% in negative control siRNA-treated cells group respectively, the rate of apoptosis was increased to 4.33% in siRNA-treated EC109 cells (Figure 6) and was further increased to 11.66% when EC109 cells were treated with both the COX-2-specific siRNA and aspirin (Figure 6). These data indicate that the COX-2-specific siRNA alone or in combination with aspirin treatment induced the apoptosis of EC109 cells.
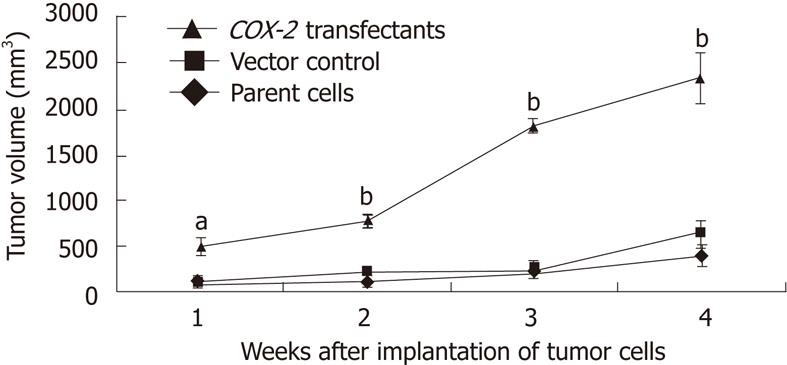
COX-2 overexpression increases tumor growth: Human COX-2 cDNA was cloned into the pcDNA3.1V5HisA vector, which was stably transfected into the EC109 cells. To assess changes in cell biology after gene transfection, cell proliferation was measured by an MTT assay. COX-2-transfected cells appeared to have a markedly different growth pattern, as compared to the EC109 parent cells or the vector control-transfected cells (Figure 3). To investigate the role of COX-2 in tumorigenicity, tumor progression was evaluated in nude mice. Following selection, G418-resistant stably transfected cells were inoculated into nude mice. The first xenografted tumor was found 3 d after the implantation of COX-2-transfected tumor cells, and tumor sizes were measured weekly. Four weeks after cell transfer, tumors established by EC109 parent cells or vector control (pcDNA3.1V5HisA) cells produced tumors of 393.43 mm3± 118.48 mm3 and 634.50 mm3± 159.29 mm3, respectively, while tumors from COX-2-transfected (pcDNA3.1V5HisA/hCOX-2) cells produced markedly larger tumors that were 2344.49 mm3± 273.52 mm3 in volume (P < 0.01 or P < 0.05, Figure 7). When mice were sacrificed at 28 d post-transfer, the EC109 parent cells and vector control cells had produced tumors that were 0.67 g ± 0.15 g and 0.89 g ± 0.29 g, respectively, while COX-2-transfected cells had produced larger tumors that were 2.58 g ± 0.26 g in weight (P < 0.05). Data are expressed as the mean ± SD of five independent samples (n = 15). These data show that COX-2 gene expression increased tumor growth.
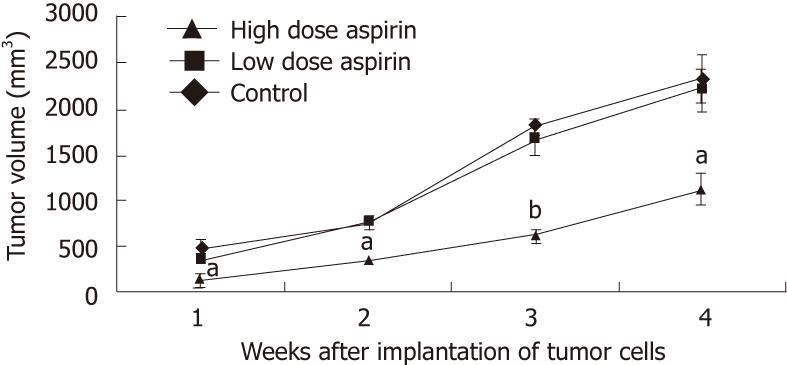
Aspirin treatment reduces tumor growth: Aspirin, an inhibitor of COX-2, was administered to nude mice starting one day before the implantation of COX-2-stably transfected EC109 cells. At day 28 after implantation, a high dose of aspirin (0.315 mg/dL-1, once per day) had significantly reduced tumor volume (P < 0.01 or P < 0.05) and tumor weight (P < 0.05) in this xenograft model (Figure 8). However, there was no significant difference in tumor volume between the low-dose aspirin (0.016 mg/dL-1, once per day) and control group. Date are expressed as the mean ± SD of five independent samples (n = 15). The tumor inhibition rate for the high-dose aspirin group was 51.80%, and the tumor inhibition rate for the low-dose aspirin group was 5.98%. No digestive hemorrhages or ulcers were found in the mice. Therefore, large doses of aspirin effectively inhibited the growth of these xenografted tumors in nude mice.
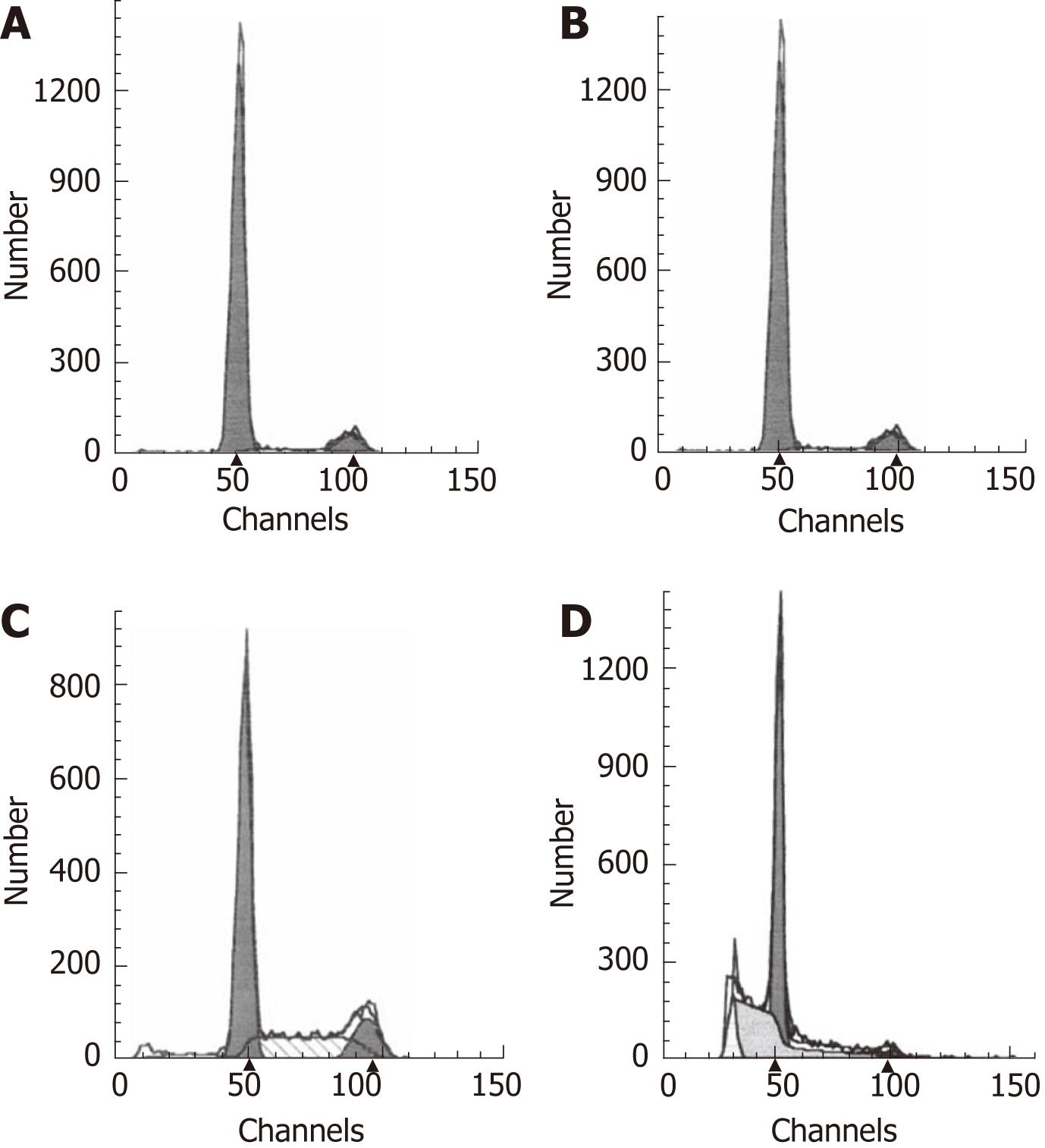
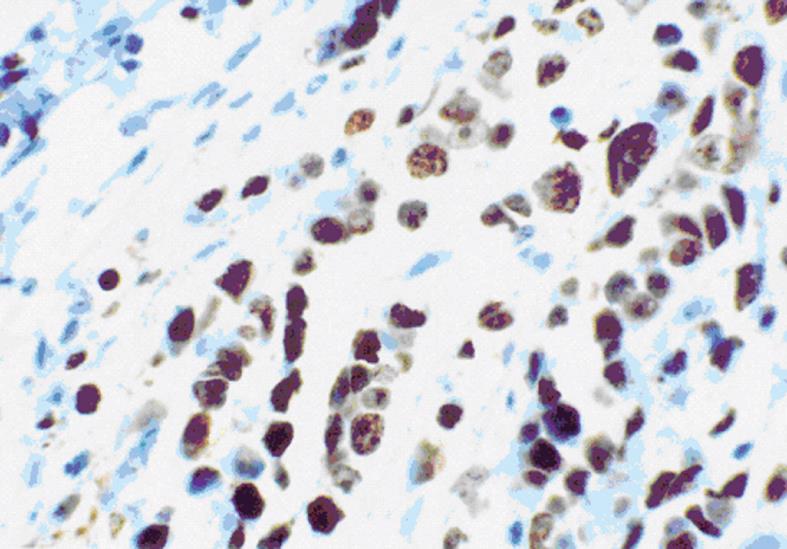
Evaluation of Ki67 immunostaining in cancer xenografts: The expression of Ki-67 protein is strictly associated with cell proliferation, which makes Ki-67 expression a biomarker and a strong predictor of cancer. We mainly observed Ki-67 protein expression in the nuclei of xenografted tumor cells (Figure 9). Immunohistochemical sections stained for Ki-67 were quantified with a computer-aided image analysis machine using the association between high gray-scale density and tumor cell proliferation. The quantification of staining intensity demonstrated that cell proliferation in tumor xenografts from cells transfected with COX-2 was greater than in tumors from parent cells and vector control-transfected cells.
A large body of evidence suggests that COX-2-overexpressing cancer cells may possess a survival advantage that facilitates tumor development and progression[15,16]. In our study, RNAi was used as a potent tool to inhibit COX-2 gene expression levels in human ESCC. COX-2 RNAi resulted in significant suppression of cellular proliferation in EC109 cells. This result, together with the observation that aspirin treatment inhibited the growth of the EC109 cells, indicates that COX-2 activity may be directly associated with proliferation in EC109 cells. To further investigate the role of COX-2 in ESCC carcinogenesis, EC109 cells were transfected with recombinant COX-2-expressing vectors to establish stably transfected cell lines. The increased COX-2 protein expression observed in transfected cells suggests that COX-2 transfection effectively increases COX-2 levels and leads to the increased proliferation of these cells.
Other studies have found an association between COX-2 overexpression and the antiapoptotic signature of cancer[17-19]. In this study, an antiapoptotic effect was observed in COX-2-transfected cells. Considering the proapoptotic effect of aspirin, our results suggest that COX-2 overexpression contributed to the antiapoptotic signature of the EC109 cells. Furthermore, COX-2 RNAi promoted apoptosis in these cells. Our data suggest that COX-2-specific siRNA treatment combined with the administration of aspirin is a promising treatment option for COX-2-expressing ESCCs. COX-2 overexpression-mediated upregulation of the antiapoptotic protein Bcl-2 has been proposed to be a mechanism that leads to the antiapoptotic signature of cancer cells[20].
In light of these results obtained from in vitro experiments, we hypothesized that COX-2 cDNA would enhance the in vivo tumorigenesis of EC109 cells. As expected, nude mice transfected with COX-2 showed faster tumor growth and larger tumors, which is in contrast to mice transfected with the parental cell line or the vector control cells. Furthermore, the immunohistochemical data support these findings, as Ki-67, a marker of cell proliferation, was found at increased levels in the COX-2-tranfected tumor xenografts. In addition, the suppression of COX-2 expression by high-dose aspirin treatment effectively attenuated the oncogenesis of EC109 cells in vivo.
Although transfection of COX-2 into EC109 cells resulted in increased cellular proliferation, stronger anti-apoptotic activity and enhanced tumorigenesis in vivo, the EC109 cell line itself had endogenous COX-2 expression. Therefore, we deduce that COX-2 function is correlated with the amount of its expression and enzyme activity.
NSAID-mediated growth inhibition of tumor cells involves COX-prostaglandin E2 (PGE2)-dependent and COX-PGE2-independent pathways[21]. In COX-PGE2-dependent pathways, COX-2 inhibitors suppress carcinogenesis via the inhibition of COX-2-derived PGE2, which promotes cell proliferation and immunosuppression[22]. The elevated levels PGE2 in COX-2-overexpressing cells are related to the metastatic potential of the cancer cells, which can be reduced in a dose-dependent manner by COX inhibitors[12]. Furthermore, the COX-2 enzyme itself can promote cancer development and progression[23]. In COX-PGE2-independent pathways, the mechanism by which COX-2 inhibitors suppress carcinogenesis involves the reduced activation of Akt, inhibition of nuclear factor-κB activation, downregulation of the antiapoptotic protein Bcl-XL, inhibition of peroxisome proliferator-activated receptor (PPAR)-δ, activation of PPAR-γ and the activation of caspase family members. One or more of these COX-PGE2-independent effects could contribute to the proapoptotic and antiproliferative effects of NSAID treatment[24]. The hypophosphorylation of the retinoblastoma tumor suppressor protein as well as the downregulation of multiple proliferation-promoting cyclins and cyclin-dependent kinases induced by NSAIDs has been reported in colon carcinoma cells[23]. The extent of the use of COX-PGE2-dependent or -independent pathways following NSAID treatment may depend on the cell type.
Gene therapy and RNAi are the most exciting and promising technologies in biomedicine today. The goal of gene therapy is to introduce genetic material (DNA or RNA) encoding a protein, which is missing or defective, into a patient’s cells or tissues. RNAi is the sequence-specific post-transcriptional silencing of gene expression mediated by small double-stranded RNA molecules. RNAi has become an exceptionally powerful method to inhibit the expression and function of disease-causing genes, and it performs better than all similar techniques previously tested in gene therapy. However, the use of RNAi is still in its infancy, and the safety of the delivery system and tissue-targeting and the potential for adverse effects or lifelong RNAi persistence should still be considered before RNAi becomes a feasible and safe therapy for patients. In fact, RNAi-mediated gene therapy remains the single most promising biomedical strategy for knocking down expression of the COX-2 gene in human ESCC, and we believe that the successful clinical implementation of RNAi gene therapy and its eventual translation into clinical benefits are merely a question of time.
In this study, no gastric mucosal hemorrhages were found in mice, even after administration of large doses of aspirin. However, the gastric mucosa of nude mice is different from human gastric mucosa. In patients, larger doses of aspirin can increase the risk of bleeding, including bleeding ulcers, and combined therapy with RNAi can allow for lower doses of aspirin. The risk for bleeding tends to decrease as the dose of aspirin is decreased. Further long-term animal experiments may be required to test the effectiveness of COX-2 inhibitors and RNAi in the treatment of ESCC.
In conclusion, our present study has indicated that COX-2 plays a crucial role in the carcinogenesis of human ESCC by increasing cell proliferation and resistance to apoptosis and subsequently enhancing tumorigenesis in vivo. These results suggest that COX-2 may be a new gene target for ESCC treatment and that inhibition of COX-2 expression by RNAi and aspirin treatment may serve as an early and effective prevention and treatment of early-stage, COX-2-expressing ESCC.
We thank Herschman H (University of California) and Smith WL (University of Michigan) for their invaluable suggestions. We thank Sharma M for critical reading of this manuscript and helpful discussions.
Esophageal cancer can be divided into two major groups including esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma. Epidemiological studies indicate that ESCC is the dominant form in Asian countries, especially China, Japan and Korea. Some studies have shown that COX-2 is overexpressed in ESCC and contributes to carcinogenesis. However, the molecular mechanisms by which COX-2 promotes carcinogenesis in squamous cells remain unclear. Given the high fatality rate and the rapidly increasing incidence of ESCC, the definition of the role of COX-2 in the pathogenesis and therapy of this cancer is highly desirable.
In this study, RNAi was used as a potential tool to inhibit COX-2 gene expression in human ESCC. COX-2 RNA interference (RNAi) significantly suppressed the proliferation of EC109 cells in a manner similar to treatment with aspirin, a non-specific COX inhibitor. Moreover, COX-2 transfection effectively increased COX-2 levels and increased the proliferation of EC109 cells. These results indicate that COX-2 may be directly associated with the proliferation of EC109 cells. Considering the proapoptotic effects of aspirin, our results favor the view that COX-2 overexpression contributes to the acquisition of antiapoptotic activity in EC109 cells. Moreover, COX-2 RNAi treatment was able to promote apoptosis. COX-2 short interfering RNA (siRNA) combined with aspirin has the potential to be an effective anticancer therapy for the treatment of ESCC. Furthermore, the tumorigenicity assay in nude mice revealed a faster tumor growth and larger xenograft tumor size in the COX-2-transfected cell group. Alternatively, the suppression of COX-2 expression by high-dose aspirin treatment effectively attenuated the oncogenesis of EC109 cells in vivo.
In the past few years, RNAi has become an important research tool to study and manipulate a particular gene and its function. Furthermore, the use of siRNA as a potent and specific inhibitor of a specific target gene provides a new therapeutic approach for many incurable diseases, particularly cancer. In addition, aspirin may boost the effectiveness of chemotherapy in the treatment of cancers. This is the first study to report that COX-2 siRNA combined with aspirin treatment has the potential to be an effective anticancer therapy for ESCC.
Gene therapy and RNAi are the most exciting and promising technologies in biomedicine today. RNAi is an exceptionally powerful method to inhibit the expression and the function of disease-causing genes, and it has out-performed all similar methodologies previously tested in gene therapy. The data presented here suggest that the use of COX-2-specific siRNA combined with aspirin is a promising treatment for COX-2-expressing ESCCs. The present research believes that the successful clinical implementation of this combination therapy is merely a question of time.
Non-steroidal antiinflammatory drug (NSAID): NSAIDs block the COX enzymes and reduce the levels of prostaglandins throughout the body. NSAIDs are used primarily to treat inflammation, mild to moderate pain, and fever. Aspirin is a unique NSAID.
The authors aimed to study the relationship between the COX-2 gene and the proliferation and apoptosis of esophageal squamous carcinoma EC109 cells. They have demonstrated that COX-2 expression was significantly reduced in EC109 cells treated with COX-2-specific siRNA but was increased in cells transfected with COX-2. They have concluded that COX-2 plays a critical role in ESCC carcinogenesis, and that COX-2 siRNA combination treatment with aspirin has the potential to be an effective anticancer therapy for ESCC. Overall, the manuscript is very interesting and adds to the body of literature on the positive effects of aspirin in carcinogenesis. It is also very well written and the conclusions are supported by the data.
Peer reviewer: Piero Marco Fisichella, MD, Assistant Professor of Surgery, Medical Director, Swallowing Center, Loyola University Medical Center, Department of Surgery, Stritch School of Medicine, 2160 South First Avenue, Room 3226, Maywood, IL 60153, United States
S- Editor Wu X L- Editor Cant MR E- Editor Xiong L
| 1. | Lv J, Cao XF, Zhu B, Ji L, Tao L, Wang DD. Effect of neoadjuvant chemoradiotherapy on prognosis and surgery for esophageal carcinoma. World J Gastroenterol. 2009;15:4962-4968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Hseu YC, Chen SC, Tsai PC, Chen CS, Lu FJ, Chang NW, Yang HL. Inhibition of cyclooxygenase-2 and induction of apoptosis in estrogen-nonresponsive breast cancer cells by Antrodia camphorata. Food Chem Toxicol. 2007;45:1107-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Moreira L, Castells A. Cyclooxygenase as a Target for Colorectal Cancer Chemoprevention. Curr Drug Targets. 2010;Epub ahead of print. [PubMed] |
| 4. | Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, Koki AT. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000;89:2637-2645. [PubMed] |
| 5. | Tucker ON, Dannenberg AJ, Yang EK, Zhang F, Teng L, Daly JM, Soslow RA, Masferrer JL, Woerner BM, Koki AT, Fahey TJ. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999;59:987-990. [PubMed] |
| 6. | van Rees BP, Saukkonen K, Ristimäki A, Polkowski W, Tytgat GN, Drillenburg P, Offerhaus GJ. Cyclooxygenase-2 expression during carcinogenesis in the human stomach. J Pathol. 2002;196:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 117] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Khuri FR, Wu H, Lee JJ, Kemp BL, Lotan R, Lippman SM, Feng L, Hong WK, Xu XC. Cyclooxygenase-2 overexpression is a marker of poor prognosis in stage I non-small cell lung cancer. Clin Cancer Res. 2001;7:861-867. [PubMed] |
| 8. | Abdullah M, Sudoyo AW, Pranowo BS, Rini D, Sutrisna B, Rani AA. Expression of NF-kappaB and COX-2 in young versus older patients with sporadic colorectal cancer. Acta Med Indones. 2009;41:70-74. [PubMed] |
| 9. | Funkhouser EM, Sharp GB. Aspirin and reduced risk of esophageal carcinoma. Cancer. 1995;76:1116-1119. [PubMed] |
| 10. | Corley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology. 2003;124:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 379] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 11. | Gasparini G, Longo R, Sarmiento R, Morabito A. Inhibitors of cyclo-oxygenase 2: a new class of anticancer agents? Lancet Oncol. 2003;4:605-615. [PubMed] |
| 12. | Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997;94:3336-3340. [PubMed] |
| 13. | Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705-716. [PubMed] |
| 14. | Yang GZ, Li L, Ding HY, Zhou JS. Cyclooxygenase-2 is over-expressed in Chinese esophageal squamous cell carcinoma, and correlated with NF-kappaB: an immunohistochemical study. Exp Mol Pathol. 2005;79:214-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Dannenberg AJ, Lippman SM, Mann JR, Subbaramaiah K, DuBois RN. Cyclooxygenase-2 and epidermal growth factor receptor: pharmacologic targets for chemoprevention. J Clin Oncol. 2005;23:254-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 314] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 16. | Zhang H, Sun XF. Overexpression of cyclooxygenase-2 correlates with advanced stages of colorectal cancer. Am J Gastroenterol. 2002;97:1037-1041. [PubMed] |
| 17. | Elder DJ, Baker JA, Banu NA, Moorghen M, Paraskeva C. Human colorectal adenomas demonstrate a size-dependent increase in epithelial cyclooxygenase-2 expression. J Pathol. 2002;198:428-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Shi H, Xu JM, Hu NZ, Xie HJ. Prognostic significance of expression of cyclooxygenase-2 and vascular endothelial growth factor in human gastric carcinoma. World J Gastroenterol. 2003;9:1421-1426. [PubMed] |
| 19. | Higashi Y, Kanekura T, Kanzaki T. Enhanced expression of cyclooxygenase (COX)-2 in human skin epidermal cancer cells: evidence for growth suppression by inhibiting COX-2 expression. Int J Cancer. 2000;86:667-671. [PubMed] |
| 20. | Sun Y, Tang XM, Half E, Kuo MT, Sinicrope FA. Cyclooxygenase-2 overexpression reduces apoptotic susceptibility by inhibiting the cytochrome c-dependent apoptotic pathway in human colon cancer cells. Cancer Res. 2002;62:6323-6328. [PubMed] |
| 21. | Ahnen DJ. Colon cancer prevention by NSAIDs: what is the mechanism of action? Eur J Surg Suppl. 1998;111-114. [PubMed] |
| 22. | Gately S, Kerbel R. Therapeutic potential of selective cyclooxygenase-2 inhibitors in the management of tumor angiogenesis. Prog Exp Tumor Res. 2003;37:179-192. [PubMed] |
| 23. | Levy GN. Prostaglandin H synthases, nonsteroidal anti-inflammatory drugs, and colon cancer. FASEB J. 1997;11:234-247. [PubMed] |
| 24. | Kern MA, Schubert D, Sahi D, Schöneweiss MM, Moll I, Haugg AM, Dienes HP, Breuhahn K, Schirmacher P. Proapoptotic and antiproliferative potential of selective cyclooxygenase-2 inhibitors in human liver tumor cells. Hepatology. 2002;36:885-894. [PubMed] |