Published online Sep 28, 2011. doi: 10.3748/wjg.v17.i36.4135
Revised: May 19, 2011
Accepted: May 26, 2011
Published online: September 28, 2011
AIM: To characterise the viral kinetics of enterovirus 71 (EV71).
METHODS: In this study, human rhabdomyosarcoma (RD) cells were infected with EV71 at different multiplicity of infection (MOI). After infection, the cytopathic effect (CPE) was monitored and recorded using a phase contrast microscope associated with a CCD camera at different time points post viral infection (0, 6, 12, 24 h post infection). Cell growth and viability were measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay in both EV71 infected and mock infected cells at each time point. EV71 replication kinetics in RD cells was determined by measuring the total intracellular viral RNA with real-time reverse-transcription polymerase chain reaction (qRT-PCR). Also, the intracellular and extracellular virion RNA was isolated and quantified at different time points to analyze the viral package and secretion. The expression of viral protein was determined by analyze the levels of viral structure protein VP1 with Western blotting.
RESULTS: EV71 infection induced a significant CPE as early as 6 h post infection (p.i.) in both RD cells infected with high ratio of virus (MOI 10) and low ratio of virus (MOI 1). In EV71 infected cells, the cell growth was inhibited and the number of viable cells was rapidly decreased in the later phase of infection. EV71 virions were uncoated immediately after entry. The intracellular viral RNA began to increase at as early as 3 h p.i. and the exponential increase was found between 3 h to 6 h p.i. in both infected groups. For viral structure protein synthesis, results from western-blot showed that intracellular viral protein VP1 could not be detected until 6 h p.i. in the cells infected at either MOI 1 or MOI 10; and reached the peak at 9 h p.i. in the cells infected with EV71 at both MOI 1 and MOI 10. Simultaneously, the viral package and secretion were also actively processed as the virus underwent rapid replication. The viral package kinetics was comparable for both MOI 1 and MOI 10 infected groups. It was observed that at 3 h p.i, the intracellular virions obviously decreased, thereafter, the intracellular virions began to increase and enter into the exponential phase until 12 h p.i. The total amounts of intracellular virons were decreased from 12 to 24 h p.i. Consistent with this result, the increase of virus secretion occurred during 6 to 12 h p.i.
CONCLUSION: The viral kinetics of EV71 were established by analyzing viral replication, package and secretion in RD cells.
- Citation: Lu J, He YQ, Yi LN, Zan H, Kung HF, He ML. Viral kinetics of Enterovirus 71 in human abdomyosarcoma cells. World J Gastroenterol 2011; 17(36): 4135-4142
- URL: https://www.wjgnet.com/1007-9327/full/v17/i36/4135.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i36.4135
Enterovirus 71 (EV71) is a member of human enterovirus species which belongs to the Picornaviridae family. It is one of the major causative agents for herpangina and hand, foot and mouth disease (HFMD)[1,2]. Among young children, acute EV71 infection may also cause severe neurological diseases such as encephalitis and meningitis that lead to significant mortality[3,4]. Recently, outbreaks of EV71 infection have been frequently reported throughout the world[5-10]. In China, a recent outbreak of EV71 infected more than 1.4 million children and caused 760 deaths from January to July, 2010. It is also noted that an adult died due to EV71 infection in Hong Kong in May, 2010 (http://www.hkcd.com.hk/content/2010-05/28/content_2531606.htm).
EV71 is a small RNA virus containing a non-enveloped capsid and a single-stranded positive genomic RNA (about 7400 bp)[11]. The life cycle of EV71 was speculated according to studies on other enteroviruses. EV71 would attach to the host cell via its specific receptors[12] and then the viral genomic RNA is released into the cytoplasm, where it directly translates into a polyprotein. This precursor protein can subsequently be cleaved into four structural (VP1, VP2, VP3 and VP4) and seven non-structural (2A, 2B, 2C, 3A, 3B, 3C and 3D) proteins[4]. For virus RNA replication, a complementary minus-strand RNA is synthesised in the cytoplasm and then this minus-strand RNA can serve as a template for new plus-strand RNA molecules. Newly synthesized virus RNA may go into another round of translation and replication, or is packaged into capsid proteins to produce infectious viral particles (see review in Ref.[13]). Studies on other viruses demonstrate that the knowledge of virus kinetics is important for clarifying viral pathogenesis and exploring effective treatments. The knowledge of the viral kinetics on human immunodeficiency virus, hepatitis B virus and hepatitis C virus has greatly improved the understanding of the cell response to these viruses and mechanisms of related antiviral therapy[14-16]. In the Picornavirus family, the kinetics of swine vesicular disease virus (SVDV) and foot-and-mouth disease virus (FMDV) have been described in several studies[17,18]. However, little information is known about EV71 infection. In this study, we used rhabdomyosarcoma (RD) cells as an in vitro model and intensively investigated the viral kinetics of EV71, including the kinetics of viral replication, viral protein synthesis, packaging and secretion.
RD cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (v/v) fetal bovine serum (FBS), 100 U/mL penicillin and 100 μg/mL streptomycin. EV71 strain SHZH98 (GenBank accession number AF302996.1) was obtained from Shen-zhen Center for Disease Control and Prevention, Shenzhen, China. To prepare virus stocks, viruses were propagated on a 90% confluent cell monolayer in DMEM with 2% FBS. The viral titres were measured by the cytopathic effect (CPE) microtitration assays and expressed as 50% tissue culture infective dose (TCID50) per millilitre (mL) according to the Kärber formula[19].
RD cells were cultured in 12-well plates and infected with nil or EV71 at multiplicity of infection (MOI) 1 and 10, respectively. Briefly, plated cells were washed twice with phosphate buffered saline (PBS) and infected with EV71. Time was set as zero after adsorption for 1 h. The culture media were removed and cells were washed twice with PBS to remove unattached virus before adding 1 mL of DMEM medium containing 10% FBS to each well. The cells were cultured at 37 °C in 5% CO2. The cell morphology was monitored and recorded using a phase-contrast microscope associated with a CCD camera and computer at different time points. The infected cells and culture supernatants were harvested to isolate RNA and proteins.
MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assays were performed to determine the cell viability upon EV71 infection. Briefly, RD cells were set in 96-well plates at 1 × 104 cells per well 24 h before infection with EV71 at MOI 1 or MOI 10. The medium was then replaced with 0.5 mg/mL MTT medium at different time points and incubated for another 4 h. The MTT solution was removed from the wells and the formazan crystals were dissolved in DMSO. Absorbances of the formazan products were measured at 550 nm with the reference wavelength at 690 nm.
For intracellular viral RNA quantification, the total cellular RNA was isolated from EV71 infected cells using TRIzol reagent (Invitrogen, United States) according to the manufacturer’s instructions. To quantitate the extracellular virions, we first isolated the virions from the culture media of infected cells. The media were harvested and briefly centrifuged to remove cell debris. Viral core particles were then precipitated with 10% polyethylene glycol 8000 containing 0.5 mol/L NaCl at 4 °C overnight. After centrifuging for 30 min at 16 000 g, viral particles were pelleted and treated with 100 μg/mL of RNase A (Sigma, United States). To isolate the intracellular virions, EV71 infected cells were lysed with lysis buffer (1% Triton 100 and 1 × Roche protease inhibitor cocktail in PBS). Then the cell lysates were used to isolate viral particles as described above. The virion-associated RNA was then isolated by using TRIzol reagent. To set up the standard curve of infectious viruses, the viral titres were first determined by CPE assay. Then the viral RNA was extracted from those infectious EV71 viruses. RNA was diluted at ten-fold serial and used to reflect the calculated PFU from 10 to 1 × 107 live virions.
The one-step quantitative real-time polymerase chain reaction (qRT-PCR) was carried out using the ABI 7500 Real-Time PCR system (Applied Biosystems) with QuantiTect SYBR Green RT-PCR Kit (Qiagen) and specific forward EV71-VP1F (5’-GCAGCCCAAAAGAACTTCAC-3’) and reverse EV71-VP1R (5’-ATTTCAGCAGCTTGGAGTGC-3’) primers targeting a conserved region of the VP1 gene[20]. PCR assay was carried out in a 20 μL volume consisting of 10 μL of 2 × Quantitect SYBR green RT-PCR Master Mix, containing HotStarTaq DNA polymerase, 1 μL of 10 μmol/L of each oligonucleotide primer, 0.2 μL of 100 × QuantiTect RT Mix (containing Omniscript and Sensiscript reverse transcriptases) and 2 μL of RNA extracted from samples or from ten-folds serial diluted virus RNA standard (from 107 to 10 copies). The target fragment amplification was carried out as follows: reverse transcription at 50 °C for 30 min; initial activation of HotStar Taq DNA Polymerase at 95 °C for 15 min; 45 cycles in four steps: 94 °C for 10 s, 56 °C for 30 s, and 72 °C for 30 s. At the end of the amplification cycles, melting temperature analysis was carried out by a slow increase in temperature (0.1 °C/s) up to 95 °C.
To prepare total cellular protein extracts, the cells were lysed with RIPA buffer (50 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 1 mmol/L EDTA, 1% Triton X-100, 0.1% SDS, 1 × Roche protease inhibitor cocktail) with occasional vortexing. Lysates were collected by centrifugation at 14 000 g for 10 min at 4 °C and protein concentrations were measured by the Bradford method (Bio-Rad, United States). Equal amounts (20 μg) of proteins from each sample were separated through 12% SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membranes (Amersham Biosciences). Membranes were blocked by 5% skim milk in Tris-Buffered Saline Tween-20 (TBST) (20 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 0.1% Tween 20) followed by incubation with specific antibodies against VP1 (PAB7631-D01P, Abnova) or GAPDH (Santa Cruz Biotechnology). Target proteins were visualized with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology) and a chemiluminescence detection system (Amersham Biosciences). Each immunoblotting was carried out at least three times.
Data are depicted as mean ± SD. All statistical analyses were carried out with SPSS 14.0 software (SPSS Inc.). Two-tailed Student’s t test was applied for two-group comparison. P < 0.05 was considered statistically significant.
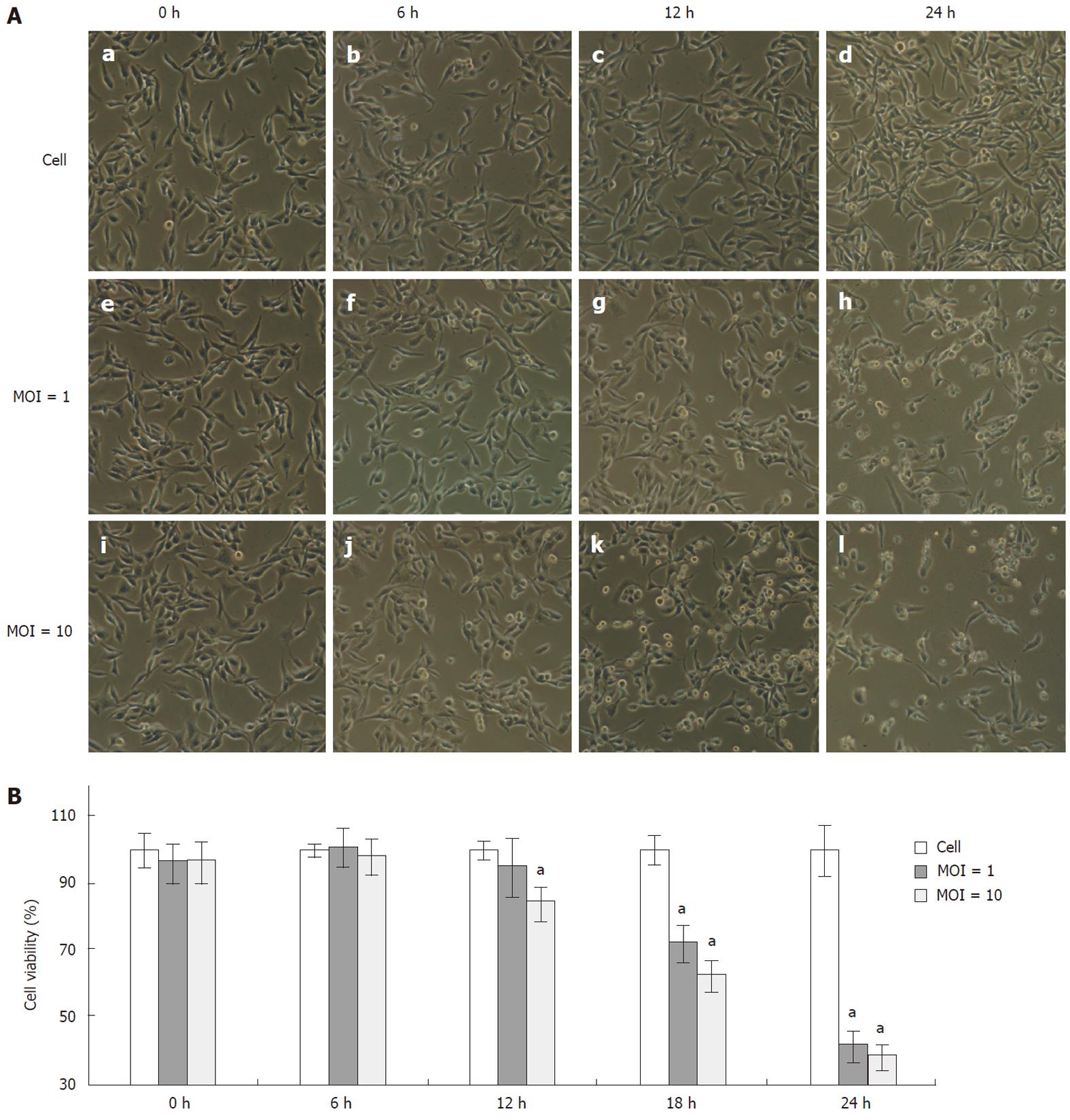
Morphological changes were observed as early as 6 h p.i. when RD cells were infected with EV71 at either MOI 1 or 10 (Figure 1A, panels f and j). Initially, the cells rounded up and became more refractile. As the culture progressed, some infected cells detached from the culture plate and floated into the medium (Figure 1A, panels g, h, k and l). Compared with the cells infected at MOI 1, more cells were unhealthy at 6 h p.i. when infected at MOI 10 (Figure 1A, panel j vs f). Later on, the cells infected with EV71 both at MOI 1 and 10 underwent significant cell death and detached from the surface of culture dishes (Figure 1A, panels g and k). At 24 h p.i, most of the cells were detached from the surface of the plate in both infected groups (Figure 1A, panels h and l). The MTT assay showed that the viability of the cells infected with EV71 at MOI 1 did not significantly decrease at 12 h p.i., but slightly reduced in cells infected at MOI 10 (Figure 1B). At 18 and 24 h p.i., the viability of the cells infected with EV71 either at MOI 1 or 10 significantly decreased.
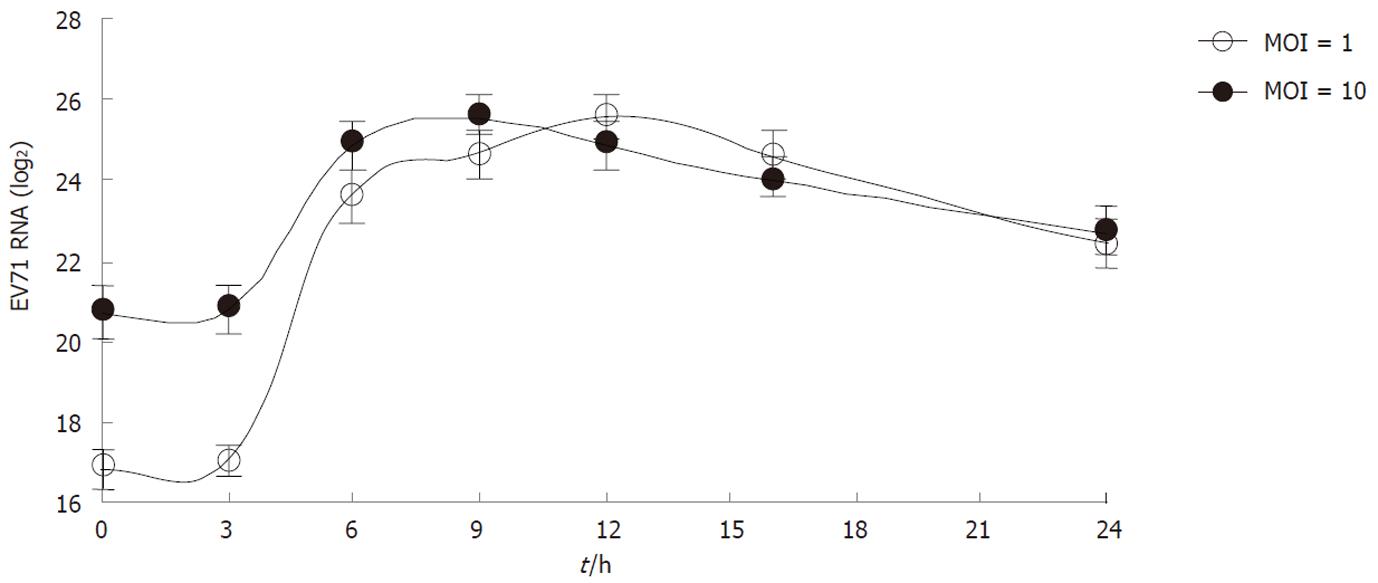
To examine the kinetics of viral replication, the levels of intracellular viral RNA were measured by qRT-PCR at each time point. As shown in Figure 2, the intracellular viral RNA began to increase as early as 3 h p.i. and the exponential phase was from 3 to 6 h p.i in both infected groups. In the case of infection at MOI 1, the intracellular viral RNA continually increased from 6 to 12 h p.i., and then gradually decreased until 24 h p.i. In the case of MOI 10, the intracellular viral RNA reached a peak between 6 and 9 h p.i., and then began to decrease.
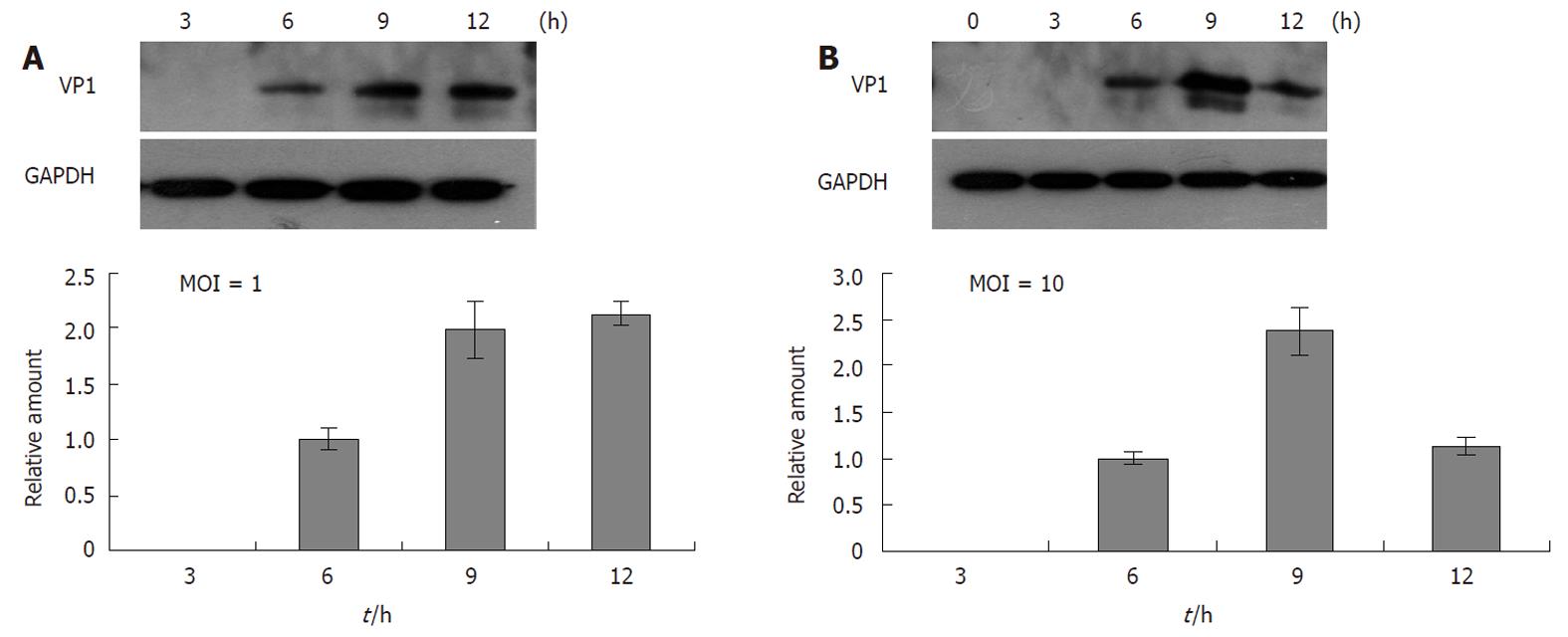
The intracellular viral protein VP1 was not detected until 6 h p.i. in the cells infected at MOI 1 and10 (Figure 3). The VP1 protein level reached a peak at 9 h p.i. in the cells infected with EV71 at both MOI 1 and MOI 10. Obviously, the VP1 levels were much higher in the cells infected at MOI 10 than at MOI 1. In the case of infection at MOI 1, the VP1 level was maintained until 12 h p.i.; whereas the VP1 protein level rapidly decreased after 9 h p.i. when the cells were infected with EV71 at MOI 10.
To determine the kinetics of virus package, the intracellular EV71 virions were quantified at different time points p.i. At 3 h p.i., the intracellular virions significantly decreased. Thereafter, the intracellular virions began to increase and enter into the exponential phase until 12 h p.i. when the amount of intracellular virions reached a peak. The viral package kinetics were comparable for both the MOI 1 and MOI 10 infected groups (Figure 4). The total amounts of intracellular virions then decreased from 12 to 24 h p.i.
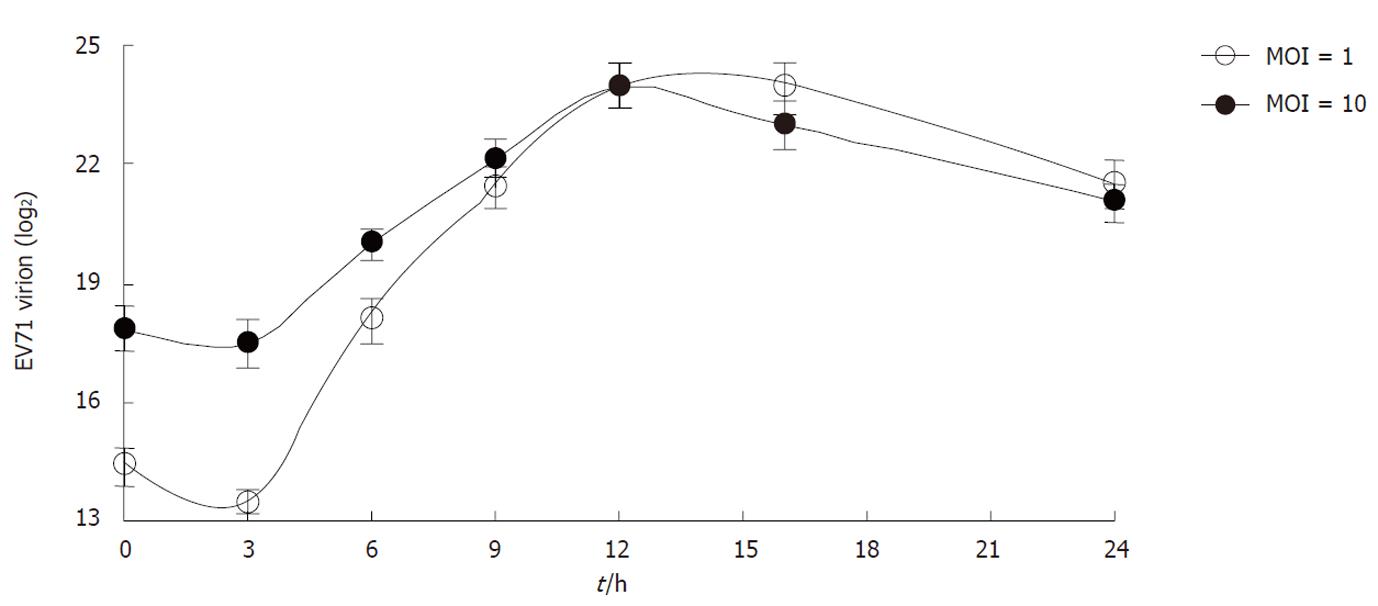
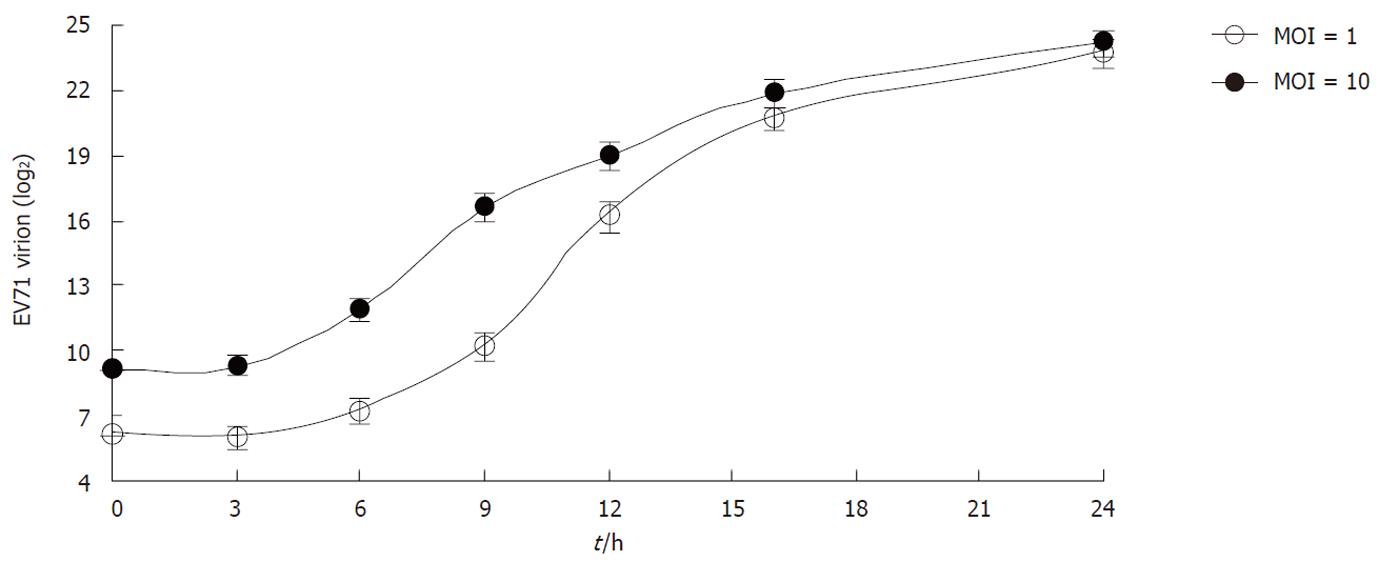
To determine the kinetics of virus secretion, the extracellular EV71 virions were quantitated. The EV71 virions began to be released from the cells infected either at MOI 1 or 10 3 h p.i. (Figure 5). The amounts of extracellular EV71 virions in the cultures of the two groups were constitutively increased. From 3 to 6 h p.i., the virions were slowly secreted into the culture media, and the virus secretion entered into the exponential phase from 6 to 12 h p.i. At 12 h p.i., the rate of increase declined and the total amount of extracellular virions reached a maximum at 24 h p.i. For cells infected at MOI 1 or 10, the virions in the culture media were similar at 24 h p.i.
Viral kinetics is an important parameter for demonstrating viral activities in the host cells and provides basic information on viral-host interactions and pathogenesis. The kinetics of some picornaviruses such as SVDV and FMDV have been described in several studies[17,18]. However, little information on EV71 is available. Some studies provided brief descriptions on EV71 RNA replication and the growth kinetics of EV71 infected cells, however, the infection ratios used in these studies were too low (MOI ≤ 0.01) to guarantee the synchronicity of infection[21,22]. In this situation, some cells were undergoing cell death, whereas others just had a chance to be infected by new EV71 viruses secreted from the first round infected cells. Therefore, the viral life cycle could not be accurately examined. In addition, with the exception of RNA synthesis, no information was provided on viral protein expression, virus package and secretion. Our study, for the first time, comprehensively described the detailed viral kinetics in human RD cells. As RD cells infected by EV71 would develop cellular pathogenesis (CPE), these cells have been extensively used to investigate the viral activities of EV71 and host responses to EV71 infection[23-26]. To obtain a synchronized infection, RD cells were pulse infected at high MOIs (MOI 1 and 10) to ensure that the majority of the cells were primarily infected in our study. Following infection, the unattached viruses were removed by washing the cells twice with PBS. This would minimize the interference of non-infectious virions. In this study, the kinetics of viral replication, gene expression, package and secretion as well as the effects of viral activities on host cells were carefully examined at different time points.
We showed here that the intracellular virions significantly decreased by over 90% at 3 h p.i. (Figure 4), while the total intracellular RNA copies remained almost at the same levels (Figure 2). These results suggested that the virions were immediately uncoated after entry and virus replication was inactive within the first 3 h after infection. During this phase, the viral RNA could be translated to generate viral proteins essential for viral replication. From 3 to 6 h p.i., the virus underwent fast replication and the total intracellular viral RNA was rapidly accumulated (Figure 2). Similar results were also reported in other poliovirus infection[27]. The total intracellular viral RNA increased by more than 64-fold within this period. In the meantime, viral gene expression was also initiated along with viral replication, as viral VP1 proteins in the host cells were clearly detected at 6 h (Figure 3). The viral package was also started (Figure 4) but very few virions were secreted (Figure 5). At 6 h p.i., about 1% (MOI 1) to 3% (MOI 10) of viral RNA was packaged into virions (Figures 2 and 4). Although the virus was rapidly replicating, the host cells were generally healthy during this period. From 6 to 9 h p.i., some cells became unhealthy (Figure 1), the viral replication entered a static stage as the total intracellular viral RNA only increased by about 2-fold in the MOI 1 and MOI 10 group. In the case of MOI 1, the total intracellular viral RNA increased a further 2-fold from 9 to 12 h p.i., but began to decrease 9 h p.i. in the cells infected at MOI 10. This suggested that viral RNA in cells infected with higher MOI reached maximal levels earlier. The viral gene expression and package were also actively processed in this period. The viral protein VP1 levels reached a peak at 9 h p.i. and rapidly decreased at 12 h p.i. in the MOI 10 group. In the case of MOI 1, the VP1 levels also reached a maximum at 9 h p.i. and maintained the same levels at 12 h p.i. Following viral protein synthesis, the intracellular virions also rapidly increased over 16-fold (MOI 10) or 64-fold (MOI 1). In both cases, the intracellular virions reached a peak and about 30% of viral RNA was packaged into the virions at 12 h p.i. (Figure 4vs Figure 2). In the case of MOI 1, the extracellularly accumulated virions increased 8- and 64-fold from 6 to 9 h, and 9 to 12 h, respectively; while the extracellularly accumulated virions increased 30- and 5-fold during the same periods in the MOI 10 group. From 12 to 24 h p.i., as more and more infected cells became unhealthy and died, the intracellular viral RNA levels significantly decreased and viral replication became less active. These findings suggested that the cells could no longer sustain further viral replication and died[28-30]. This was further supported by the data on intracellular and extracellular virion levels. We showed here that the intracellular virions maintained high levels at 16 h p.i. although more and more virions were secreted into the culture media. After that, the ratio of packaged viral RNA to total viral RNA was constant at about 50% in both the MOI 1 and MOI 10 group.
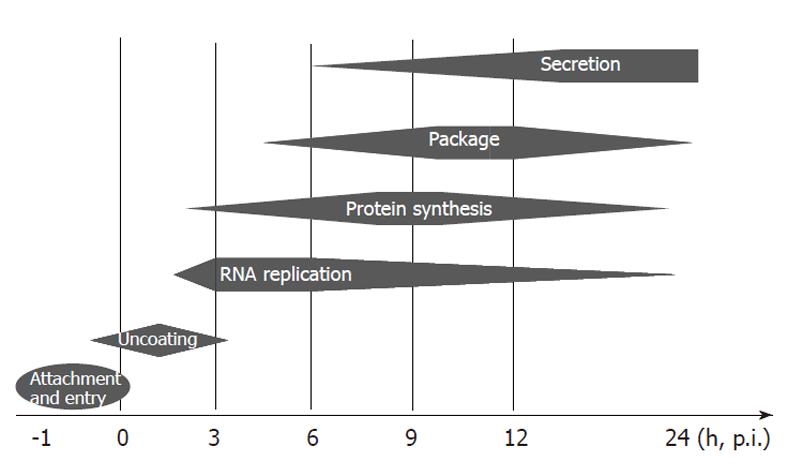
In summary, we have established a viral kinetics model of EV71 in human RD cells (Figure 6). We showed that upon infection, the virus uncoated within the first 3 h and started to synthesize the essential viral proteins for replication. From 3 to 6 h p.i., the virus rapidly replicated its genomic RNA and initiated viral package. The fast viral package displayed from 3 to 12 h p.i. and virion secretion from 6 h p.i. continued until death of the host cells. Host cells started to become unhealthy as early as 6 h p.i. but still supported viral replication, package and secretion until death. Thus, our study provides important information for further investigations into virus-host interactions and host pathogenesis.
Enterovirus 71 (EV71) infection causes hand-foot-and-mouth disease (HFMD) and neurological disease. Recently, EV71 infection has become a major health threat in China. However, the mechanisms of these diseases caused by EV71 infection are still largely unknown.
Studies on other human viruses such as human immunodeficiency virus, hepatitis C virus and hepatitis B virus have highlighted the importance of understanding viral kinetics. In this study, for the first time, the authors fully described the viral kinetics of EV71 in rhabdomyosarcoma (RD) cells and characterized the activities during each step of viral replication in detail.
Accurate information on viral kinetics will provide a valuable reference for investigating EV71-host interactions and the pathogenic mechanisms of diseases caused by EV71 infection.
EV71 is a small positive RNA virus. During viral replication, the complementary minus-strand RNA is synthesised first and serves as a template for the next round of translation and replication. Thus, the viral RNA levels in cells, viral particles and culture supernatants represent the relative levels of viral replication, package and secretion.
The authors provided detailed information on EV71 replication, package, secretion and viral protein expression in RD cells. The rapid increase of intracellular EV71 viral RNA and viral particles revealed that EV71 RNA was synthesized soon after infection and the viral particles could immediately package and released. These results will provide important information for further understanding the viral pathogenesis and EV71-host interactions.
Peer reviewer: Dr. Mohamed Hassan, Department of Tumour Therapy, University Hospital of Dusseldorf, 40225 Dusseldorf, Germany
S- Editor Lv S L- Editor Webster JR E- Editor Zhang DN
| 1. | Fowlkes AL, Honarmand S, Glaser C, Yagi S, Schnurr D, Oberste MS, Anderson L, Pallansch MA, Khetsuriani N. Enterovirus-associated encephalitis in the California encephalitis project, 1998-2005. J Infect Dis. 2008;198:1685-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Hamaguchi T, Fujisawa H, Sakai K, Okino S, Kurosaki N, Nishimura Y, Shimizu H, Yamada M. Acute encephalitis caused by intrafamilial transmission of enterovirus 71 in adult. Emerg Infect Dis. 2008;14:828-830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Ishimaru Y, Nakano S, Yamaoka K, Takami S. Outbreaks of hand, foot, and mouth disease by enterovirus 71. High incidence of complication disorders of central nervous system. Arch Dis Child. 1980;55:583-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 168] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | McMinn PC. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol Rev. 2002;26:91-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 585] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 5. | Huang SW, Hsu YW, Smith DJ, Kiang D, Tsai HP, Lin KH, Wang SM, Liu CC, Su IJ, Wang JR. Reemergence of enterovirus 71 in 2008 in taiwan: dynamics of genetic and antigenic evolution from 1998 to 2008. J Clin Microbiol. 2009;47:3653-3662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 167] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 6. | Yang F, Ren L, Xiong Z, Li J, Xiao Y, Zhao R, He Y, Bu G, Zhou S, Wang J. Enterovirus 71 outbreak in the People's Republic of China in 2008. J Clin Microbiol. 2009;47:2351-2352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 245] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 7. | AbuBakar S, Chee HY, Al-Kobaisi MF, Xiaoshan J, Chua KB, Lam SK. Identification of enterovirus 71 isolates from an outbreak of hand, foot and mouth disease (HFMD) with fatal cases of encephalomyelitis in Malaysia. Virus Res. 1999;61:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 165] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | McMinn P, Lindsay K, Perera D, Chan HM, Chan KP, Cardosa MJ. Phylogenetic analysis of enterovirus 71 strains isolated during linked epidemics in Malaysia, Singapore, and Western Australia. J Virol. 2001;75:7732-7738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 225] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 9. | Bible JM, Iturriza-Gomara M, Megson B, Brown D, Pantelidis P, Earl P, Bendig J, Tong CY. Molecular epidemiology of human enterovirus 71 in the United Kingdom from 1998 to 2006. J Clin Microbiol. 2008;46:3192-3200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Singh S, Chow VT, Phoon MC, Chan KP, Poh CL. Direct detection of enterovirus 71 (EV71) in clinical specimens from a hand, foot, and mouth disease outbreak in Singapore by reverse transcription-PCR with universal enterovirus and EV71-specific primers. J Clin Microbiol. 2002;40:2823-2827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Muir P, Kämmerer U, Korn K, Mulders MN, Pöyry T, Weissbrich B, Kandolf R, Cleator GM, van Loon AM. Molecular typing of enteroviruses: current status and future requirements. The European Union Concerted Action on Virus Meningitis and Encephalitis. Clin Microbiol Rev. 1998;11:202-227. [PubMed] |
| 12. | Yamayoshi S, Yamashita Y, Li J, Hanagata N, Minowa T, Takemura T, Koike S. Scavenger receptor B2 is a cellular receptor for enterovirus 71. Nat Med. 2009;15:798-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 423] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 13. | Zoll J, Heus HA, van Kuppeveld FJ, Melchers WJ. The structure-function relationship of the enterovirus 3'-UTR. Virus Res. 2009;139:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Herrmann E, Lee JH, Marinos G, Modi M, Zeuzem S. Effect of ribavirin on hepatitis C viral kinetics in patients treated with pegylated interferon. Hepatology. 2003;37:1351-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 192] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 15. | Layden-Almer JE, Ribeiro RM, Wiley T, Perelson AS, Layden TJ. Viral dynamics and response differences in HCV-infected African American and white patients treated with IFN and ribavirin. Hepatology. 2003;37:1343-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 138] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Zeuzem S, Herrmann E, Lee JH, Fricke J, Neumann AU, Modi M, Colucci G, Roth WK. Viral kinetics in patients with chronic hepatitis C treated with standard or peginterferon alpha2a. Gastroenterology. 2001;120:1438-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 199] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Belsham GJ, Normann P. Dynamics of picornavirus RNA replication within infected cells. J Gen Virol. 2008;89:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Egger D, Bienz K. Intracellular location and translocation of silent and active poliovirus replication complexes. J Gen Virol. 2005;86:707-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Cohen BJ, Audet S, Andrews N, Beeler J. Plaque reduction neutralization test for measles antibodies: Description of a standardised laboratory method for use in immunogenicity studies of aerosol vaccination. Vaccine. 2007;26:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 155] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 20. | Yi L, He Y, Chen Y, Kung HF, He ML. Potent inhibition of human enterovirus 71 replication by type I interferon subtypes. Antivir Ther. 2011;16:51-58. [PubMed] |
| 21. | Leong WF, Chow VT. Transcriptomic and proteomic analyses of rhabdomyosarcoma cells reveal differential cellular gene expression in response to enterovirus 71 infection. Cell Microbiol. 2006;8:565-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Shih SR, Stollar V, Lin JY, Chang SC, Chen GW, Li ML. Identification of genes involved in the host response to enterovirus 71 infection. J Neurovirol. 2004;10:293-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Kok TW, Pryor T, Payne L. Comparison of rhabdomyosarcoma, buffalo green monkey kidney epithelial, A549 (human lung epithelial) cells and human embryonic lung fibroblasts for isolation of enteroviruses from clinical samples. J Clin Virol. 1998;11:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | She RC, Crist G, Billetdeaux E, Langer J, Petti CA. Comparison of multiple shell vial cell lines for isolation of enteroviruses: a national perspective. J Clin Virol. 2006;37:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Johansson ES, Xing L, Cheng RH, Shafren DR. Enhanced cellular receptor usage by a bioselected variant of coxsackievirus a21. J Virol. 2004;78:12603-12612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Pérez-Ruiz M, Navarro-Marí JM, Palacios del Valle E, Rosa-Fraile M. Human rhabdomyosarcoma cells for rapid detection of enteroviruses by shell-vial assay. J Med Microbiol. 2003;52:789-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Pliaka V, Dedepsidis E, Kyriakopoulou Z, Papadi G, Tsakogiannis D, Pratti A, Levidiotou-Stefanou S, Markoulatos P. Growth kinetic analysis of bi-recombinant poliovirus vaccine strains. Virus Genes. 2010;40:200-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Chen LC, Shyu HW, Chen SH, Lei HY, Yu CK, Yeh TM. Enterovirus 71 infection induces Fas ligand expression and apoptosis of Jurkat cells. J Med Virol. 2006;78:780-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Liang CC, Sun MJ, Lei HY, Chen SH, Yu CK, Liu CC, Wang JR, Yeh TM. Human endothelial cell activation and apoptosis induced by enterovirus 71 infection. J Med Virol. 2004;74:597-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Chang SC, Lin JY, Lo LY, Li ML, Shih SR. Diverse apoptotic pathways in enterovirus 71-infected cells. J Neurovirol. 2004;10:338-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |