Published online Jul 21, 2011. doi: 10.3748/wjg.v17.i27.3184
Revised: March 1, 2011
Accepted: March 8, 2011
Published online: July 21, 2011
The risk of developing neoplasia leading to colorectal cancer is significantly increased in ulcerative colitis (UC) and most likely in Crohn’s disease. Several endoscopic surveillance strategies have been implemented to identify these lesions. The main issue is that colitis-associated neoplasms often occurs in flat mucosa, often being detected on taking random biopsies rather than by identification of these lesions via endoscopic imaging. The standard diagnostic procedure in long lasting UC is to take four biopsies every 10 cm. Image enhancement methods, such as chromoendoscopy and virtual histology using endomicroscopy, have greatly improved neoplasia detection rates and may contribute to reduced random biopsies by taking targeted “smart” biopsies. Chromoendoscopy may effectively be performed by experienced endoscopists for routine screening of UC patients. By contrast, endomicroscopy is often only available in selected specialized endoscopic centers. Importantly, advanced endoscopic imaging has the potential to increase the detection rate of neoplasia whereas the interplay between endoscopic experience and interpretation of histological biopsy evaluation allows the physician to make a proper diagnosis and to find the appropriate therapeutic approach. Colitis-associated intraepithelial neoplasms may occur in flat mucosa of endoscopically normal appearance or may arise as dysplasia-associated lesion or mass (DALM), which may be indistinguishable from sporadic adenomas in healthy or non-colitis mucosa [adenoma-like mass (ALM)]. The aim of this review was to summarize endoscopic and histological characteristics of DALM and ALM in the context of therapeutic procedures.
- Citation: Neumann H, Vieth M, Langner C, Neurath MF, Mudter J. Cancer risk in IBD: How to diagnose and how to manage DALM and ALM. World J Gastroenterol 2011; 17(27): 3184-3191
- URL: https://www.wjgnet.com/1007-9327/full/v17/i27/3184.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i27.3184
Inflammatory bowel disease (IBD) encompasses two major forms of chronic intestinal disorders, Crohn’s disease and ulcerative colitis (UC). Evidence suggests that IBD results from an inappropriate inflammatory response to intestinal microbes in a genetically susceptible host environment[1]. Increased production of proinflammatory cytokines and increased resistance to apoptosis finally lead to an uncontrolled chronic activation of the mucosal immune system[2]. Previously, it was shown that proinflammatory cytokines, such as Interleukin-6 produced by lamina propria mononuclear cells, directly contribute to tumor growth and to cancer development in UC[3,4]. Although the association between IBD and colorectal cancer (CRC) is well-established, there are still concerns regarding adequate diagnosis and treatment of early pre-neoplastic and neoplastic lesions.
The overall prevalence of CRC in UC patients was recently analyzed in a large meta-analysis and estimated to be 3.7%[5]. In this context, the duration and anatomical extent are well-established risk factors for cancer development. Patients with a course of disease longer than 10 years and with pancolitis are at the highest risk. Additionally, patients with left-sided UC or those with more proximal disease are considered to be at high risk for cancer development[6]. Therefore, surveillance colonoscopy is considered to be the gold standard in diagnosing intraepithelial neoplasia (formerly termed dysplasia) and cancer in IBD patients. Nevertheless, to date, no randomized controlled studies have shown a reduced risk of CRC development by surveillance colonoscopy in IBD patients.
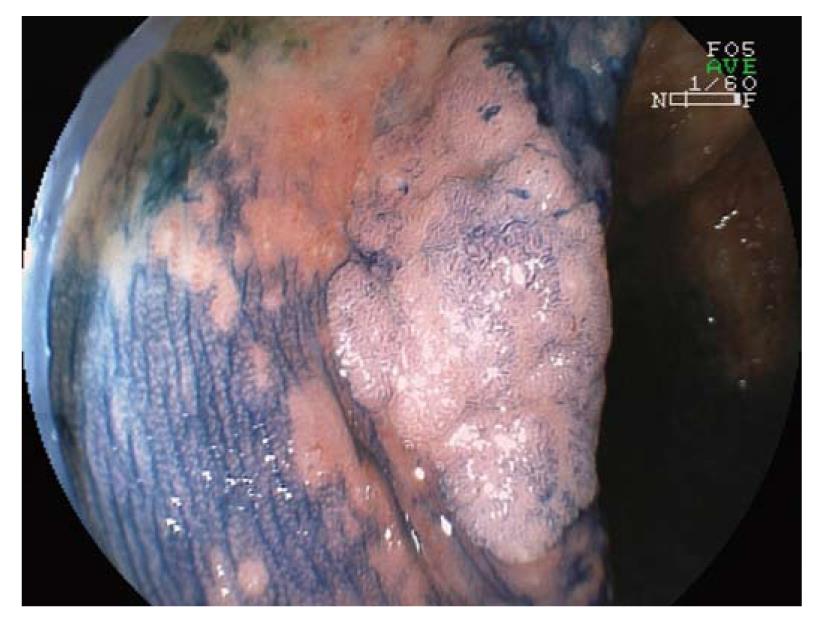
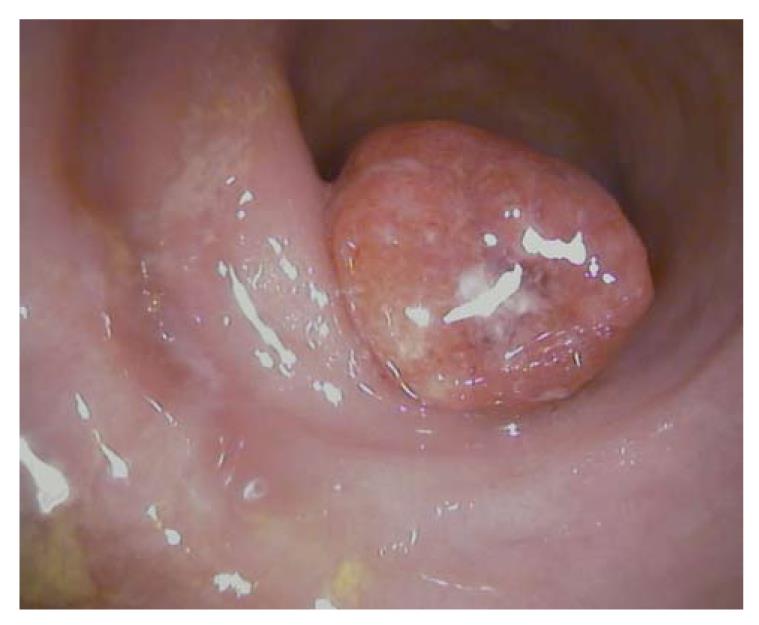
The main aim of surveillance programs in IBD is to detect early dysplastic alterations. Dysplastic alterations of the intestinal mucosa in IBD patients may occur in flat or raised mucosal lesions, and are differentiated by the terms dysplasia-associated lesion or mass (DALM, Figure 1) and adenoma-like mass (ALM, Figure 2)[6-8].
The differential diagnosis between colitis associated intraepithelial neoplasms and sporadic adenoma in patients with UC may be difficult, especially if only biopsy specimens are obtained[9,10]. Even carcinomas are difficult to diagnose because of their commonly well-differentiated morphology, and accurate diagnosis may only be possible in a resection specimen showing unequivocal invasion into the submucosal layer. Thus, in approximately 40% of cases with high-grade intraepithelial neoplasia on biopsy, diagnosis of the corresponding resection specimen shows invasive adenocarcinoma[11-13]. In low-grade intraepithelial neoplasia, the prevalence of carcinoma in the resection specimen has historically been believed to be up to 19%[14]. More recent data, however, indicate a significantly lower prevalence, indicating that a diagnosis of low-grade intraepithelial neoplasia does not justify prophylactic colectomy and local endoscopic treatment should not be ruled out[15].
Often the macroscopic endoscopic appearance of a lesion does not render the crucial clue to differential diagnosis, because colitis associated neoplasms may show subtle macroscopic features mimicking a broad range of lesions, ranging from inflammatory appearance to suspicion of carcinoma. Data from the literature indicate that in 50%-80% of cases with colitis-associated neoplasms, the lesions are not visible upon endoscopy[11].
Adenomas are mainly sharply delineated, with or without a stalk, and often show a smooth surface with Kudo surface pit pattern IIIS (small roundish or tubular pits), IIIL (large roundish or tubular pits), or IV (branch-like or gyrus-like pits)[16,17]. The gross appearance of colitis-associated neoplasms varies from case to case. These lesions can be endoscopically invisible or may be encountered as irregularly delineated, plaque-like, or irregularly elevated, lesions or verrucous structures. Some cases even show a combination of different types (Figures 1 and 2).
Previously, it was reported that to exclude dysplasia in colonic mucosal biopsies, at least 56 or 33 non-targeted jumbo-forceps biopsies have to be taken (95% and 90% confidence, respectively)[6]. In 2005, an international consensus conference agreed that a minimum of 32 biopsies should be performed at each surveillance colonoscopy by obtaining four-quadrant biopsies every 10 cm. In addition, separate jars should be used for each quartet and suspicious lesions[18].
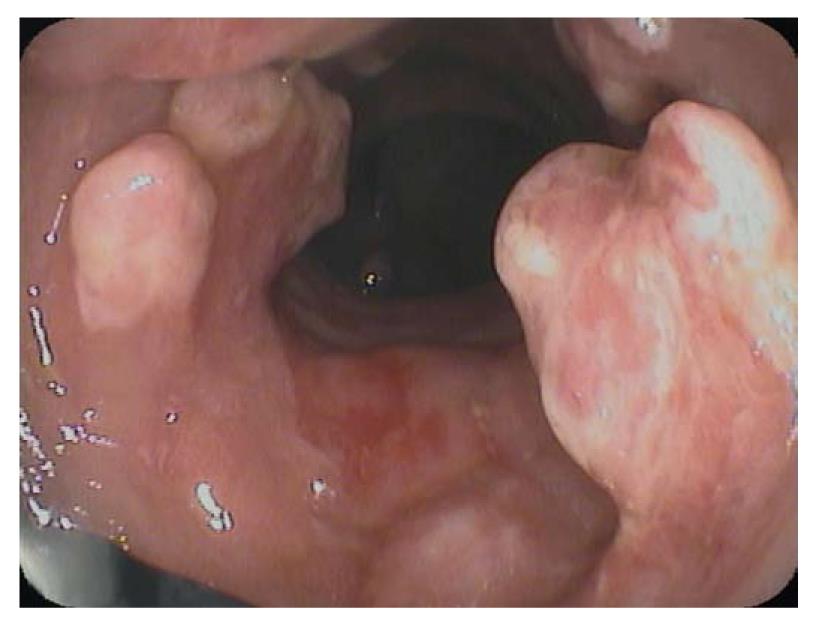
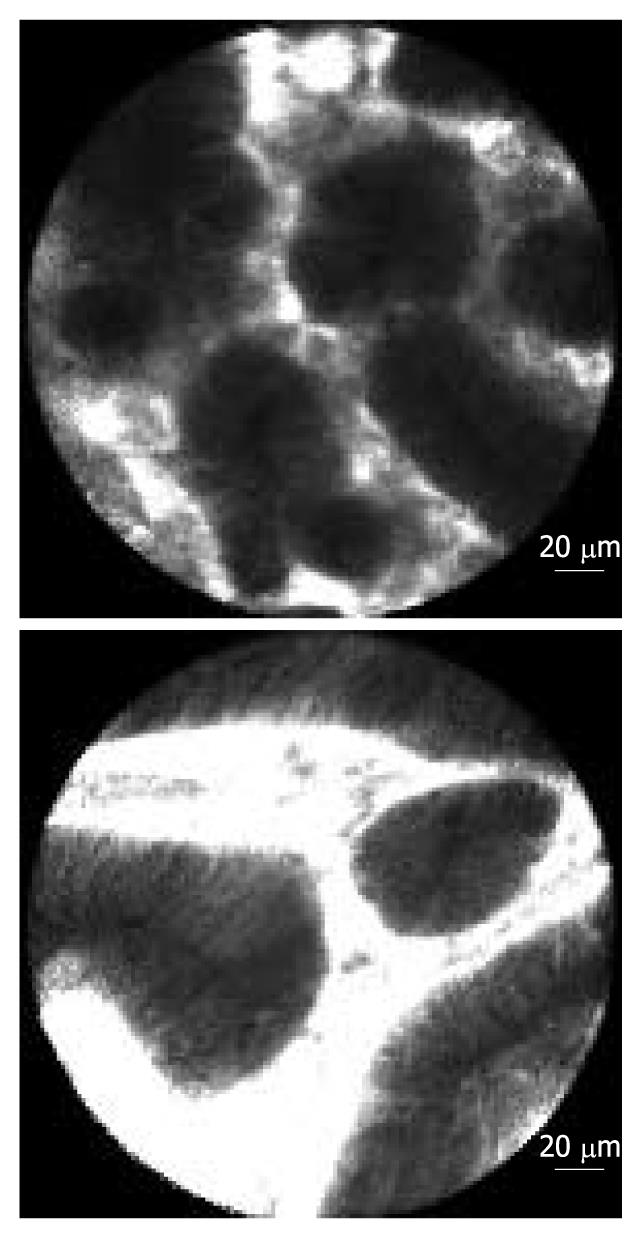
To avoid sampling error in IBD-cancer surveillance, new endoscopic imaging techniques were introduced, including chromoendoscopy, magnification endoscopy, and confocal laser endomicroscopy (Table 1, Figures 3, 4, 5, 6)[19].
| Author and reference | Study design | Patients | Technique | Results |
| Kiesslich et al[20] | Prospective | 165 | Chromoendoscopy (methylene blue) | Chromoendoscopy detects significantly more IEN compared to conventional colonoscopy |
| Rutter et al[21] | Prospective | 100 | Chromoendoscopy (indigo carmine) | Targeted mucosal biopsies detect significantly more IEN than non-targeted biopsies |
| van den Broek et al[24] | Prospective | 50 | AFI + NBI + WLE | AFI improves detection of colitis-associated neoplasia. |
| Hurlstone et al[25] | Prospective | 162 | MCE (indigo carmine) | MCE significantly increases diagnostic yield of IEN and number of flat lesions with IEN |
| Hurlstone et al[26] | Prospective | 350 | MCE (indigo carmine) | MCE significantly increases diagnostic yield of IEN and number of flat lesions with IEN |
| Kiesslich et al[29] | Prospective | 153 | Chromoendoscopy (methylene blue) + endomicroscopy | Combination of both techniques detects 4.75-fold more neoplasms and requires 50% less biopsy specimen |
| Hurlstone et al[30] | Prospective | 36 | Endomicroscopy | Endomicroscopy can differentiate ALM and DALM in vivo with high accuracy (97%) |
In 2003, Kiesslich and colleagues conducted the first study of chromoendoscopy in IBD patients[20]. One hundred and sixty-five patients with long standing UC were randomized in a 1:1 ratio to undergo conventional colonoscopy or colonoscopy with chromoendoscopy using 0.1% methylene blue. In the chromoendoscopy group, significantly more intraepithelial neoplasms were detected compared to the conventional colonoscopy group (32 vs 10, P = 0.003). Another “back-to-back” colonoscopy study included 100 patients with longstanding UC[21]. Patients received both random and directed biopsies, followed by spraying of the entire mucosa with 0.1% indigo carmine, and subsequent biopsy of any additional visible abnormality. There was a strong trend towards statistically increased dysplasia detection following dye spraying (7/100 patients vs 2/100 patients, P = 0.06). The targeted biopsy protocol detected dysplasia in significantly more patients than the non-targeted protocol (7/100 patients vs 0/100 patients, P = 0.02). Additionally, the targeted biopsy protocol with pancolonic chromoendoscopy required fewer biopsies than taking multiple non-targeted biopsies (157 biopsies vs 2904 biopsies).
However, dye-based chromoendoscopy has some potential limitations, as it harbors additional costs for the equipment needed for dye spraying and is a time consuming procedure. Additionally, the dye often does not coat the entire surface and it does not allow for a detailed analysis of subepithelial capillary network, which is an important feature in the early diagnosis of gastrointestinal neoplasia (Figure 4).
Therefore, dye-less chromoendoscopy (also called virtual chromoendoscopy) has been developed, including narrow band imaging (NBI; Olympus, Tokyo, Japan), Fujinon intelligent color enhancement (FICE; Fujinon, Tokyo, Japan), and i-Scan (Pentax, Tokyo, Japan). NBI is based on optical filters within the light source of the endoscope, which narrow the bandwidth of spectral transmittance such that the blood vessels are enhanced and are thus seen more easily. FICE and i-Scan use an endoscopic image from the video processor and reconstruct virtual images in real time, resulting in an improved contrast of the capillary patterns and enhancement of the mucosal surface[19,22].
The first pilot study on NBI in patients with UC included 46 patients. Suspicious lesions were observed by magnifying NBI-colonoscopy and were subsequently classified based on their surface appearance into honeycomb-like, villous, or tortuous patterns. The tortuous pattern determined by NBI-colonoscopy may be a clue for the identification of dysplasia during surveillance for UC[23].
One recently published study assessed the value of endoscopic tri-modal imaging, incorporating white light endoscopy (WLE), autofluorescence imaging (AFI), and NBI for the detection and classification of neoplasia in 50 patients with longstanding UC[24]. Neoplasia miss-rates for AFI and WLE were 0% and 50%, respectively (P = 0.036). The Kudo classification by NBI had a sensitivity and specificity of 75% and 81%, respectively. Thus, in this study, AFI improved the detection of neoplasia in patients with UC and decreased the yield of random biopsies, while pit pattern analysis by NBI had a moderate accuracy for the prediction of histology.
While both vital and virtual chromoendoscopy offer the identification of subtle lesions and their borderlines, subsequent magnification endoscopy has the capability to enable detailed surface analysis and pit pattern classification of those lesions.
Indeed, Hurlstone and colleagues prospectively analyzed 162 patients with longstanding UC using magnifying chromoendoscopy with 0.5% indigo carmine[25]. Selective chromoendoscopy was used, following detection of subtle mucosal changes. Magnification chromoendoscopy with targeted biopsies significantly increased diagnostic yield for intraepithelial neoplasia (42 lesions vs 11 lesions, P < 0.001) and the number of flat lesions with intraepithelial neoplasia as compared to conventional colonoscopy (31 lesions vs 6 lesions, P < 0.001). The overall sensitivity and specificity of magnification chromoendoscopy in predicting neoplasia was 97% and 93%, respectively. These data were also confirmed in a larger cohort of patients[26]. A total of 350 patients with long-standing UC underwent surveillance colonoscopy using high-magnification chromoendoscopy. Quadrantic biopsies at 10-cm intervals were taken on extubation, in addition to targeted biopsies of abnormal mucosal areas, and data were compared to 350 disease duration- and disease extent-matched control patients who had undergone conventional colonoscopic surveillance. Magnification chromoendoscopy detected significantly more intraepithelial neoplastic lesions compared with controls (69 lesions vs 24 lesions, P < 0.0001). In addition, chromoendoscopy detected more flat lesions with intraepithelial neoplasia compared with controls (53 lesions vs 14 lesions, P < 0.001).
Taken together, this study indicates that magnification chromoendoscopy has the capability to predict neoplastic and non-neoplastic mucosal changes with a high overall accuracy.
Although high-magnification endoscopy enables detailed surface analysis and pit pattern classification, it cannot visualize cellular and subcellular details. Certainly, diagnosis of intraepithelial neoplasia is mostly based on alterations of cellular and subcellular structures. Additionally, magnifying chromoendoscopy cannot differentiate between colitis-associated intraepithelial neoplasms and adenoma, because the staining pattern of both entities is similar[27].
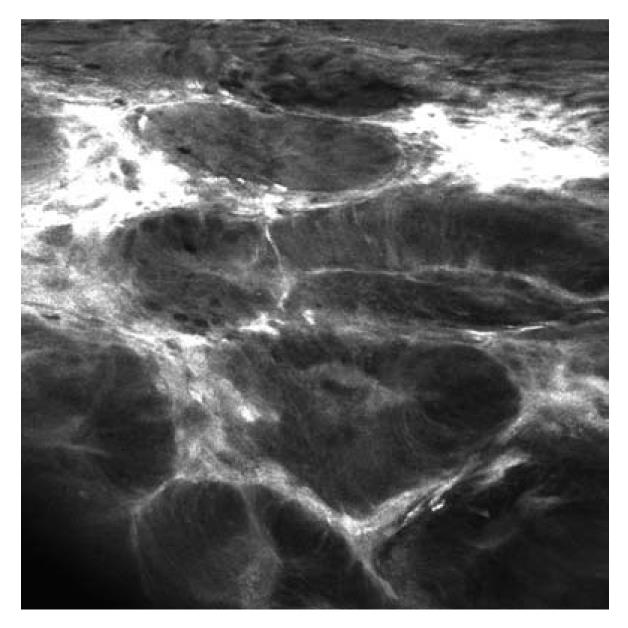
Recently, confocal laser endomicroscopy was introduced, allowing real-time in vivo imaging of the gastrointestinal mucosa at 1000-fold magnification, thereby providing an optical biopsy (Figures 5 and 6)[28].
One randomized controlled trial assessed the value of combined chromoendoscopy (0.1% methylene blue) and endomicroscopy for the in vivo diagnosis of intraepithelial neoplasia in patients with UC[29]. One hundred and fifty-three patients with long-term UC in clinical remission were randomized in a 1:1 ratio to undergo conventional colonoscopy or chromoendoscopy with endomicroscopy. In the combined group, 4.75-fold more neoplasms were detected compared to conventional colonoscopy (P = 0.005) and 50% less biopsy specimens were required (P = 0.008). Moreover, if only circumscribed lesions would have been biopsied, the total number of biopsy specimens could have been reduced by more than 90%. Overall, endomicroscopy predicted the presence of neoplastic changes with high sensitivity, specificity, and accuracy (94.7%, 98.3%, and 97.8%, respectively).
Recently, a study by Hurlstone and colleagues evaluated the clinical applicability and predictive power of endomicroscopy for the in vivo differentiation of DALM and ALM. Thirty-six patients with 36 lesions were prospectively included. The accuracy of endomicroscopy was 97%, and there was an excellent agreement between endomicroscopy and histopathological diagnosis (κ = 0.91)[30].
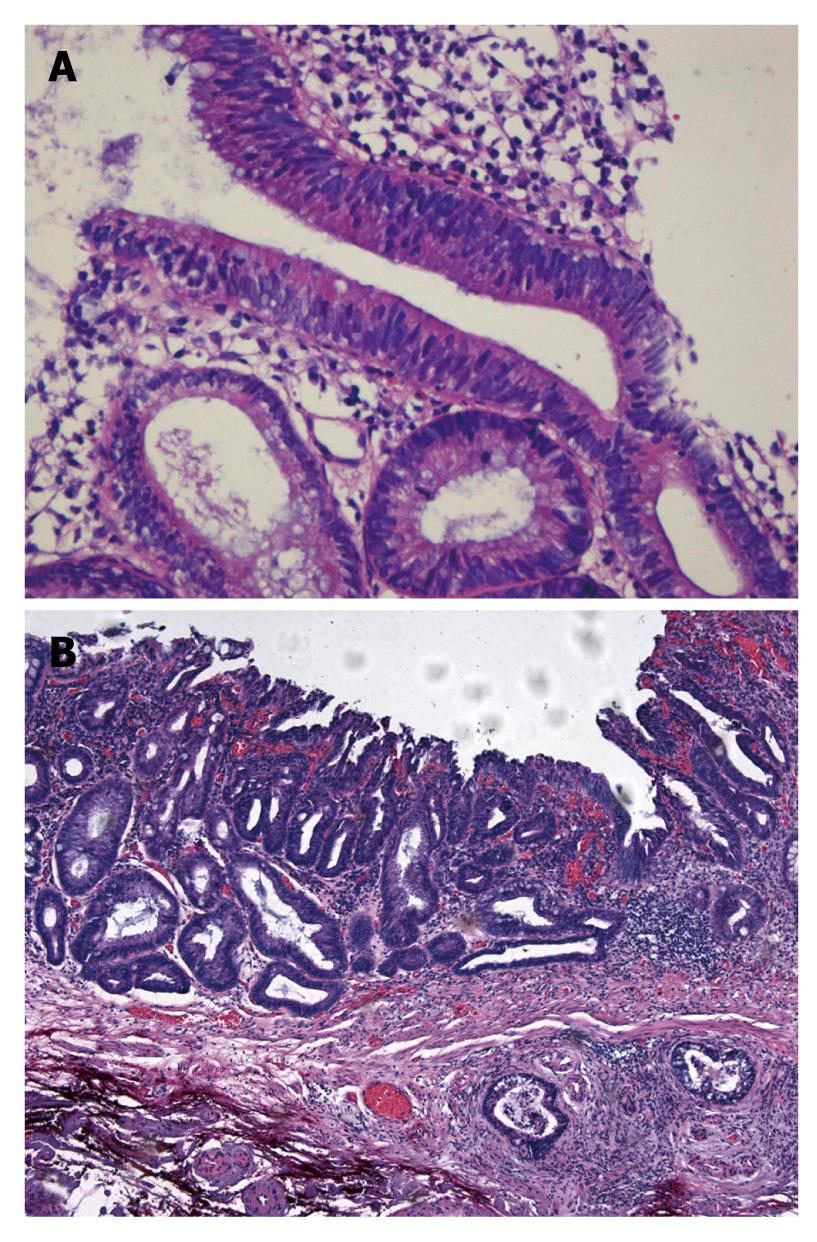
The histopathological differential diagnosis between colitis-associated neoplasms and sporadic adenoma is difficult (Figure 7)[31]. Both entities are defined as unequivocal intraepithelial neoplasms that can show either low-grade or high-grade intraepithelial neoplasia. The lesions are mainly differentiated by combined analysis of histological growth pattern and gross appearance[32]. Clinically, accurate pathological diagnosis is very important with respect to different therapeutic consequences: endoscopic polypectomy vs potential surgical proctocolectomy.
Clinically-based criteria for the differentiation of patients with sporadic adenoma from those with colitis-associated neoplasia have been known from the early 1990s. Thus, patients with adenomas differ from those with colitis-associated neoplasms with respect to age, onset age of colitis, number of lesions and extent of colitis[33].
In sporadic adenomas, the glands are round or oval shaped with regular structure, equal configuration, and similar diameter. Furthermore, sporadic adenomas show a “top-down” morphology, whereas colitis associated neoplasms show a “down-top” morphology, with glands that are irregular in size, shape, configuration, and diameter.
The proliferation zone in sporadic adenomas starts from the apical/luminal part of the mucosa (“top-down”). In contrast, the proliferation zone of colitis-associated neoplasms starts from the base of the mucosa (“down-top”); the neoplastic epithelium ultimately covers the whole length of the crypts, finally reaching the mucosal surface. Horizontal tubular proliferations above the muscularis mucosae are typically found in colitis-associated neoplasms.
In adenomas, mucin vacuoles are evenly distributed within the neoplastic epithelium and are mostly found in the apical part of the cytoplasm. Colitis-associated neoplasms show irregularly distributed mucin vacuoles. Often so-called “dystrophic” goblet cells are found, which are characterized by mucin vacuoles located in the basal parts of the cytoplasm, i.e. between nucleus and basal membrane.
Sporadic adenomas show palisading of elongated hyperchromatic nuclei, which are regular in shape and diameter. In colitis-associated neoplasms, nuclei are oval to round, less densely packed, and vary in diameter and configuration. These nuclei are often more irregularly arranged within the glands compared with sporadic adenomas, and may show greater variation in chromatin content.
In between the glands of a sporadic adenoma, the stromal tissue is loosely and evenly arranged, whereas in colitis-associated intraepithelial neoplasms, the stromal tissue is often irregularly packed between the glands, with bands of connective tissue largely varying in shape and thickness. Depending on the activity of the underlying disease, mixed inflammatory infiltrates, with cryptitis and crypt abscess formation, may be observed.
Sporadic adenomas commonly show sharp and abrupt delineation between neoplastic glands and adjacent non-neoplastic epithelium. In colitis-associated neoplasms, this delineation is more irregular and less well defined.
The question of whether a lesion is a sporadic adenoma or a colitis-associated neoplasm depends on all of the above named criteria. Making a definite diagnosis based upon a single criterion is largely unreliable in this respect. In addition, other factors should be taken into account, such as the endoscopic appearance of a particular lesion, the age of the patient, duration and extent of the underlying disease, and the localization within or above the segment of colon affected by colitis. The use of immunohistochemistry is not very helpful in differentiating the two entities and consequently cannot be recommended[31].
This tool may help to differentiate sporadic adenomas from colitis-associated neoplasms in selected cases. A small study assessing 19 patients showed that all sporadic adenomas evaluated were euploid, in contrast to only four out of 19 colitis-associated neoplasms. Fourteen of the remaining 15 lesions were unequivocally aneuploid[34].
While APC-mutations (adenomatous polyposis coli protein) and shift from cytoplasmic to nuclear β-catenin expression are common findings in sporadic adenomas, they are infrequent in colitis-associated neoplasms[35-37]. In contrast, p53 mutations are less common in sporadic adenomas compared with colitis-associated neoplasms (4% vs 40%), where they have been identified as the most common initiating mutation within micro-dissected individual neoplastic crypts[31,38]. Similarly, abnormalities of the p16 gene locus are far more common in colitis-associated neoplasms compared with sporadic adenomas[39]. Cyclin-D1 upregulation and p21 (WAF/CIP1) downregulation occur early in colitis-associated carcinogenesis. However, cyclin-D1 upregulation in colitis-associated cancers is less common than in sporadic colon cancers[40]. Finally, LOH analyses (loss of heterozygosity) have shown more frequent deletions on chromosome 3p within the von-Hippel-Lindau gene locus in colitis-associated neoplasms compared with sporadic adenomas in colitis patients[41].
These molecular features may only give a first insight into ongoing research work. However, nuclear β catenin expression appears to be a promising marker, especially if used in combination with ki67 immunohistochemistry, which may facilitate detection of “down-top” or “top-down” morphology as mentioned above[42]. Of note, however, no molecular marker, including those mentioned above, has yet entered daily practice to aid the differential diagnosis between sporadic adenomas and colitis-associated intraepithelial neoplasms. The skills of an experienced histopathologist remain the golden standard. In critical cases, obtaining a second opinion may be helpful to ensure the diagnosis and subsequent therapeutic approaches.
Macroscopically flat or raised lesions without proper delineation to the surrounding mucosa that occur in long standing IBD are mostly diagnosed as DALM and harbor a high risk of progression to CRC (Figure 1). Furthermore, the occurrence of DALM is frequently associated with synchronic or metachronic neoplasia. Therefore, patients with DALM are recommended to undergo prophylactic proctocolectomy with ileoanal pouch. In contrast, the term ALM describes sporadic adenomas that are similar to those observed in non-IBD patients and which are treated by standard polypectomy (Figure 2).
From the clinical perspective, the endoscopic resectability of a lesion is more important than whether it is thought to be a sporadic adenoma (ALM) or a DALM. One essential point is that the lesion, whether DALM or ALM, should be removed in its entirety.
Early data support the hypothesis that ALMs can be successfully removed using standard polypectomy techniques, with little risk of subsequent malignancy on follow-up[43-45].
In a retrospective study, including 525 patients with UC, a total of 110 neoplastic areas were detected in 56 patients. Eighty-five (77.3%) of the lesions were macroscopically visible at colonoscopy. Fifty patients (89.3%) had macroscopically detectable neoplasia, and six (10.7%) had macroscopically invisible lesions. Importantly, the frequency of cancer in patients who had endoscopic resection of neoplasia did not differ from that of the surveillance population as a whole, irrespective of whether the lesion was thought to be an adenoma or a DALM. Conversely, a high proportion of unresectable lesions harbored cancer[46].
Additionally, in a large retrospective study Vieth and coworkers showed that endoscopic resection of ALMs represents an adequate treatment for those lesions[47]. Furthermore, it was shown that an adenoma based on biopsy material from a patient with UC must be subjected to endoscopic resection, both to confirm the biopsy-based adenoma diagnosis and to exclude colitis-associated intraepithelial neoplasms. In this large retrospective cohort, 2.3% of patients developed a colitis-associated carcinoma (follow up 6 years). Importantly, these carcinomas were located in a segment of the colon other than that bearing the primarily endoscopically resected adenoma.
Considerable progress has been made recently in both the diagnosis and treatment of adenomatous and non-adenomatous lesions in UC. New and emerging endoscopic imaging techniques, like chromoendoscopy, magnification endoscopy, and confocal laser endomicroscopy, offer the potential for real time in vivo diagnosis of surface, vascular, and cellular patterns, and to perform tactical and targeted biopsies thereby increasing the diagnostic yield for intraepithelial neoplasia.
The following seven basic rules for the detection of neoplasia should be taken into account and applied in accordance with international guidelines, respectively. (1) Experienced gastroenterologist; (2) Endoscopic and bioptic control in remission phase; (3) Examination outside routine schedule without time limitation; (4) Ileocolonoscopy with special focus on the detection of DALMs and step (quadrant) biopsies from the rectum to the cecum in 10 cm intervals (sigmoid and rectum: quadrant biopsies at 5 cm intervals); (5) ALMs with low-grade intraepithelial neoplasia and clear cut margins can be resected endoscopically; (6) Experienced histopathologist who has all clinical and endoscopy data readily available; and (7) Second opinion recommended in cases of histological diagnosis of neoplasia.
In any event, patient follow-up will disclose whether the histopathological diagnosis of sporadic adenoma or colitis-associated neoplasms was justified. Nevertheless, the biopsy-based diagnosis of adenoma is uncertain. Therefore, endoscopic resection of suspicious lesions should be preferred. Several studies are available indicating that polypectomy of sporadic adenomas is an adequate and curative treatment. Of note, all patients with UC should undergo regular surveillance colonoscopies 8 years after the first appearance of symptoms (in the case of pancolitis); repeat colonoscopy with random biopsies in 10 cm intervals should be recommended every 1 to 2 years. Alternatively, chromoendoscopy with targeted biopsies may be applied in specialized centers. Patients that have been diagnosed with left-sided colitis should undergo colonoscopy in year eight after the first appearance of symptoms. If the colitis is still restricted to the distal colon (left-sided) surveillance may start at year 15. If colitis has progressed to pancolitis surveillance colonoscopies should be performed every 1 to 2 years. In general, if intraepithelial neoplasia is present in random biopsy specimens, colectomy should be recommended. If an adenomatous lesion with intraepithelial neoplasia was resected endoscopically control colonoscopy should be performed within 6 mo.
Peer reviewer: Wojciech Blonski, MD, PhD, University of Pennsylvania, GI Research-Ground Centrex, 3400 Spruce St, Philadelphia, PA 19104, United States
S- Editor Sun H L- Editor Stewart GJ E- Editor Zheng XM
| 1. | Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066-2078. |
| 2. | Mudter J, Neurath MF. Apoptosis of T cells and the control of inflammatory bowel disease: therapeutic implications. Gut. 2007;56:293-303. |
| 3. | Atreya R, Neurath MF. Signaling molecules: the pathogenic role of the IL-6/STAT-3 trans signaling pathway in intestinal inflammation and in colonic cancer. Curr Drug Targets. 2008;9:369-374. |
| 4. | Mudter J, Neurath MF. Il-6 signaling in inflammatory bowel disease: pathophysiological role and clinical relevance. Inflamm Bowel Dis. 2007;13:1016-1023. |
| 5. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. |
| 6. | Ullman T, Odze R, Farraye FA. Diagnosis and management of dysplasia in patients with ulcerative colitis and Crohn’s disease of the colon. Inflamm Bowel Dis. 2009;15:630-638. |
| 7. | Loddenkemper C. Diagnostic standards in the pathology of inflammatory bowel disease. Dig Dis. 2009;27:576-583. |
| 8. | Odze RD. Adenomas and adenoma-like DALMs in chronic ulcerative colitis: a clinical, pathological, and molecular review. Am J Gastroenterol. 1999;94:1746-1750. |
| 9. | Nagasako K, Iizuka B, Ishii F, Miyazaki J, Fujimori T. Colonoscopic diagnosis of dysplasia and early cancer in longstanding colitis. J Gastroenterol. 1995;30 Suppl 8:36-39. |
| 10. | Riddell RH, Goldman H, Ransohoff DF, Appelman HD, Fenoglio CM, Haggitt RC, Ahren C, Correa P, Hamilton SR, Morson BC. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983;14:931-968. |
| 11. | Bernstein CN, Shanahan F, Weinstein WM. Are we telling patients the truth about surveillance colonoscopy in ulcerative colitis? Lancet. 1994;343:71-74. |
| 12. | Connell WR, Lennard-Jones JE, Williams CB, Talbot IC, Price AB, Wilkinson KH. Factors affecting the outcome of endoscopic surveillance for cancer in ulcerative colitis. Gastroenterology. 1994;107:934-944. |
| 13. | Nugent FW, Haggitt RC, Gilpin PA. Cancer surveillance in ulcerative colitis. Gastroenterology. 1991;100:1241-1248. |
| 14. | Desaint B, Legendre C, Florent C. Dysplasia and cancer in ulcerative colitis. Hepatogastroenterology. 1989;36:219-226. |
| 15. | Lim CH, Dixon MF, Vail A, Forman D, Lynch DA, Axon AT. Ten year follow up of ulcerative colitis patients with and without low grade dysplasia. Gut. 2003;52:1127-1132. |
| 16. | Kudo S, Lambert R, Allen JI, Fujii H, Fujii T, Kashida H, Matsuda T, Mori M, Saito H, Shimoda T. Nonpolypoid neoplastic lesions of the colorectal mucosa. Gastrointest Endosc. 2008;68:S3-S47. |
| 17. | Lambert R, Kudo SE, Vieth M, Allen JI, Fujii H, Fujii T, Kashida H, Matsuda T, Mori M, Saito H. Pragmatic classification of superficial neoplastic colorectal lesions. Gastrointest Endosc. 2009;70:1182-1199. |
| 18. | Itzkowitz SH, Present DH. Consensus conference: Colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:314-321. |
| 19. | Neumann H, Neurath MF, Mudter J. New endoscopic approaches in IBD. World J Gastroenterol. 2011;17:63-68. |
| 20. | Kiesslich R, Fritsch J, Holtmann M, Koehler HH, Stolte M, Kanzler S, Nafe B, Jung M, Galle PR, Neurath MF. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology. 2003;124:880-888. |
| 21. | Rutter MD, Saunders BP, Schofield G, Forbes A, Price AB, Talbot IC. Pancolonic indigo carmine dye spraying for the detection of dysplasia in ulcerative colitis. Gut. 2004;53:256-260. |
| 22. | Neumann H, Fry LC, Bellutti M, Malfertheiner P, Mönkemüller K. Double-balloon enteroscopy-assisted virtual chromoendoscopy for small-bowel disorders: a case series. Endoscopy. 2009;41:468-471. |
| 23. | Matsumoto T, Kudo T, Jo Y, Esaki M, Yao T, Iida M. Magnifying colonoscopy with narrow band imaging system for the diagnosis of dysplasia in ulcerative colitis: a pilot study. Gastrointest Endosc. 2007;66:957-965. |
| 24. | van den Broek FJ, Fockens P, van Eeden S, Reitsma JB, Hardwick JC, Stokkers PC, Dekker E. Endoscopic tri-modal imaging for surveillance in ulcerative colitis: randomised comparison of high-resolution endoscopy and autofluorescence imaging for neoplasia detection; and evaluation of narrow-band imaging for classification of lesions. Gut. 2008;57:1083-1089. |
| 25. | Hurlstone DP, McAlindon ME, Sanders DS, Koegh R, Lobo AJ, Cross SS. Further validation of high-magnification chromoscopic-colonoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology. 2004;126:376-378. |
| 26. | Hurlstone DP, Sanders DS, Lobo AJ, McAlindon ME, Cross SS. Indigo carmine-assisted high-magnification chromoscopic colonoscopy for the detection and characterisation of intraepithelial neoplasia in ulcerative colitis: a prospective evaluation. Endoscopy. 2005;37:1186-1192. |
| 27. | Kiesslich R, Neurath MF. Surveillance colonoscopy in ulcerative colitis: magnifying chromoendoscopy in the spotlight. Gut. 2004;53:165-167. |
| 28. | Neumann H, Kiesslich R, Wallace MB, Neurath MF. Confocal laser endomicroscopy: technical advances and clinical applications. Gastroenterology. 2010;139:388-392, 392.e1-392.e2. |
| 29. | Kiesslich R, Goetz M, Lammersdorf K, Schneider C, Burg J, Stolte M, Vieth M, Nafe B, Galle PR, Neurath MF. Chromoscopy-guided endomicroscopy increases the diagnostic yield of intraepithelial neoplasia in ulcerative colitis. Gastroenterology. 2007;132:874-882. |
| 30. | Hurlstone DP, Thomson M, Brown S, Tiffin N, Cross SS, Hunter MD. Confocal endomicroscopy in ulcerative colitis: differentiating dysplasia-associated lesional mass and adenoma-like mass. Clin Gastroenterol Hepatol. 2007;5:1235-1241. |
| 31. | Mueller E, Vieth M, Stolte M, Mueller J. The differentiation of true adenomas from colitis-associated dysplasia in ulcerative colitis: a comparative immunohistochemical study. Hum Pathol. 1999;30:898-905. |
| 32. | Tarmin L, Yin J, Harpaz N, Kozam M, Noordzij J, Antonio LB, Jiang HY, Chan O, Cymes K, Meltzer SJ. Adenomatous polyposis coli gene mutations in ulcerative colitis-associated dysplasias and cancers versus sporadic colon neoplasms. Cancer Res. 1995;55:2035-2038. |
| 33. | Schneider A, Stolte M. Clinical and pathomorphological findings in patients with colorectal carcinoma complicating ulcerative colitis. Z Gastroenterol. 1993;31:192-197. |
| 34. | Vieth M, Behrens H, Stolte M. [Sporadic adenoma and colitis-associated intraepithelial neoplasia: a difficult differential diagnosis]. Pathologe. 2003;24:36-43. |
| 35. | Mikami T, Mitomi H, Hara A, Yanagisawa N, Yoshida T, Tsuruta O, Okayasu I. Decreased expression of CD44, alpha-catenin, and deleted colon carcinoma and altered expression of beta-catenin in ulcerative colitis-associated dysplasia and carcinoma, as compared with sporadic colon neoplasms. Cancer. 2000;89:733-740. |
| 36. | Aust DE, Terdiman JP, Willenbucher RF, Chew K, Ferrell L, Florendo C, Molinaro-Clark A, Baretton GB, Löhrs U, Waldman FM. Altered distribution of beta-catenin, and its binding proteins E-cadherin and APC, in ulcerative colitis-related colorectal cancers. Mod Pathol. 2001;14:29-39. |
| 37. | Aust DE, Terdiman JP, Willenbucher RF, Chang CG, Molinaro-Clark A, Baretton GB, Loehrs U, Waldman FM. The APC/beta-catenin pathway in ulcerative colitis-related colorectal carcinomas: a mutational analysis. Cancer. 2002;94:1421-1427. |
| 38. | Leedham SJ, Graham TA, Oukrif D, McDonald SA, Rodriguez-Justo M, Harrison RF, Shepherd NA, Novelli MR, Jankowski JA, Wright NA. Clonality, founder mutations, and field cancerization in human ulcerative colitis-associated neoplasia. Gastroenterology. 2009;136:542-550.e6. |
| 39. | Odze RD, Brown CA, Hartmann CJ, Noffsinger AE, Fogt F. Genetic alterations in chronic ulcerative colitis-associated adenoma-like DALMs are similar to non-colitic sporadic adenomas. Am J Surg Pathol. 2000;24:1209-1216. |
| 40. | Wong NA, Mayer NJ, Anderson CE, McKenzie HC, Morris RG, Diebold J, Mayr D, Brock IW, Royds JA, Gilmour HM. Cyclin D1 and p21 in ulcerative colitis-related inflammation and epithelial neoplasia: a study of aberrant expression and underlying mechanisms. Hum Pathol. 2003;34:580-588. |
| 41. | Fogt F, Vortmeyer AO, Stolte M, Mueller E, Mueller J, Noffsinger A, Poremba C, Zhuang Z. Loss of heterozygosity of the von Hippel Lindau gene locus in polypoid dysplasia but not flat dysplasia in ulcerative colitis or sporadic adenomas. Hum Pathol. 1998;29:961-964. |
| 42. | Andersen SN, Rognum TO, Bakka A, Clausen OP. Ki-67: a useful marker for the evaluation of dysplasia in ulcerative colitis. Mol Pathol. 1998;51:327-332. |
| 43. | Engelsgjerd M, Farraye FA, Odze RD. Polypectomy may be adequate treatment for adenoma-like dysplastic lesions in chronic ulcerative colitis. Gastroenterology. 1999;117:1288-1294; discussion 1488-1491. |
| 44. | Mönkemüller K, Neumann H, Malfertheiner P, Fry LC. Advanced colon polypectomy. Clin Gastroenterol Hepatol. 2009;7:641-652. |
| 45. | Odze RD, Farraye FA, Hecht JL, Hornick JL. Long-term follow-up after polypectomy treatment for adenoma-like dysplastic lesions in ulcerative colitis. Clin Gastroenterol Hepatol. 2004;2:534-541. |
| 46. | Rutter MD, Saunders BP, Wilkinson KH, Kamm MA, Williams CB, Forbes A. Most dysplasia in ulcerative colitis is visible at colonoscopy. Gastrointest Endosc. 2004;60:334-339. |