Published online Jun 21, 2011. doi: 10.3748/wjg.v17.i23.2801
Revised: November 17, 2010
Accepted: November 24, 2010
Published online: June 21, 2011
Non-alcoholic fatty liver disease (NAFLD) comprising hepatic steatosis, non-alcoholic steatohepatitis (NASH), and progressive liver fibrosis is considered the most common liver disease in western countries. Fatty liver is more prevalent in overweight than normal-weight people and liver fat positively correlates with hepatic insulin resistance. Hepatic steatosis is regarded as a benign stage of NAFLD but may progress to NASH in a subgroup of patients. Besides liver biopsy no diagnostic tools to identify patients with NASH are available, and no effective treatment has been established. Visceral obesity is a main risk factor for NAFLD and inappropriate storage of triglycerides in adipocytes and higher concentrations of free fatty acids may add to increased hepatic lipid storage, insulin resistance, and progressive liver damage. Most of the adipose tissue-derived proteins are elevated in obesity and may contribute to systemic inflammation and liver damage. Adiponectin is highly abundant in human serum but its levels are reduced in obesity and are even lower in patients with hepatic steatosis or NASH. Adiponectin antagonizes excess lipid storage in the liver and protects from inflammation and fibrosis. This review aims to give a short survey on NAFLD and the hepatoprotective effects of adiponectin.
- Citation: Buechler C, Wanninger J, Neumeier M. Adiponectin, a key adipokine in obesity related liver diseases. World J Gastroenterol 2011; 17(23): 2801-2811
- URL: https://www.wjgnet.com/1007-9327/full/v17/i23/2801.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i23.2801
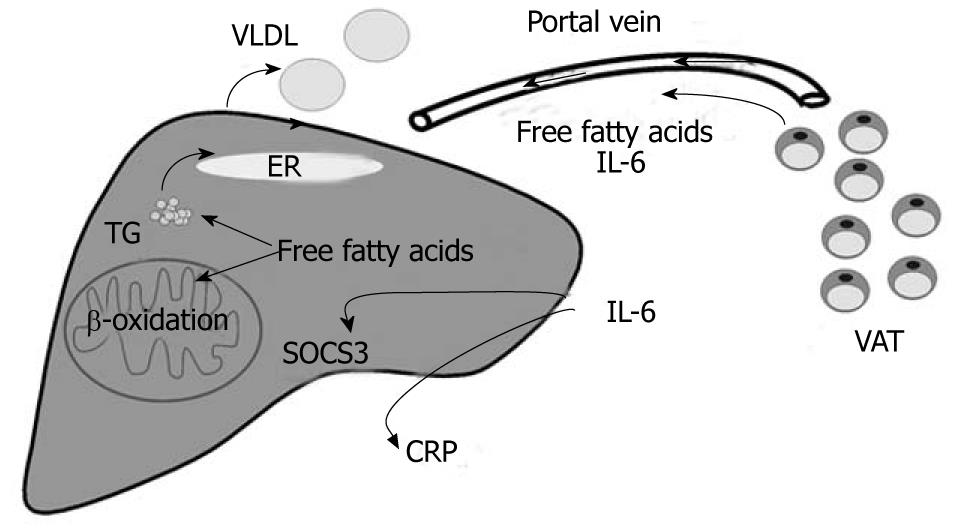
Obesity is associated with insulin resistance, a common risk factor for type 2 diabetes, cardiovascular disease, hepatic steatosis and non-alcoholic steatohepatitis (NASH)[1,2]. Hypertrophied adipocytes in obesity fail to appropriately store excess triglycerides and excessive ectopic accumulation of lipids in skeletal muscle and liver disturbs insulin signalling[3]. Body fat distribution appears to be even more important than the total amount of adipose tissue, and visceral fat mass is strongly linked to insulin resistance and non-alcoholic fatty liver disease (NAFLD)[4]. Visceral fat released free fatty acids are transported to the liver by the portal vein and may contribute to hepatic steatosis, production of triglyceride rich very low density lipoproteins (VLDL) and elevated β-oxidation[5,6] (Figure 1). Metabolically healthy but obese (MHO) individuals are insulin sensitive and hepatic fat accumulation is significantly lower compared to similarly overweight subjects that develop insulin resistance[7,8]. Despite comparable fatness between MHO and control cohorts that develop insulin resistance, MHO subjects have 49% less visceral fat which further emphasizes the unfavourable characteristics of this fat depot[9]. Lean body mass may be associated with a higher insulin sensitivity and is significantly lower in MHO subjects[9]. A recent study even describes an independent association of lean body mass with impaired glucose disposal and systemic C-reactive protein (CRP) levels in centrally obese postmenopausal women that may exacerbate the harmful effects of visceral fat mass[10]. These studies further point to the highly complex interplay of various factors associated with metabolic diseases like the metabolic syndrome.
Various epidemiological studies have identified central obesity as an independent risk factor for metabolic diseases and highlight the crucial role of impaired production or activity of adipose tissue released proteins[6,11]. Most of the adipokines identified so far are elevated in obesity and raised chemokine C-C motif ligand 2 (CCL2) contributes to the increasing number of adipose tissue resident macrophages[11,12]. They produce inflammatory proteins like interleukin-6 (IL-6) and tumour necrosis factor (TNF) whose circulating levels are increased in obesity, a state of low-grade, chronic inflammation[13]. TNF impairs insulin signalling and plays a crucial role in non-alcoholic steatohepatitis (NASH) progression[14,15]. Visceral fat released proteins are directly transported to the liver by the portal vein and the anatomical feature of this fat depot may explain the harmful metabolic effects of visceral adiposity[6]. IL-6 is preferentially released from visceral fat and upregulates suppressor of cytokine signalling 3 (SOCS3) in the liver that causes hepatic insulin resistance[16-18]. Furthermore, IL-6 is a well known inducer of CRP, a marker protein for systemic inflammation[19] (Figure 1). Leptin is mainly produced by adipocytes, and obesity is characterized by elevated systemic levels and central and peripheral leptin resistance[6]. Leptin prevents lipid accumulation in non-adipose tissues like the liver. Leptin lowers stearoyl-CoA desaturase that catalyzes the rate-limiting reaction of monounsaturated fatty acid synthesis and thereby may ameliorate hepatic insulin sensitivity[20]. Animal studies have proven that leptin directly promotes fibrogenesis. Leptin induces transforming growth factor β (TGF-β) and connective tissue growth factor (CTGF) production in hepatic stellate cells through indirect effects on Kupffer cells[21]. In humans, a direct association of circulating leptin and liver fibrosis has not been confirmed yet and locally produced leptin and/or leptin resistance may have to be taken into account[22].
The adipokine adiponectin is highly abundant in human serum and is secreted by adipose tissue in inverse proportion to the body mass index[23]. Adiponectin circulates as trimer, hexamer and higher order multimer in serum and isoform-specific effects have been described[24-26]. Adiponectin may also form hetero-oligomers with additional members of the C1q/TNF-related protein (CTRP) family like the recently described CTRP9[27]. Early studies indicate that globular adiponectin, the globular C1q domain of this protein generated by proteolysis of the full-length protein, may also exist in serum[28]. However, circulating levels seem to be rather low, questioning the biological significance of this protein[29] that may nevertheless be of therapeutic relevance. Epidemiological studies revealed that low adiponectin levels are associated with NASH independent of insulin resistance and body mass index, and hepatoprotective effects of adiponectin have been identified in animal studies or with isolated liver cells[30-32]. MHO individuals are insulin sensitive and have adiponectin levels similar to normal-weight controls despite excessive weight and body fat, and this association may further underline the protective effects of this adipokine[33].
NAFLD not only compromises the hepatic manifestation of the metabolic syndrome but is linked to a higher risk of develop metabolic disorders like type 2 diabetes or cardiovascular disease[34,35]. Fatty liver is even associated with dyslipidemia, metabolic syndrome and low adiponectin independent of body mass index (BMI), waist to hip ratio and visceral fat mass[36]. NAFLD has been predicted to increase along with the growing epidemic of obesity[37] and understanding of its pathophysiology is a prerequisite to develop non-invasive diagnostic tools and to establishing effective treatment regimes. Rising adiponectin levels may be beneficial in liver disease and its protective effects in hepatic steatosis and NASH[38] are summarized in the current review article.
Diagnosis of NAFLD requires a careful anamnesis to exclude other liver diseases or drug-mediated liver damage. Moderate alcohol intake, defined by most physicians as 20 to 40 g/d in men and 20 g/d in women, has to be enquired about[39]. Liver biopsy is essential for diagnosis and staging but the use of this invasive method is limited to a subgroup of patients[39].
When NAFLD is defined as elevation of aspartate aminotransferase (AST) or alanine aminotransferase (ALT) and transferrin saturation of less than 50% the frequency is 5.4% in the general population of the United States[40]. When elevated gamma-glutamyltranspeptidase (GGT) is included and lower cut-off values are used the prevalence is 24%[41]. Studies performed in gastroenterology units restricted cohorts identified 11% of the patients as having NAFLD[42]. In bariatric surgery patients hepatic steatosis ranges from 65% to 90%[43,44]; NASH has been diagnosed in 15 to 55% and fibrosis in 34% to 47%[41,45].
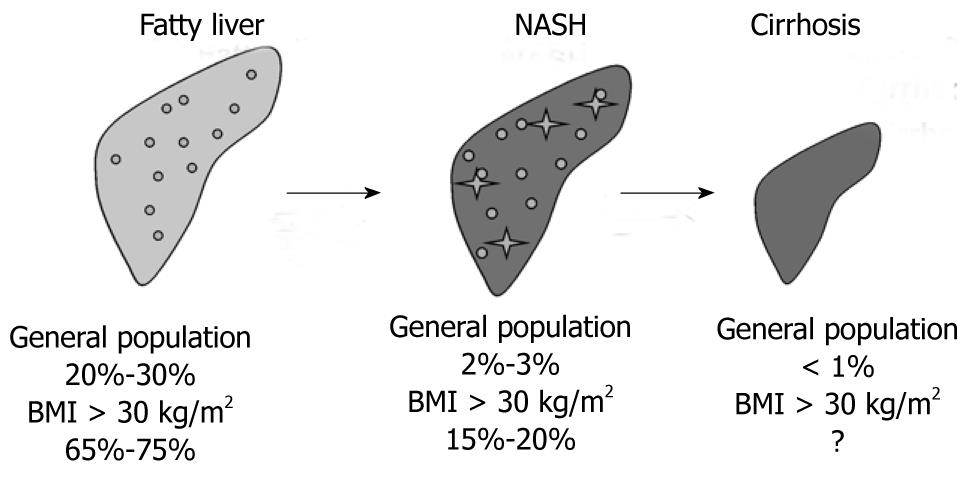
Current estimates based on different studies in unselected and selected populations indicate that about 20% to 30% of adults in Western countries have excess fat accumulation in the liver, 2% to 3% of adults are thought to meet current diagnostic criteria for NASH and eventually up to one third of those with NASH suffer from progressive fibrosis or even cirrhosis[41,45,46]. In obesity defined as BMI above 30 kg/m2 and in morbidly obese patients these values are much higher and patients with NASH are over-represented in these populations[41,45] (Figure 2).
Visceral adiposity and insulin resistance are clearly related to NAFLD[47] and genetic variations associated with obesity and disproportionate body fat distribution may predispose to development of steatotic liver. Chemerin is a recently identified adipokine and a common genetic variation is associated with increased visceral fat mass in non-obese subjects but epidemiological studies to link chemerin alleles with NAFLD are still lacking[48]. The adiponectin 45T→G variant contributes to overall fatness and abdominal obesity but is not an important determinant of NAFLD at least in Chinese people[49,50].
Gene variations may influence NAFLD stage, progression and even occurrence. NAFLD is much more likely in Hispanic Americans than among whites[51] and African Americans have a lower degree of hepatic steatosis relative to whites[52]. Familial clustering of NAFLD has been demonstrated, and fatty liver is more common in siblings and parents of children with NAFLD indicating that NAFLD, similar to type 2 diabetes, is a multifactorial disease[53]. Environmental and genetic factors define the individual risk of developing NAFLD and may also explain why only a subgroup of patients develop more progressive liver damage.
Studies in small cohorts have identified genetic associations of microsomal triglyceride transfer protein, an enzyme regulating hepatic VLDL release, the antioxidant mitochondrial enzyme superoxide dismutase 2, the inflammatory cytokine TNF and the main profibrotic cytokine TGF-β with NAFLD[54,55]. Genome-wide association studies find that variations of patatin-like phospholipase domain containing 3 (PNPLA3, adiponectin), a protein with close homology to adipose triglyceride lipase but so far unknown function, contributes to ethnic and inter-individual differences in hepatic steatosis and susceptibility to NAFLD[56,57].
This association has not been confirmed in a recent study in non-Hispanic, Caucasian, women with liver biopsy proven NAFLD, where an association between NASH activity score and single nucleotide polymorphisms (SNPs) within the squalene synthase (FDFT1) gene, a key regulator of cholesterol biosynthesis, is described. Polymorphisms of the pregnancy zone protein, a proteinase involved in clearance of TGF-β, are linked to systemic AST levels, and variants of platelet-derived growth factor α are linked to liver fibrosis[58].
Genetic variations of adiponectin are found to be associated with NAFLD[50,59], and single nucleotide polymorphisms in adiponectin receptor 1 (AdipoR1) and AdipoR2 contribute to variations in hepatic fat accumulation in humans[60,61].
Systemic adiponectin concentrations are in the μg/mLrange indicating that adiponectin constitutes a substantial fraction of plasma proteins, and these high levels are remarkably constant. Despite its abundant presence in plasma, adiponectin is cleared rapidly by the liver with a half-life of about 75 min[62].
Visceral adiposity is associated with elevated circulating free fatty acids and higher concentrations of most adipose tissue released proteins[63]. Adiponectin differs from the adipokines described so far because its systemic levels are decreased in obesity[23]. In high fat diet induced obese rodents and in ob/ob mice adiponectin levels are reduced in plasma and clearance is significantly prolonged, indicating markedly impaired adiponectin synthesis in obesity[62,64,65]. Visceral fat accumulation is associated with hypoadiponectinemia and negative associations of visceral fat with systemic adiponectin have been identified[66,67].
Besides adiponectin, circulating levels of omentin predominantly released from the stromovascular cells of visceral fat are found reduced in obesity and serum omentin levels are increased in patients with NAFLD and independently predict hepatocyte ballooning[68].
In healthy Caucasians, BMI and adiponectin, but not insulin resistance, predict serum concentrations of both ALT and GGT[69]. Low adiponectin levels are even found associated with NASH independent of insulin resistance and BMI[30]. Multivariate regression analysis identifies decreased adiponectin as an independent predictor of liver steatosis and elevated ALT and GGT levels in healthy obese individuals[32]. In NAFLD patients low adiponectin levels are closely associated with the degree of hepatic steatosis, necroinflammation and fibrosis[30,32]. Shimada et al[70] reported that 90% of patients with early-stage NASH can be predicted by a combined evaluation of the serum adiponectin level, homeostasis assessment model-insulin resistance (HOMA-IR) score, and serum type IV collagen 7S level.
Circulating adiponectin levels in the μg/mL range by far exceed concentrations commonly required for receptor-dependent signalling. This may indicate receptor independent functions of adiponectin and binding to growth factors like platelet derived growth factor (PDGF), extracellular matrix proteins, low density lipoprotein (LDL) and opsonization of apoptotic cells to stimulate phagocytosis have been described[71-74].
Systemic adiponectin is about 20% to 60% lower in NAFLD than healthy controls[75-77] but considering the high levels in the circulation and a half-maximal effective dose of 0.85 μg/mL full-length adiponectin for AdipoR2 stimulated fatty acid oxidation[78] the question arises whether impaired receptor-mediated signalling due to reduced concentrations is a reasonable explanation for metabolic complications associated with hypoadiponectinemia. Therefore, it is likely that adiponectin receptor signal transduction pathways are also impaired in NAFLD.
Two 7-transmembrane proteins, AdipoR1 and AdipoR2, have been identified to function as adiponectin receptors[78]. Although initial studies using rodent tissues reveal preferential expression of AdipoR2 in the liver, in human tissues AdipoR1 and AdipoR2 mRNAs are most abundant in skeletal muscle and both are moderately expressed in the liver[78]. AdipoR1 protein is easily detected in human hepatocytes indicating that both receptors may play a role in liver physiology[79].
Although there is a well documented relationship between low adiponectin and liver disease, an association of NAFLD and reduced expression of hepatic adiponectin receptors is not consistently reported. Furthermore, mainly mRNA expression has been analysed and this may not necessarily predict protein abundance[80,81].
In animal models of obesity, hepatic adiponectin receptor mRNAs are found unchanged or even increased[65,82,83]. In human biopsies, hepatic adiponectin receptor mRNAs are increased in biopsy-proven NASH compared to steatotic livers[84]. Other studies, however, describe similar levels of adiponectin receptor mRNA in normal liver, steatotic liver and NASH[85,86]. There are also reports on reduced AdipoR2 mRNA in NASH compared to simple steatosis or lower AdipoR2 mRNA in fatty liver with no further reduction in NASH[87,88].
Data on AdipoR2 proteins are sparse and one study demonstrates reduced AdipoR2 protein in human NASH compared to steatotic liver[88]. Treatment of hepatocytes with palmitate is used as an in vitro model for hepatocyte steatosis and 200 μmol/L of this fatty acid reduce AdipoR2 protein in Huh7 cells[89]. Activating transcription factor 3 (ATF3) is induced upon endoplasmic reticulum stress and in the liver of ob/ob mice, and suppresses AdipoR2 in HepG2 cells[90]. Therefore, besides low circulating adiponectin, AdipoR2 may be reduced in hepatic steatosis and NASH indicating a possible adiponectin resistant state.
Dyslipidemia is characterized by high circulating triglycerides[91] and low high density lipoprotein (HDL) cholesterol levels, and is frequently accompanied by hepatic steatosis[92]. Adiponectin negatively correlates with serum triglycerides and apolipoprotein B (ApoB), the main apolipoprotein of the triglyceride rich VLDL[93,94]. Hepatocyte ApoB and triglycerides are reduced by adiponectin indicating lower hepatic VLDL release[28,95,96]. Furthermore, VLDL catabolism is enhanced by an increased skeletal muscle lipoprotein lipase and VLDL receptor expression[97]. This more favourable lipid profile may be linked to lower hepatic lipid storage.
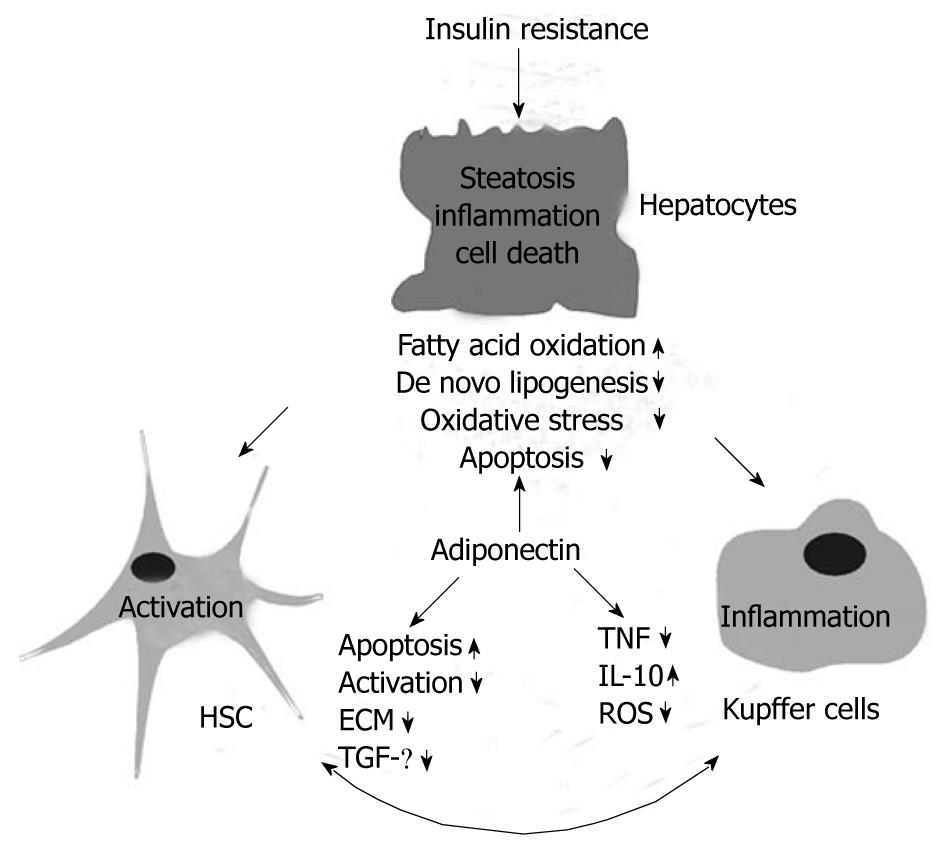
A choline and L-amino acid deficient diet induces more severe hepatic steatosis in adiponectin deficient mice compared to wild type animals[14]. Adenoviral expression of adiponectin ameliorates lipid deposition in the liver[95]. SREBP-1c is a central regulator of fatty acid synthesis, and is suppressed by adiponectin in hepatocytes and in the liver of db/db mice[95]. AMP-activated protein kinase (AMPK) is physiologically activated by low energy status, and switches on ATP-producing catabolic pathways (such as fatty acid oxidation and glycolysis), and switches off ATP-consuming anabolic pathways (such as lipogenesis)[98]. Adiponectin activates AMPK by binding to AdipoR1[78].Suppression of SREBP-1c by adiponectin is mediated through AdipoR1/LKB1, an upstream kinase of AMPK, and AMPK pathway[95]. AMPK in addition phosphorylates acetyl-CoA carboxylase (ACC) and this is subsequently associated with a higher activity of carnitine palmitoyltransferase 1 (CPT-1), a rate limiting enzyme in fatty acid oxidation[98].
Signalling via AdipoR2 enhances peroxisome-proliferator activated receptor α (PPARα) activity[78,99]. PPARα upregulates CPT-1, stimulates β-oxidation, reduces lipid synthesis and thereby prevents excess triglyceride storage[100].
Lipopolysaccharide (LPS) is involved in the pathogenesis of NAFLD and elevated levels of circulating LPS are found in obesity[101,102]. Increased gut permeability and a higher prevalence of small intestinal bacterial overgrowth correlates with the severity of steatosis but not with NASH[103]. Besides age, inflammation was identified as an independent predictor of progression to advanced fibrosis in NASH patients[104]. Hepatic steatosis may be accompanied by inflammatory cell infiltrates composed of neutrophils and mononuclear cells. In several mouse models of immune mediated hepatitis, adiponectin reduces TNF and induces interleukin-10 (IL-10) release from Kupffer cells[105]. Adiponectin lowers CRP synthesis in cytokine stimulated rat hepatocytes, and an inverse correlation of systemic adiponectin and CRP has been identified in obese patients[106,107]. Adiponectin may exert its anti-inflammatory activity by lowering nuclear factor kappa B (NFκB) action in preactivated cells or by inducing tolerance to inflammatory stimuli by a rapid and transient activation of NFκB that subsequently renders the cells inert to further activation[108-111].
Nevertheless, in patients suffering from chronic inflammatory diseases like inflammatory bowel disease or type 1 diabetes that are not associated with adiposity elevated circulating adiponectin levels that even correlate with inflammatory markers are found[112-114], and an induction of inflammatory proteins and activation of NFκB by recombinant adiponectin is described in several studies[25,108,113,115]. Therefore, adiponectin seems to be regulated in the opposite direction in classic versus obesity-associated chronic inflammatory diseases and may even exert opposite activities in resting compared to activated cells[116].
NFκB promotes cell survival and NEMO-mediated NFκB activation in hepatocytes has an essential physiological function to prevent the spontaneous development of steatohepatitis and hepatocellular carcinoma[117]. Adiponectin activates NFκB in human hepatocytes, and thereby may prevent hepatocyte apoptosis. Adiponectin further upregulates the chemokine interleukin 8 (CXCL8) via AdipoR1 and NFκB dependent pathways in primary human hepatocytes[118]. CXCL8 is an antiapoptotic protein[119] and overexpression of the rodent CXCL8 homologous protein protects the liver from galactosamine and endotoxin induced damage[120].
Adiponectin further antagonizes hepatocyte death by blocking fatty acid-induced activation of c-Jun NH2 terminal kinase[121], by reducing TNF levels[105] and by inhibiting fatty acid mediated upregulation of CD95[122].
Fatty liver is thought to represent the first incident towards the subsequent development of liver fibrosis[6]. Accelerated β-oxidation of fatty acids in hepatic steatosis is associated with excess reactive oxygen species (ROS), lipid peroxidation, the release of inflammatory cytokines, death of hepatocytes and activation of hepatic stellate cells[1]. ROS and lipid peroxidation are thought to contribute to the progression of liver injury partly by accelerating inflammation that in turn causes ROS production[1]. Oxidative stress is enhanced in human hypoadiponectinemia and in adiponectin knock-out mice fed a choline-deficient L-amino acid deficient diet[123,124]. Hepatic cytochrome P450 2E1 (CYP2E1) is elevated in these animals and in human NASH and may contribute to higher ROS levels[125,126].
Aldehyde oxidase 1 (AOX1) is a xenobiotic metabolizing protein whose physiological role has not been evaluated in detail so far[127]. AOX1 activity has been identified as an important source of ROS[128] and is reduced in hepatocytes by adiponectin via activation of PPARα[31] Adiponectin also increases ROS detoxifying enzymes and AdipoR2 is involved in the induction of superoxide dismutase 1 and catalase[129].
Liver injury causes activation of otherwise “quiescent” hepatic stellate cells (HSC) and activated cells proliferate, synthesize CTGF and extracellular matrix proteins[130]. TGF-β is the main profibrotic factor in fibrosis and induces CTGF synthesis. CTGF stimulates binding of TGF-β to its receptor and thereby enhances TGF-β activity[130]. CTGF is induced by TGF-β indicating an autocrine or paracrine loop that mutually enhances synthesis of both proteins[130]. Knock-down of AdipoR2 in mice fed a methionine-choline deficient diet to cause progressive fibrosing steatohepatitis is associated with higher levels of steatosis, inflammation and fibrosis[131]. Overexpression of AdipoR2 is protective, and this mechanistically includes inhibition of TGF-β signaling and stimulation of PPARα activity[131].
Expression of recombinant adiponectin in activated HSC reduces proliferation and lowers α-smooth muscle actin that is induced in activated HSC[132]. Furthermore, apoptotic cell death of activated HSC is augmented[132]. Exogenously added recombinant adiponectin suppresses PDGF-stimulated HSC proliferation by activation of AMPK[133]. Adiponectin may also bind to growth factors like PDGF and thereby inhibits binding to their corresponding receptors[71]. Leptin is a well described profibrotic adipokine and several studies have shown that adiponectin antagonizes leptin bioactivity[134,135]. Adiponectin blocks leptin-induced STAT3 phosphorylation in activated HSC and leptin-mediated upregulation of TIMP-1 release and these in-vitro findings have been confirmed in-vivo[134].
Studies analysing the impact of changes in life style and medications in NAFLD have been performed in small patient groups sometimes even lacking suitable controls. Currently weight loss and exercise are recommended as initial strategies to improve NASH[136]. Diet and diet in conjunction with exercise for 6 mo cause a similar reduction in body weight and intrahepatic fat[137]. In 19 sedentary obese men and women four weeks of aerobic exercise improved hepatic steatosis even in the absence of weight loss[138]. In a randomized controlled trial enrolling 31 patients with biopsy-proven NASH intensive changes in life style with the objective of at least 7% weight loss and educational training without weight reduction have been compared[139]. Weight loss significantly correlates with improvement in NASH histological activity score and weight loss of 7% or even more is recommended as a treatment strategy for these patients[139]. Another study also reports improvements of histological and laboratory parameters when body weight is reduced by 10% in NASH patients[140]. Adiponectin concentrations increase by about 36% in type 2 diabetic patients by 13% weight loss[141], and this may partly contribute to the metabolic improvements observed in these patients.
Clinical trials using fibrates have revealed inconsistent results so far. Treatment of sixteen NASH patients with clofibrate did not ameliorate biochemical or histological parameters[142], whereas a second study demonstrated biochemical and ultrasound improvements with fenofibrate[143]. Emerging data on thiazolidinediones have demonstrated improvement in both liver enzymes and histology[144,145]. These drugs activate PPARγ and thereby inhibit growth of HSC and TGF-β mediated induction of CTGF, respectively[146]. PPARγ is the main adipogenic transcription factor and its agonists stimulate adipogenesis[147]. Thiazolidinediones strongly stimulate adiponectin synthesis and elevate systemic adiponectin[147]. Increase of adiponectin by pioglitazone is related to histological improvement of steatosis, inflammation and fibrosis confirming the crucial role of adiponectin in NAFLD[148]. The PPARγ agonist rosiglitazone even induces AdipoR2 in hepatocytes[146]. A recent study reports that pioglitazone therapy improves adipose tissue insulin sensitivity and this correlates with a reduction in hepatic fat and necroinflammation[149]. Activation of PPARγ primes human monocytes into alternative M2 macrophages with anti-inflammatory properties and patients may also benefit from reduced inflammation[150]. In line with this hypothesis pentoxifylline with multiple pharmacological effects including antioxidant and antiinflammatory activity[151] has been tested in small clinical trials, and biochemical and histological improvements have been reported[151,152]. Vitamin E therapy decreases AST and ALT levels and hepatic steatosis but does not improve necroinflammation and fibrosis[153]. Antioxidants may even prevent health-promoting effects of physical exercise namely insulin sensitivity and rise of systemic adiponectin in untrained and pre-trained individuals, and therefore, may be more effective in patients with low physical activity[154]. In summary to date no pharmacologic treatment has been reliably shown to be effective for the treatment of NASH patients.
Adiponectin has emerged as a protective adipokine in insulin resistance and obesity related liver diseases (Figure 3), and drugs that elevate systemic adiponectin may be useful as therapeutics for NAFLD. Adiponectin receptor signalling pathways and potential hepatic adiponectin resistance in NASH, however, have been poorly investigated so far. Identification of molecules downstream of AdipoR1/2 and strategies to enhance adiponectin receptor activity may constitute promising approaches towards treatment of NAFLD.
Peer reviewer: Christopher O'Brien, MD, Professor of Clinical Medicine, Chief of Clinical Hepatology, Center for Liver Diseases, Divisions of Liver and GI Transplantation, University of Miami School of Medicine, 1500 Northwest 12th Ave., Suite #1101, Miami, FL 33136, United States
S- Editor Tian L L- Editor O’Neill M E- Editor Ma WH
| 1. | Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147-152. [Cited in This Article: ] |
| 2. | Gil-Campos M, Cañete RR, Gil A. Adiponectin, the missing link in insulin resistance and obesity. Clin Nutr. 2004;23:963-974. [Cited in This Article: ] |
| 3. | Goossens GH. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav. 2008;94:206-218. [Cited in This Article: ] |
| 4. | Calamita G, Portincasa P. Present and future therapeutic strategies in non-alcoholic fatty liver disease. Expert Opin Ther Targets. 2007;11:1231-1249. [Cited in This Article: ] |
| 5. | Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93:S57-S63. [Cited in This Article: ] |
| 6. | Schäffler A, Schölmerich J, Büchler C. Mechanisms of disease: adipocytokines and visceral adipose tissue--emerging role in nonalcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:273-280. [Cited in This Article: ] |
| 7. | Messier V, Karelis AD, Robillard ME, Bellefeuille P, Brochu M, Lavoie JM, Rabasa-Lhoret R. Metabolically healthy but obese individuals: relationship with hepatic enzymes. Metabolism. 2010;59:20-24. [Cited in This Article: ] |
| 8. | Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, Balletshofer B, Machicao F, Fritsche A, Häring HU. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609-1616. [Cited in This Article: ] |
| 9. | Brochu M, Tchernof A, Dionne IJ, Sites CK, Eltabbakh GH, Sims EA, Poehlman ET. What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women? J Clin Endocrinol Metab. 2001;86:1020-1025. [Cited in This Article: ] |
| 10. | Brochu M, Mathieu ME, Karelis AD, Doucet E, Lavoie ME, Garrel D, Rabasa-Lhoret R. Contribution of the lean body mass to insulin resistance in postmenopausal women with visceral obesity: a Monet study. Obesity (Silver Spring). 2008;16:1085-1093. [Cited in This Article: ] |
| 11. | Catalán V, Gómez-Ambrosi J, Rodríguez A, Salvador J, Frühbeck G. Adipokines in the treatment of diabetes mellitus and obesity. Expert Opin Pharmacother. 2009;10:239-254. [Cited in This Article: ] |
| 12. | Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494-1505. [Cited in This Article: ] |
| 13. | Zeyda M, Stulnig TM. Adipose tissue macrophages. Immunol Lett. 2007;112:61-67. [Cited in This Article: ] |
| 14. | Kamada Y, Takehara T, Hayashi N. Adipocytokines and liver disease. J Gastroenterol. 2008;43:811-822. [Cited in This Article: ] |
| 15. | Borst SE. The role of TNF-alpha in insulin resistance. Endocrine. 2004;23:177-182. [Cited in This Article: ] |
| 16. | Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010-1013. [Cited in This Article: ] |
| 17. | Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, Barrett T, Kim JK, Davis RJ. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539-1543. [Cited in This Article: ] |
| 18. | Wiest R, Weigert J, Wanninger J, Neumeier M, Bauer S, Schmidhofer S, Farkas S, Scherer MN, Schäffler A, Schölmerich J. Impaired hepatic removal of interleukin-6 in patients with liver cirrhosis. Cytokine. 2011;53:178-183. [Cited in This Article: ] |
| 19. | Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621-636. [Cited in This Article: ] |
| 20. | Biddinger SB, Miyazaki M, Boucher J, Ntambi JM, Kahn CR. Leptin suppresses stearoyl-CoA desaturase 1 by mechanisms independent of insulin and sterol regulatory element-binding protein-1c. Diabetes. 2006;55:2032-2041. [Cited in This Article: ] |
| 21. | Wang J, Leclercq I, Brymora JM, Xu N, Ramezani-Moghadam M, London RM, Brigstock D, George J. Kupffer cells mediate leptin-induced liver fibrosis. Gastroenterology. 2009;137:713-723. [Cited in This Article: ] |
| 22. | Lanthier N, Horsmans Y, Leclercq IA. The metabolic syndrome: how it may influence hepatic stellate cell activation and hepatic fibrosis. Curr Opin Clin Nutr Metab Care. 2009;12:404-411. [Cited in This Article: ] |
| 23. | Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79-83. [Cited in This Article: ] |
| 24. | Wang Y, Lam KS, Yau MH, Xu A. Post-translational modifications of adiponectin: mechanisms and functional implications. Biochem J. 2008;409:623-633. [Cited in This Article: ] |
| 25. | Neumeier M, Weigert J, Schäffler A, Wehrwein G, Müller-Ladner U, Schölmerich J, Wrede C, Buechler C. Different effects of adiponectin isoforms in human monocytic cells. J Leukoc Biol. 2006;79:803-808. [Cited in This Article: ] |
| 26. | Schober F, Neumeier M, Weigert J, Wurm S, Wanninger J, Schäffler A, Dada A, Liebisch G, Schmitz G, Aslanidis C. Low molecular weight adiponectin negatively correlates with the waist circumference and monocytic IL-6 release. Biochem Biophys Res Commun. 2007;361:968-973. [Cited in This Article: ] |
| 27. | Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Ge G, Spooner E, Hug C, Gimeno R, Lodish HF. Identification and characterization of CTRP9, a novel secreted glycoprotein, from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin. FASEB J. 2009;23:241-258. [Cited in This Article: ] |
| 28. | Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA. 2001;98:2005-2010. [Cited in This Article: ] |
| 29. | Kusminski CM, McTernan PG, Schraw T, Kos K, O‘Hare JP, Ahima R, Kumar S, Scherer PE. Adiponectin complexes in human cerebrospinal fluid: distinct complex distribution from serum. Diabetologia. 2007;50:634-642. [Cited in This Article: ] |
| 30. | Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology. 2004;40:46-54. [Cited in This Article: ] |
| 31. | Neumeier M, Weigert J, Schäffler A, Weiss TS, Schmidl C, Büttner R, Bollheimer C, Aslanidis C, Schölmerich J, Buechler C. Aldehyde oxidase 1 is highly abundant in hepatic steatosis and is downregulated by adiponectin and fenofibric acid in hepatocytes in vitro. Biochem Biophys Res Commun. 2006;350:731-735. [Cited in This Article: ] |
| 32. | Targher G, Bertolini L, Scala L, Poli F, Zenari L, Falezza G. Decreased plasma adiponectin concentrations are closely associated with nonalcoholic hepatic steatosis in obese individuals. Clin Endocrinol (Oxf). 2004;61:700-703. [Cited in This Article: ] |
| 33. | Aguilar-Salinas CA, García EG, Robles L, Riaño D, Ruiz-Gomez DG, García-Ulloa AC, Melgarejo MA, Zamora M, Guillen-Pineda LE, Mehta R. High adiponectin concentrations are associated with the metabolically healthy obese phenotype. J Clin Endocrinol Metab. 2008;93:4075-4079. [Cited in This Article: ] |
| 34. | Rubinstein E, Lavine JE, Schwimmer JB. Hepatic, cardiovascular, and endocrine outcomes of the histological subphenotypes of nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:380-385. [Cited in This Article: ] |
| 35. | Bellentani S, Bedogni G, Tiribelli C. Liver and heart: a new link? J Hepatol. 2008;49:300-302. [Cited in This Article: ] |
| 36. | Speliotes EK, Massaro JM, Hoffmann U, Vasan RS, Meigs JB, Sahani DV, Hirschhorn JN, O‘Donnell CJ, Fox CS. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham Heart Study. Hepatology. 2010;51:1979-1987. [Cited in This Article: ] |
| 37. | Roberts EA. Non-alcoholic steatohepatitis in children. Clin Liver Dis. 2007;11:155-172, x. [Cited in This Article: ] |
| 38. | Wang Y, Zhou M, Lam KS, Xu A. Protective roles of adiponectin in obesity-related fatty liver diseases: mechanisms and therapeutic implications. Arq Bras Endocrinol Metabol. 2009;53:201-212. [Cited in This Article: ] |
| 39. | Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202-1219. [Cited in This Article: ] |
| 40. | Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960-967. [Cited in This Article: ] |
| 41. | Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40 Suppl 1:S5-S10. [Cited in This Article: ] |
| 42. | Byron D, Minuk GY. Clinical hepatology: profile of an urban, hospital-based practice. Hepatology. 1996;24:813-815. [Cited in This Article: ] |
| 43. | Andersen T, Christoffersen P, Gluud C. The liver in consecutive patients with morbid obesity: a clinical, morphological, and biochemical study. Int J Obes. 1984;8:107-115. [Cited in This Article: ] |
| 44. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [Cited in This Article: ] |
| 45. | Preiss D, Sattar N. Non-alcoholic fatty liver disease: an overview of prevalence, diagnosis, pathogenesis and treatment considerations. Clin Sci (Lond). 2008;115:141-150. [Cited in This Article: ] |
| 46. | Erickson SK. Nonalcoholic fatty liver disease. J Lipid Res. 2009;50 Suppl:S412-S416. [Cited in This Article: ] |
| 47. | Marchesini G, Marzocchi R, Agostini F, Bugianesi E. Nonalcoholic fatty liver disease and the metabolic syndrome. Curr Opin Lipidol. 2005;16:421-427. [Cited in This Article: ] |
| 48. | Müssig K, Staiger H, Machicao F, Thamer C, Machann J, Schick F, Claussen CD, Stefan N, Fritsche A, Häring HU. RARRES2, encoding the novel adipokine chemerin, is a genetic determinant of disproportionate regional body fat distribution: a comparative magnetic resonance imaging study. Metabolism. 2009;58:519-524. [Cited in This Article: ] |
| 49. | Loos RJ, Ruchat S, Rankinen T, Tremblay A, Pérusse L, Bouchard C. Adiponectin and adiponectin receptor gene variants in relation to resting metabolic rate, respiratory quotient, and adiposity-related phenotypes in the Quebec Family Study. Am J Clin Nutr. 2007;85:26-34. [Cited in This Article: ] |
| 50. | Wang ZL, Xia B, Shrestha U, Jiang L, Ma CW, Chen Q, Chen H, Hu ZG. Correlation between adiponectin polymorphisms and non-alcoholic fatty liver disease with or without metabolic syndrome in Chinese population. J Endocrinol Invest. 2008;31:1086-1091. [Cited in This Article: ] |
| 51. | Weston SR, Leyden W, Murphy R, Bass NM, Bell BP, Manos MM, Terrault NA. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005;41:372-379. [Cited in This Article: ] |
| 52. | Mohanty SR, Troy TN, Huo D, O‘Brien BL, Jensen DM, Hart J. Influence of ethnicity on histological differences in non-alcoholic fatty liver disease. J Hepatol. 2009;50:797-804. [Cited in This Article: ] |
| 53. | Schwimmer JB, Celedon MA, Lavine JE, Salem R, Campbell N, Schork NJ, Shiehmorteza M, Yokoo T, Chavez A, Middleton MS. Heritability of nonalcoholic fatty liver disease. Gastroenterology. 2009;136:1585-1592. [Cited in This Article: ] |
| 54. | Wilfred de Alwis NM, Day CP. Genes and nonalcoholic fatty liver disease. Curr Diab Rep. 2008;8:156-163. [Cited in This Article: ] |
| 55. | Wilfred de Alwis NM, Day CP. Genetics of alcoholic liver disease and nonalcoholic fatty liver disease. Semin Liver Dis. 2007;27:44-54. [Cited in This Article: ] |
| 56. | Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461-1465. [Cited in This Article: ] |
| 57. | Rotman Y, Koh C, Zmuda JM, Kleiner DE, Liang TJ. The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology. 2010;52:894-903. [Cited in This Article: ] |
| 58. | Chalasani N, Guo X, Loomba R, Goodarzi MO, Haritunians T, Kwon S, Cui J, Taylor KD, Wilson L, Cummings OW. Genome-wide association study identifies variants associated with histologic features of nonalcoholic Fatty liver disease. Gastroenterology. 2010;139:1567-1576, 1576.e1-e6. [Cited in This Article: ] |
| 59. | Musso G, Gambino R, De Michieli F, Durazzo M, Pagano G, Cassader M. Adiponectin gene polymorphisms modulate acute adiponectin response to dietary fat: Possible pathogenetic role in NASH. Hepatology. 2008;47:1167-1177. [Cited in This Article: ] |
| 60. | Kotronen A, Yki-Järvinen H, Aminoff A, Bergholm R, Pietiläinen KH, Westerbacka J, Talmud PJ, Humphries SE, Hamsten A, Isomaa B. Genetic variation in the ADIPOR2 gene is associated with liver fat content and its surrogate markers in three independent cohorts. Eur J Endocrinol. 2009;160:593-602. [Cited in This Article: ] |
| 61. | Stefan N, Machicao F, Staiger H, Machann J, Schick F, Tschritter O, Spieth C, Weigert C, Fritsche A, Stumvoll M. Polymorphisms in the gene encoding adiponectin receptor 1 are associated with insulin resistance and high liver fat. Diabetologia. 2005;48:2282-2291. [Cited in This Article: ] |
| 62. | Halberg N, Schraw TD, Wang ZV, Kim JY, Yi J, Hamilton MP, Luby-Phelps K, Scherer PE. Systemic fate of the adipocyte-derived factor adiponectin. Diabetes. 2009;58:1961-1970. [Cited in This Article: ] |
| 63. | Phillips LK, Prins JB. The link between abdominal obesity and the metabolic syndrome. Curr Hypertens Rep. 2008;10:156-164. [Cited in This Article: ] |
| 64. | Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K. Adiponectin and adiponectin receptors in obesity-linked insulin resistance. Novartis Found Symp. 2007;286:164-176; discussion 176-182, 200-203. [Cited in This Article: ] |
| 65. | Neumeier M, Hellerbrand C, Gäbele E, Buettner R, Bollheimer C, Weigert J, Schäffler A, Weiss TS, Lichtenauer M, Schölmerich J. Adiponectin and its receptors in rodent models of fatty liver disease and liver cirrhosis. World J Gastroenterol. 2006;12:5490-5494. [Cited in This Article: ] |
| 66. | Matsuzawa Y. The role of fat topology in the risk of disease. Int J Obes (Lond). 2008;32 Suppl 7:S83-S92. [Cited in This Article: ] |
| 67. | Nakamura Y, Sekikawa A, Kadowaki T, Kadota A, Kadowaki S, Maegawa H, Kita Y, Evans RW, Edmundowicz D, Curb JD. Visceral and subcutaneous adiposity and adiponectin in middle-aged Japanese men: the ERA JUMP study. Obesity (Silver Spring). 2009;17:1269-1273. [Cited in This Article: ] |
| 68. | Yilmaz Y, Yonal O, Kurt R, Alahdab YO, Eren F, Ozdogan O, Celikel CA, Imeryuz N, Kalayci C, Avsar E. Serum levels of omentin, chemerin and adipsin in patients with biopsy-proven nonalcoholic fatty liver disease. Scand J Gastroenterol. 2011;46:91-97. [Cited in This Article: ] |
| 69. | López-Bermejo A, Botas P, Funahashi T, Delgado E, Kihara S, Ricart W, Fernández-Real JM. Adiponectin, hepatocellular dysfunction and insulin sensitivity. Clin Endocrinol (Oxf). 2004;60:256-263. [Cited in This Article: ] |
| 70. | Shimada M, Kawahara H, Ozaki K, Fukura M, Yano H, Tsuchishima M, Tsutsumi M, Takase S. Usefulness of a combined evaluation of the serum adiponectin level, HOMA-IR, and serum type IV collagen 7S level to predict the early stage of nonalcoholic steatohepatitis. Am J Gastroenterol. 2007;102:1931-1938. [Cited in This Article: ] |
| 71. | Wang Y, Lam KS, Xu JY, Lu G, Xu LY, Cooper GJ, Xu A. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem. 2005;280:18341-18347. [Cited in This Article: ] |
| 72. | Okamoto Y, Arita Y, Nishida M, Muraguchi M, Ouchi N, Takahashi M, Igura T, Inui Y, Kihara S, Nakamura T. An adipocyte-derived plasma protein, adiponectin, adheres to injured vascular walls. Horm Metab Res. 2000;32:47-50. [Cited in This Article: ] |
| 73. | Kobayashi K, Inoguchi T, Sonoda N, Sekiguchi N, Nawata H. Adiponectin inhibits the binding of low-density lipoprotein to biglycan, a vascular proteoglycan. Biochem Biophys Res Commun. 2005;335:66-70. [Cited in This Article: ] |
| 74. | Takemura Y, Ouchi N, Shibata R, Aprahamian T, Kirber MT, Summer RS, Kihara S, Walsh K. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest. 2007;117:375-386. [Cited in This Article: ] |
| 75. | Bugianesi E, Pagotto U, Manini R, Vanni E, Gastaldelli A, de Iasio R, Gentilcore E, Natale S, Cassader M, Rizzetto M. Plasma adiponectin in nonalcoholic fatty liver is related to hepatic insulin resistance and hepatic fat content, not to liver disease severity. J Clin Endocrinol Metab. 2005;90:3498-3504. [Cited in This Article: ] |
| 76. | Pagano C, Soardo G, Esposito W, Fallo F, Basan L, Donnini D, Federspil G, Sechi LA, Vettor R. Plasma adiponectin is decreased in nonalcoholic fatty liver disease. Eur J Endocrinol. 2005;152:113-118. [Cited in This Article: ] |
| 77. | Vuppalanchi R, Marri S, Kolwankar D, Considine RV, Chalasani N. Is adiponectin involved in the pathogenesis of nonalcoholic steatohepatitis? A preliminary human study. J Clin Gastroenterol. 2005;39:237-242. [Cited in This Article: ] |
| 78. | Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762-769. [Cited in This Article: ] |
| 79. | Neumeier M, Weigert J, Schäffler A, Weiss T, Kirchner S, Laberer S, Schölmerich J, Buechler C. Regulation of adiponectin receptor 1 in human hepatocytes by agonists of nuclear receptors. Biochem Biophys Res Commun. 2005;334:924-929. [Cited in This Article: ] |
| 80. | Bauer S, Weigert J, Neumeier M, Wanninger J, Schäffler A, Luchner A, Schnitzbauer AA, Aslanidis C, Buechler C. Low-abundant adiponectin receptors in visceral adipose tissue of humans and rats are further reduced in diabetic animals. Arch Med Res. 2010;41:75-82. [Cited in This Article: ] |
| 81. | Weigert J, Neumeier M, Wanninger J, Wurm S, Kopp A, Schober F, Filarsky M, Schäffler A, Zeitoun M, Aslanidis C. Reduced response to adiponectin and lower abundance of adiponectin receptor proteins in type 2 diabetic monocytes. FEBS Lett. 2008;582:1777-1782. [Cited in This Article: ] |
| 82. | Inukai K, Nakashima Y, Watanabe M, Takata N, Sawa T, Kurihara S, Awata T, Katayama S. Regulation of adiponectin receptor gene expression in diabetic mice. Am J Physiol Endocrinol Metab. 2005;288:E876-E882. [Cited in This Article: ] |
| 83. | Tsuchida A, Yamauchi T, Ito Y, Hada Y, Maki T, Takekawa S, Kamon J, Kobayashi M, Suzuki R, Hara K. Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J Biol Chem. 2004;279:30817-30822. [Cited in This Article: ] |
| 84. | Nannipieri M, Cecchetti F, Anselmino M, Mancini E, Marchetti G, Bonotti A, Baldi S, Solito B, Giannetti M, Pinchera A. Pattern of expression of adiponectin receptors in human liver and its relation to nonalcoholic steatohepatitis. Obes Surg. 2009;19:467-474. [Cited in This Article: ] |
| 85. | Ma H, Gomez V, Lu L, Yang X, Wu X, Xiao SY. Expression of adiponectin and its receptors in livers of morbidly obese patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2009;24:233-237. [Cited in This Article: ] |
| 86. | Uribe M, Zamora-Valdés D, Moreno-Portillo M, Bermejo-Martínez L, Pichardo-Bahena R, Baptista-González HA, Ponciano-Rodríguez G, Uribe MH, Medina-Santillán R, Méndez-Sánchez N. Hepatic expression of ghrelin and adiponectin and their receptors in patients with nonalcoholic fatty liver disease. Ann Hepatol. 2008;7:67-71. [Cited in This Article: ] |
| 87. | Shimizu A, Takamura T, Matsuzawa N, Nakamura S, Nabemoto S, Takeshita Y, Misu H, Kurita S, Sakurai M, Yokoyama M. Regulation of adiponectin receptor expression in human liver and a hepatocyte cell line. Metabolism. 2007;56:1478-1485. [Cited in This Article: ] |
| 88. | Kaser S, Moschen A, Cayon A, Kaser A, Crespo J, Pons-Romero F, Ebenbichler CF, Patsch JR, Tilg H. Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut. 2005;54:117-121. [Cited in This Article: ] |
| 89. | Rahman SM, Qadri I, Janssen RC, Friedman JE. Fenofibrate and PBA prevent fatty acid-induced loss of adiponectin receptor and pAMPK in human hepatoma cells and in hepatitis C virus-induced steatosis. J Lipid Res. 2009;50:2193-2202. [Cited in This Article: ] |
| 90. | Koh IU, Lim JH, Joe MK, Kim WH, Jung MH, Yoon JB, Song J. AdipoR2 is transcriptionally regulated by ER stress-inducible ATF3 in HepG2 human hepatocyte cells. FEBS J. 2010;277:2304-2317. [Cited in This Article: ] |
| 91. | Tietge UJ, Böker KH, Manns MP, Bahr MJ. Elevated circulating adiponectin levels in liver cirrhosis are associated with reduced liver function and altered hepatic hemodynamics. Am J Physiol Endocrinol Metab. 2004;287:E82-E89. [Cited in This Article: ] |
| 92. | Qureshi K, Abrams GA. Metabolic liver disease of obesity and role of adipose tissue in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol. 2007;13:3540-3553. [Cited in This Article: ] |
| 93. | Matsubara M, Maruoka S, Katayose S. Inverse relationship between plasma adiponectin and leptin concentrations in normal-weight and obese women. Eur J Endocrinol. 2002;147:173-180. [Cited in This Article: ] |
| 94. | Zietz B, Herfarth H, Paul G, Ehling A, Müller-Ladner U, Schölmerich J, Schäffler A. Adiponectin represents an independent cardiovascular risk factor predicting serum HDL-cholesterol levels in type 2 diabetes. FEBS Lett. 2003;545:103-104. [Cited in This Article: ] |
| 95. | Awazawa M, Ueki K, Inabe K, Yamauchi T, Kaneko K, Okazaki Y, Bardeesy N, Ohnishi S, Nagai R, Kadowaki T. Adiponectin suppresses hepatic SREBP1c expression in an AdipoR1/LKB1/AMPK dependent pathway. Biochem Biophys Res Commun. 2009;382:51-56. [Cited in This Article: ] |
| 96. | Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91-100. [Cited in This Article: ] |
| 97. | Qiao L, Zou C, van der Westhuyzen DR, Shao J. Adiponectin reduces plasma triglyceride by increasing VLDL triglyceride catabolism. Diabetes. 2008;57:1824-1833. [Cited in This Article: ] |
| 98. | Hardie DG, Pan DA. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem Soc Trans. 2002;30:1064-1070. [Cited in This Article: ] |
| 99. | Yamauchi T, Kadowaki T. Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int J Obes (Lond). 2008;32 Suppl 7:S13-S18. [Cited in This Article: ] |
| 100. | Fruchart JC, Duriez P, Staels B. Peroxisome proliferator-activated receptor-alpha activators regulate genes governing lipoprotein metabolism, vascular inflammation and atherosclerosis. Curr Opin Lipidol. 1999;10:245-257. [Cited in This Article: ] |
| 101. | Al-Attas OS, Al-Daghri NM, Al-Rubeaan K, da Silva NF, Sabico SL, Kumar S, McTernan PG, Harte AL. Changes in endotoxin levels in T2DM subjects on anti-diabetic therapies. Cardiovasc Diabetol. 2009;8:20. [Cited in This Article: ] |
| 102. | Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci USA. 1997;94:2557-2562. [Cited in This Article: ] |
| 103. | Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877-1887. [Cited in This Article: ] |
| 104. | Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol. 2009;51:371-379. [Cited in This Article: ] |
| 105. | Matsumoto H, Tamura S, Kamada Y, Kiso S, Fukushima J, Wada A, Maeda N, Kihara S, Funahashi T, Matsuzawa Y. Adiponectin deficiency exacerbates lipopolysaccharide/D-galactosamine-induced liver injury in mice. World J Gastroenterol. 2006;12:3352-3358. [Cited in This Article: ] |
| 106. | Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta. 2007;380:24-30. [Cited in This Article: ] |
| 107. | Devaraj S, Torok N, Dasu MR, Samols D, Jialal I. Adiponectin decreases C-reactive protein synthesis and secretion from endothelial cells: evidence for an adipose tissue-vascular loop. Arterioscler Thromb Vasc Biol. 2008;28:1368-1374. [Cited in This Article: ] |
| 108. | Tsao TS, Murrey HE, Hug C, Lee DH, Lodish HF. Oligomerization state-dependent activation of NF-kappa B signaling pathway by adipocyte complement-related protein of 30 kDa (Acrp30). J Biol Chem. 2002;277:29359-29362. [Cited in This Article: ] |
| 109. | Park PH, McMullen MR, Huang H, Thakur V, Nagy LE. Short-term treatment of RAW264.7 macrophages with adiponectin increases tumor necrosis factor-alpha (TNF-alpha) expression via ERK1/2 activation and Egr-1 expression: role of TNF-alpha in adiponectin-stimulated interleukin-10 production. J Biol Chem. 2007;282:21695-21703. [Cited in This Article: ] |
| 110. | Saijo S, Nagata K, Nakano Y, Tobe T, Kobayashi Y. Inhibition by adiponectin of IL-8 production by human macrophages upon coculturing with late apoptotic cells. Biochem Biophys Res Commun. 2005;334:1180-1183. [Cited in This Article: ] |
| 111. | Tsatsanis C, Zacharioudaki V, Androulidaki A, Dermitzaki E, Charalampopoulos I, Minas V, Gravanis A, Margioris AN. Adiponectin induces TNF-alpha and IL-6 in macrophages and promotes tolerance to itself and other pro-inflammatory stimuli. Biochem Biophys Res Commun. 2005;335:1254-1263. [Cited in This Article: ] |
| 112. | Weigert J, Obermeier F, Neumeier M, Wanninger J, Filarsky M, Bauer S, Aslanidis C, Rogler G, Ott C, Schäffler A. Circulating levels of chemerin and adiponectin are higher in ulcerative colitis and chemerin is elevated in Crohn‘s disease. Inflamm Bowel Dis. 2010;16:630-637. [Cited in This Article: ] |
| 113. | Abke S, Neumeier M, Weigert J, Wehrwein G, Eggenhofer E, Schäffler A, Maier K, Aslanidis C, Schölmerich J, Buechler C. Adiponectin-induced secretion of interleukin-6 (IL-6), monocyte chemotactic protein-1 (MCP-1, CCL2) and interleukin-8 (IL-8, CXCL8) is impaired in monocytes from patients with type I diabetes. Cardiovasc Diabetol. 2006;5:17. [Cited in This Article: ] |
| 114. | Karmiris K, Koutroubakis IE, Xidakis C, Polychronaki M, Voudouri T, Kouroumalis EA. Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:100-105. [Cited in This Article: ] |
| 115. | Rovin BH, Song H. Chemokine induction by the adipocyte-derived cytokine adiponectin. Clin Immunol. 2006;120:99-105. [Cited in This Article: ] |
| 116. | Fantuzzi G. Adiponectin and inflammation: consensus and controversy. J Allergy Clin Immunol. 2008;121:326-330. [Cited in This Article: ] |
| 117. | Luedde T, Beraza N, Kotsikoris V, van Loo G, Nenci A, De Vos R, Roskams T, Trautwein C, Pasparakis M. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11:119-132. [Cited in This Article: ] |
| 118. | Wanninger J, Neumeier M, Weigert J, Bauer S, Weiss TS, Schäffler A, Krempl C, Bleyl C, Aslanidis C, Schölmerich J. Adiponectin-stimulated CXCL8 release in primary human hepatocytes is regulated by ERK1/ERK2, p38 MAPK, NF-kappaB, and STAT3 signaling pathways. Am J Physiol Gastrointest Liver Physiol. 2009;297:G611-G618. [Cited in This Article: ] |
| 119. | Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605-1621. [Cited in This Article: ] |
| 120. | Hanson JC, Bostick MK, Campe CB, Kodali P, Lee G, Yan J, Maher JJ. Transgenic overexpression of interleukin-8 in mouse liver protects against galactosamine/endotoxin toxicity. J Hepatol. 2006;44:359-367. [Cited in This Article: ] |
| 121. | Jung TW, Lee YJ, Lee MW, Kim SM, Jung TW. Full-length adiponectin protects hepatocytes from palmitate-induced apoptosis via inhibition of c-Jun NH2 terminal kinase. FEBS J. 2009;276:2278-2284. [Cited in This Article: ] |
| 122. | Wedemeyer I, Bechmann LP, Odenthal M, Jochum C, Marquitan G, Drebber U, Gerken G, Gieseler RK, Dienes HP, Canbay A. Adiponectin inhibits steatotic CD95/Fas up-regulation by hepatocytes: therapeutic implications for hepatitis C. J Hepatol. 2009;50:140-149. [Cited in This Article: ] |
| 123. | Fujita K, Nishizawa H, Funahashi T, Shimomura I, Shimabukuro M. Systemic oxidative stress is associated with visceral fat accumulation and the metabolic syndrome. Circ J. 2006;70:1437-1442. [Cited in This Article: ] |
| 124. | Kamada Y, Tamura S, Kiso S, Matsumoto H, Saji Y, Yoshida Y, Fukui K, Maeda N, Nishizawa H, Nagaretani H. Enhanced carbon tetrachloride-induced liver fibrosis in mice lacking adiponectin. Gastroenterology. 2003;125:1796-1807. [Cited in This Article: ] |
| 125. | Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105:1067-1075. [Cited in This Article: ] |
| 126. | Weltman MD, Farrell GC, Liddle C. Increased hepatocyte CYP2E1 expression in a rat nutritional model of hepatic steatosis with inflammation. Gastroenterology. 1996;111:1645-1653. [Cited in This Article: ] |
| 127. | Garattini E, Fratelli M, Terao M. Mammalian aldehyde oxidases: genetics, evolution and biochemistry. Cell Mol Life Sci. 2008;65:1019-1048. [Cited in This Article: ] |
| 128. | Kundu TK, Hille R, Velayutham M, Zweier JL. Characterization of superoxide production from aldehyde oxidase: an important source of oxidants in biological tissues. Arch Biochem Biophys. 2007;460:113-121. [Cited in This Article: ] |
| 129. | Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332-339. [Cited in This Article: ] |
| 130. | Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med. 2006;10:76-99. [Cited in This Article: ] |
| 131. | Tomita K, Oike Y, Teratani T, Taguchi T, Noguchi M, Suzuki T, Mizutani A, Yokoyama H, Irie R, Sumimoto H. Hepatic AdipoR2 signaling plays a protective role against progression of nonalcoholic steatohepatitis in mice. Hepatology. 2008;48:458-473. [Cited in This Article: ] |
| 132. | Ding X, Saxena NK, Lin S, Xu A, Srinivasan S, Anania FA. The roles of leptin and adiponectin: a novel paradigm in adipocytokine regulation of liver fibrosis and stellate cell biology. Am J Pathol. 2005;166:1655-1669. [Cited in This Article: ] |
| 133. | Adachi M, Brenner DA. High molecular weight adiponectin inhibits proliferation of hepatic stellate cells via activation of adenosine monophosphate-activated protein kinase. Hepatology. 2008;47:677-685. [Cited in This Article: ] |
| 134. | Handy JA, Saxena NK, Fu P, Lin S, Mells JE, Gupta NA, Anania FA. Adiponectin activation of AMPK disrupts leptin-mediated hepatic fibrosis via suppressors of cytokine signaling (SOCS-3). J Cell Biochem. 2010;110:1195-1207. [Cited in This Article: ] |
| 135. | Jardé T, Caldefie-Chézet F, Goncalves-Mendes N, Mishellany F, Buechler C, Penault-Llorca F, Vasson MP. Involvement of adiponectin and leptin in breast cancer: clinical and in vitro studies. Endocr Relat Cancer. 2009;16:1197-1210. [Cited in This Article: ] |
| 136. | Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705-1725. [Cited in This Article: ] |
| 137. | Shah K, Stufflebam A, Hilton TN, Sinacore DR, Klein S, Villareal DT. Diet and exercise interventions reduce intrahepatic fat content and improve insulin sensitivity in obese older adults. Obesity (Silver Spring). 2009;17:2162-2168. [Cited in This Article: ] |
| 138. | Johnson NA, Sachinwalla T, Walton DW, Smith K, Armstrong A, Thompson MW, George J. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology. 2009;50:1105-1112. [Cited in This Article: ] |
| 139. | Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, Fava JL, Wing RR. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121-129. [Cited in This Article: ] |
| 140. | Palmer M, Schaffner F. Effect of weight reduction on hepatic abnormalities in overweight patients. Gastroenterology. 1990;99:1408-1413. [Cited in This Article: ] |
| 141. | Pasarica M, Tchoukalova YD, Heilbronn LK, Fang X, Albu JB, Kelley DE, Smith SR, Ravussin E. Differential effect of weight loss on adipocyte size subfractions in patients with type 2 diabetes. Obesity (Silver Spring). 2009;17:1976-1978. [Cited in This Article: ] |
| 142. | Laurin J, Lindor KD, Crippin JS, Gossard A, Gores GJ, Ludwig J, Rakela J, McGill DB. Ursodeoxycholic acid or clofibrate in the treatment of non-alcohol-induced steatohepatitis: a pilot study. Hepatology. 1996;23:1464-1467. [Cited in This Article: ] |
| 143. | Athyros VG, Mikhailidis DP, Didangelos TP, Giouleme OI, Liberopoulos EN, Karagiannis A, Kakafika AI, Tziomalos K, Burroughs AK, Elisaf MS. Effect of multifactorial treatment on non-alcoholic fatty liver disease in metabolic syndrome: a randomised study. Curr Med Res Opin. 2006;22:873-883. [Cited in This Article: ] |
| 144. | Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003;38:1008-1017. [Cited in This Article: ] |
| 145. | Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Sponseller CA, Hampton K, Bacon BR. Interim results of a pilot study demonstrating the early effects of the PPAR-gamma ligand rosiglitazone on insulin sensitivity, aminotransferases, hepatic steatosis and body weight in patients with non-alcoholic steatohepatitis. J Hepatol. 2003;38:434-440. [Cited in This Article: ] |
| 146. | Sun K, Wang Q, Huang XH. PPAR gamma inhibits growth of rat hepatic stellate cells and TGF beta-induced connective tissue growth factor expression. Acta Pharmacol Sin. 2006;27:715-723. [Cited in This Article: ] |
| 147. | Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, Ouchi N. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094-2099. [Cited in This Article: ] |
| 148. | Gastaldelli A, Harrison S, Belfort-Aguiar R, Hardies J, Balas B, Schenker S, Cusi K. Pioglitazone in the treatment of NASH: the role of adiponectin. Aliment Pharmacol Ther. 2010;32:769-775. [Cited in This Article: ] |
| 149. | Gastaldelli A, Harrison SA, Belfort-Aguilar R, Hardies LJ, Balas B, Schenker S, Cusi K. Importance of changes in adipose tissue insulin resistance to histological response during thiazolidinedione treatment of patients with nonalcoholic steatohepatitis. Hepatology. 2009;50:1087-1093. [Cited in This Article: ] |
| 150. | Bouhlel MA, Derudas B, Rigamonti E, Dièvart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137-143. [Cited in This Article: ] |
| 151. | Satapathy SK, Sakhuja P, Malhotra V, Sharma BC, Sarin SK. Beneficial effects of pentoxifylline on hepatic steatosis, fibrosis and necroinflammation in patients with non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2007;22:634-638. [Cited in This Article: ] |
| 152. | Lee YM, Sutedja DS, Wai CT, Dan YY, Aung MO, Zhou L, Cheng CL, Wee A, Lim SG. A randomized controlled pilot study of Pentoxifylline in patients with non-alcoholic steatohepatitis (NASH). Hepatol Int. 2008;2:196-201. [Cited in This Article: ] |
| 153. | Yakaryilmaz F, Guliter S, Savas B, Erdem O, Ersoy R, Erden E, Akyol G, Bozkaya H, Ozenirler S. Effects of vitamin E treatment on peroxisome proliferator-activated receptor-alpha expression and insulin resistance in patients with non-alcoholic steatohepatitis: results of a pilot study. Intern Med J. 2007;37:229-235. [Cited in This Article: ] |
| 154. | Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA. 2009;106:8665-8670. [Cited in This Article: ] |