Published online Jan 14, 2011. doi: 10.3748/wjg.v17.i2.249
Revised: September 26, 2010
Accepted: October 3, 2010
Published online: January 14, 2011
AIM: To study the association between hilar cholangiocarcinoma (HC) and pre-existing medical conditions.
METHODS: Three hundred and thirteen HC patients admitted to the Eastern Hepatobiliary Surgery Hospital (Shanghai, China) in 2000-2005 and 608 healthy controls were enrolled in this study. Association between HC and pre-existing medical conditions was studied with their adjusted odds ratio (OR) calculated by logistic regression analysis.
RESULTS: The prevalence of choledocholithiasis (adjusted OR = 2.704, P = 0.039), hepatolithiasis (adjusted OR = 3.278, P = 0.018), cholecystolithiasis (adjusted OR = 4.499, P < 0.0001), cholecystectomy (adjusted OR = 7.012, P = 0.004), biliary ascariasis (adjusted OR = 7.188, P = 0.001), liver fluke (adjusted OR = 10.088, P = 0.042) and liver schistosomiasis (adjusted OR = 9.913, P = 0.001) was higher in HC patients than in healthy controls.
CONCLUSION: Biliary tract stone disease (choledocholithiasis, hepatolithiasis, cholecystolithiasis) and parasitic liver disease (biliary ascariasis, liver fluke, liver schistosomiasis) are the risk factors for HC in Chinese population.
- Citation: Cai WK, Sima H, Chen BD, Yang GS. Risk factors for hilar cholangiocarcinoma: A case-control study in China. World J Gastroenterol 2011; 17(2): 249-253
- URL: https://www.wjgnet.com/1007-9327/full/v17/i2/249.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i2.249
Hilar cholangiocarcinoma (HC) is a rare and highly malignant cancer of the bile duct, accounting for less than 2% of all human malignancies. Although the entire biliary tree is a potential risk, tumors involving the biliary confluence or the right or left hepatic ducts are most common, accounting for 40%-60% of all tumors. Its etiology remains poorly understood and its incidence has been increasing in China. The long-term prognosis of HC is poor due to its early metastasis. Complete resection has been recognized as the most effective therapy for HC. However, surgical management still remains a major challenge because of its location close to the portal vein, hepatic artery, and liver parenchyma. So far, most studies have been focused on the risk factors for intrahepatic cholangiocarcinoma (ICC)[1-3], while few studies on the risk factors for HC are available[4]. Due to the increasing incidence and poor long-term prognosis of HC, it is extremely important to evaluate the risk factors for HC in order to decrease its incidence.
Three hundred and thirteen HC patients who received surgical dissection at the Eastern Hepatobiliary Surgery Hospital of the Second Military Medical University (Shanghai, China) from January 2000 to December 2005 were included in this study. HC was diagnosed by pathological examination of samples taken from HC patients. Those who were diagnosed as HC but did not undergo surgery were excluded from the study.
Six hundred and eight healthy individuals who visited the Eastern hepatobiliary Surgery Hospital of the Second Military Medical University for a routine checkup served as controls and were matched to the HC patients for sex and age (± 4 years). Moreover, the years of search were matched in HC patients and controls for risk factors to minimize their difference.
Several potential risk factors for HC were studied and divided into 3 broad categories: biliary tract condition and operation, infectious diseases, and miscellaneous potential risk factors. Biliary tract condition and operation included choledocholithiasis, hepatolithiasis, cholecystolithiasis, primary sclerosing cholangitis (PSC), parasitic liver disease and cholecystectomy. Infectious diseases included hepatitis B virus (HBV) and hepatitis C virus (HCV) infection. Miscellaneous potential risk factors included alcoholic liver disease, type II diabetes mellitus, smoking and ulcerative colitis.
Prevalence of HC and potential risk factors for HC were compared in HC patients and controls. χ2 test or Fisher’s exact test was used for categorical variables, and t test was used for discrete variables.
Variables with a P value < 0.05 were considered statistically significant. Odds ratio (OR) and 95% CI of each risk factor for HC were computed as estimate of the relative risk by unconditional logistic regression analysis, using the maximum-likelihood estimate.
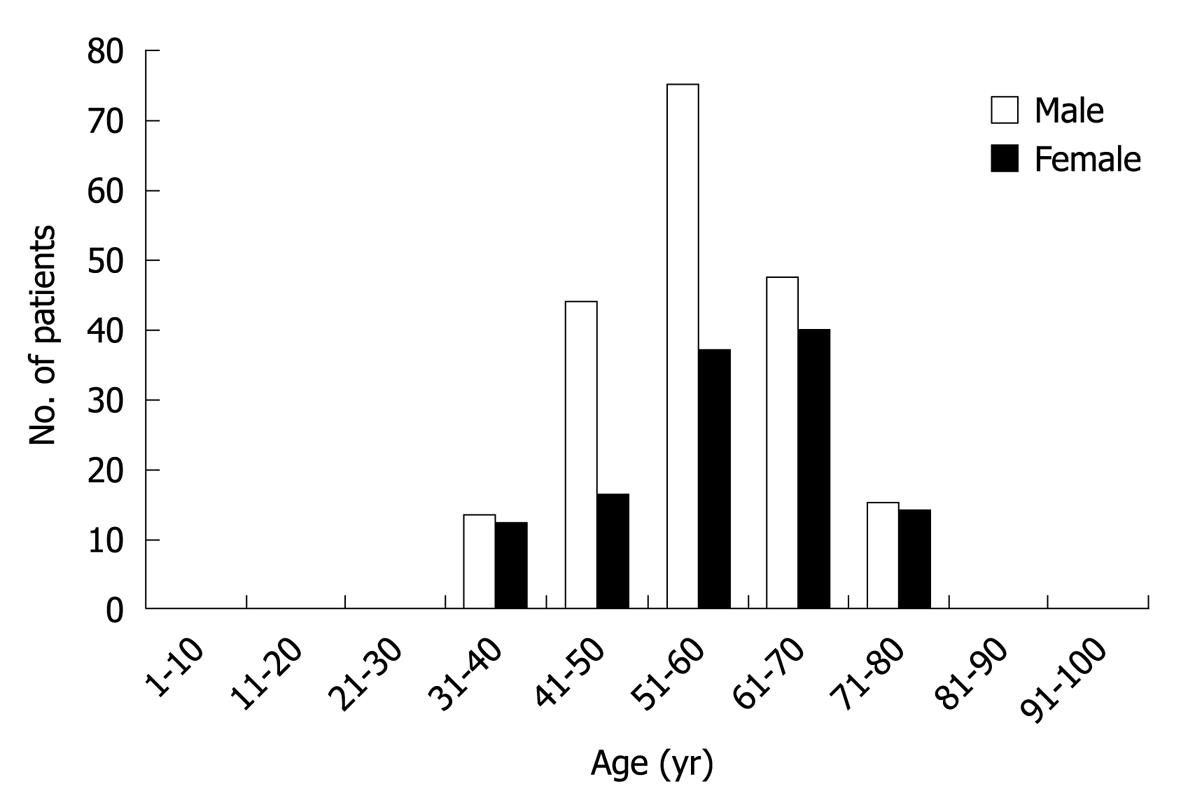
A total of 313 patients (194 men and 119 women with male-to-female ratio of 1.63:1) diagnosed as HC were enrolled in the present study. Their mean age was 56.64 ± 10.585 years (range 32-80 years). Most HC developed during the fourth to sixth decade, and reached the peak at the age of 55 years (Figure 1).
The distribution of HC in controls and HC patients according to their age and gender is shown in Table 1. No significant difference was found in gender and mean age between HC patients and controls, suggesting that the pairing is effective.
| Variable | HC patients (n = 313) | Controls (n = 608) | P |
| Mean age | 56.64 ± 10.585 | 55.58 ± 10.792 | 0.895 |
| Gender | |||
| Male | 194 | 380 | 0.878 |
| Female | 119 | 228 |
The risk factors were divided into 3 categories: biliary tract condition and operation, infectious diseases and other risk factors. In biliary tract condition and operation, the prevalence of choledocholithiasis, hepatolithiasis cholecystolithiasis, cholecystectomy, biliary ascariasis, liver schistosomiasis and liver fluke was higher in HC patients than in controls (P < 0.05), while no significant difference was found in the prevalence of PSC between HC patients and controls. In infectious diseases, the prevalence of HBV infection was 7.3% in HC patients and 6.3% in controls (P = 0.526), the prevalence of HCV infection was1.0% in HC patients and 2.1% in controls (P = 0.151). In other risk factors, the prevalence of ulcerative colitis (UC), alcoholic liver disease, type II diabetes mellitus and smoking was 0.3%, 0.6%, 5.4% and 22%, respectively, in HC patients, and 0.0%, 0.3%, 6.1% and 20.0%, respectively, in controls.
The distribution of risk factors in each category is summarized in Table 2.
| Risk factors | Controls (n = 608) | HC patients (n = 313) | P |
| Biliary tract condition and operation | |||
| Choledocholithiasis | 8 (1.3) | 28 (8.9) | < 0.0001 |
| Hepatolithiasis | 7 (1.2) | 25 (8.0) | < 0.0001 |
| Cholecystolithiasis | 45 (7.4) | 98 (31.3) | < 0.0001 |
| Cholecystectomy | 3 (0.5) | 23 (7.3) | < 0.0001 |
| PSC | 0 (0.0) | 2 (0.6) | 0.115 |
| Biliary ascariasis | 4 (0.7) | 15 (4.8) | < 0.0001 |
| Liver fluke | 1 (0.2) | 4 (1.3) | 0.048 |
| Liver schistosomiasis | 3 (0.5) | 12 (3.8) | < 0.0001 |
| Infectious etiologies | |||
| HBV infection | 38 (6.3) | 23 (7.3) | 0.526 |
| HCV infection | 13 (2.1) | 3 (1.0) | 0.151 |
| Other risk factors | |||
| UC | 0 (0.0) | 1 (0.3) | 0.340 |
| Alcoholic liver disease | 2 (0.3) | 2 (0.6) | 0.498 |
| Diabetes mellitus type II | 37 (6.1) | 17 (5.4) | 0.689 |
| Smoking | 134 (22.0) | 63 (20.0) | 0.503 |
Logistic regression model was used to adjust the demographics (age, sex), and the adjusted OR and 95% CI for the different risk factors were calculated. Multivariate analysis showed that choledocholithiasis, hepatolithiasis, cholecystolithiasis, cholecystectomy, biliary ascariasis, liver fluke and liver schistosomiasis were the risk factors for HC, while HBV infection, HCV infection, PSC, UC, alcoholic liver disease, type II diabetes mellitus and smoking were not the significant risk factors for HC (Table 3).
| Risk factors | P | OR | 95% CI | |
| Lower | Upper | |||
| Choledocholithiasis | 0.039 | 2.704 | 1.054 | 6.941 |
| Hepatolithiasis | 0.018 | 3.278 | 1.226 | 8.766 |
| Cholecystolithiasis | < 0.0001 | 4.499 | 2.990 | 6.769 |
| Cholecystectomy | 0.004 | 7.012 | 1.895 | 25.954 |
| Biliary ascariasis | 0.001 | 7.188 | 2.245 | 23.015 |
| Liver fluke | 0.042 | 10.088 | 1.085 | 93.775 |
| Liver schistosomiasis | 0.001 | 9.913 | 2.702 | 36.369 |
In this case-control study, the risk factors for HC were examined in Chinese population, including choledocholithiasis, hepatolithiasis, cholecystolithiasis, cholecystectomy, biliary ascariasis, liver fluke and liver schistosomiasis. Although HBV and HCV infection have been reported to be intimately associated with intrahepatic cholangiocarcinoma (ICC)[1,2], they were not demonstrated to be significantly associated with HC in our study. In addition, PSC, UC, alcoholic liver disease, type II diabetes mellitus and smoking were not significantly related to HC.
The prevalence of biliary tract stone disease (hepatolithiasis, cholecystolithiasis and choledocholithiasis) and cholecystectomy was higher in HC patients than in controls. Hepatolithiasis is more frequently seen in East Asian countries than in Western countries, and represents a high-risk factor for ICC due to inflammation and epithelial proliferation[3]. In parallel, extrahepatic lithiasis may promote chronic inflammatory changes in extrahepatic bile ducts, thus increasing the risk of developing extrahepatic cholangiocarcinoma (ECC), which can explain why choledocholithiasis increases the risk of developing HC. However, the reasons why hepatolithiasis increases the risk of developing HC are less clear. It was reported that cholecystolithiasis is a risk factor for ECC according to its ecological and epidemiological evidence, and the incidence of HC is decreased 10 or more years after cholecystectomy for cholecystolithiasis[5,6]. In our study, the prevalence of both cholecystolithiasis and cholecystectomy was higher in HC patients than in controls. Cholecystectomy was not a risk factor for HC because the majority of surgical procedures (76%) were performed within a year prior to tumor diagnosis, which is consistent with the findings in a recent US case-control study[7].
Chronic infectious liver diseases, such as HBV and HCV infection, were not significantly associated with HC in our study. The prevalence of HBV and HCV infection was 7.3% and 1.0%, respectively, in HC patients, and 6.3% and 2.1%, respectively, in controls. The prevalence of HBV is rather high in China, and HBV is strongly associated with hepatocellular carcinoma (HCC). Moreover, the HBV genome has been detected not only in infected hepatocytes but also in bile duct epithelial, endothelial, and smooth muscle cells[8]. It has been shown that HBV is also an independent risk factor for ICC in Chinese population[9]. In the present study, HBV was not associated with HC, which is, however, not consistent with the reported findings in China[10], suggesting that HBV is related to ECC. It was reported that HCV can also damage the bile duct epithelial cells, thus leading to a range of proliferative, inflammatory, and generative changes[11]. Recent studies from Korea, Japan and Italy showed that HCV is associated with cholangiocarcinoma[12-14]. A population-based case-control study in US[6] showed that HCV is associated with ICC, but not with ECC. This study also showed that HCV was not a risk factor for HC. Although both HBV and HCV can be detected in hepatic bile ducts, most studies demonstrated that they are related only to intrahepatic bile duct carcinoma[15,16]. The detailed mechanism still needs further investigation.
It has been shown that parasitic liver disease is related with cholangiocarcinoma[17-19]. In the present study, biliary ascariasis, liver fluke (Clonorchis sinensis) and liver schistosomiasis were significantly associated with HC in Chinese population. Ascariasis is endemic in China and 4.8% cases had a history of biliary ascariasis in our study. Ascaris invasion into the bile duct may cause biliary colic, pyogenic cholangitis, pancreatitis and septicemia. A residual dead worm may destroy the biliary epithelial cells, resulting in fibrosis or nidi that may form stones. It was reported that ova and fragments of ascaris can serve as for stone formation[20] and choledocholithiasis is a risk factor for HC. The bile duct damage induced by ascaris, worm and stone formation may play an important role in genesis and development of cholangiocarcinoma. Clonorchis sinensis infestation is common in East Asia countries, including China, Korea, and Far East Russia, where the local inhabitants are used to eat raw fish or shrimp[21]. In our study, 4 patients were infected with Clonorchis sinensis and had a history of eating fresh fish and shrimp. An epidemiological study from Thailand suggested that liver fluke (opisthorchis viverrini) is associated with cholangiocarcinoma[22]. A hospital-based case control study in Korea also revealed that Clonorchis sinensis is strongly associated with cholangiocarcinoma[23]. Schistosomiasis is the second most common parasitic infection worldwide after malaria. Schistosomiasis Japonica, occurring in Japan and China, may be a risk factor and an independent adverse prognostic factor for HCC[24,25]. Besides, it has been shown that schistosomiasis Japonica is a risk factor for HC in Egyptian[4]. Our study also confirmed that schistosomiasis Japonica was associated with HC in Chinese population. Carcinogenesis occurs in patients with parasitic infection, because the existence of parasites within the host induces chronic inflammation[26,27]. Phagocytes release reactive oxygen and nitrogen species in patients with chronic inflammation are potential to damage DNA, proteins and cell membranes, modulate enzyme activities and gene expression, thus promoting carcinogenesis[28-30].
In the Western world, the most common risk factor for HC is PSC[31,32], an inflammatory disease of the bile duct, which is in turn closely associated with UC. The prevalence of UC in patients with PSC is 70%-100%[33-35]. It was reported that the lifetime risk of developing cholangiocarcinoma in patients with PSC is 10%-15%[36]. Parker et al[37] described the association between UC and cholangiocarcinoma in 1954. Converse et al[38] in 1971 found that the incidence of HC is higher in patients with PSC than in those with UC. The prevalence of PSC and UC is higher in Western world than in Asia[39]. No epidemiological investigation of PSC and UC is available in China. In our study, PSC was diagnosed in 2 patients and UC in 1 patient. However, no PSC or UC was observed in controls.
It was reported that alcoholic liver disease and type II diabetes mellitus are related to extrahepatic cholangiocarcinoma in US population[40]. However, our study demonstrated that they were not significantly associated with HC in Chinese population.
Our study had two potential limitations. One is that clinical records were used as the source for risk factor information, and the other is that most patients and controls came from certain geographic regions, which may omit some risk factors prevalent in other regions of China.
In summary, cholecystolithiasis, hepatolithiasis, choledocholithiasis, biliary ascariasis, liver fluke and liver schistosomiasis are the risk factors for HC, while PSC and UC are the suspected risk factors for HC in China. Further study is needed to explore the role of these risk factors in the development of HC.
Although several risk factors are associated with the development of hilar cholangiocarcinoma (HC), such as primary sclerosing cholangitis (PSC), liver fluke or biliary tract stone disease, the risk factors for HC in Chinese patients have not been fully studied.
The etiology of cholangiocarcinoma has been well studied, and several risk factors for HC have been documented. Chronic infectious liver diseases, such as hepatitis B virus (HBV) and hepatitis C virus (HCV) infection, have been fully investigated in recent studies and are considered as the important risk factors for cholangiocarcinoma.
In this study, neither HBV infection nor HCV infection was associated with HC in Chinese population, which is not consistent with previous studies. PSC and ulcerative colitis were not the risk factors for HC possibly due to their low incidence in Chinese population. In addition to liver fluke, biliary ascariasis and liver schistosomiasis are also the risk factors for HC, which have not been reported in previous studies.
HC is a rare malignant disease with a poor prognosis. The findings in this study may contribute to its control.
The authors investigated the potential risk factors associated with HC, and demonstrated that biliary tract stone and parasitic liver disease are the risk factor for HC, which may contribute to its control.
Peer reviewer: Ching-Chung Lin, Division of Gastroenterology, Department of Internal Medicine, Mackay Memorial Hospital, Taipei 111, Taiwan, China
S- Editor Sun H L- Editor Wang XL E- Editor Zheng XM
| 1. | Gatselis NK, Tepetes K, Loukopoulos A, Vasiou K, Zafiriou A, Gioti C, Dalekos GN. Hepatitis B virus and intrahepatic cholangiocarcinoma. Cancer Invest. 2007;25:55-58. |
| 2. | Shaib YH, El-Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005;128:620-626. |
| 3. | Kuroki T, Tajima Y, Kanematsu T. Hepatolithiasis and intrahepatic cholangiocarcinoma: carcinogenesis based on molecular mechanisms. J Hepatobiliary Pancreat Surg. 2005;12:463-466. |
| 4. | Abdel Wahab M, Mostafa M, Salah T, Fouud A, Kandeel T, Elshobary M, Abd Allah OF, Elghawalby N, Sultan A, Ezzat F. Epidemiology of hilar cholangiocarcinoma in Egypt: single center study. Hepatogastroenterology. 2007;54:1626-1631. |
| 5. | Chow WH, Johansen C, Gridley G, Mellemkjaer L, Olsen JH, Fraumeni JF Jr. Gallstones, cholecystectomy and risk of cancers of the liver, biliary tract and pancreas. Br J Cancer. 1999;79:640-644. |
| 6. | Ekbom A, Hsieh CC, Yuen J, Trichopoulos D, McLaughlin JK, Lan SJ, Adami HO. Risk of extrahepatic bileduct cancer after cholecystectomy. Lancet. 1993;342:1262-1265. |
| 7. | Welzel TM, Graubard BI, El-Serag HB, Shaib YH, Hsing AW, Davila JA, McGlynn KA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol. 2007;5:1221-1228. |
| 8. | Blum HE, Stowring L, Figus A, Montgomery CK, Haase AT, Vyas GN. Detection of hepatitis B virus DNA in hepatocytes, bile duct epithelium, and vascular elements by in situ hybridization. Proc Natl Acad Sci USA. 1983;80:6685-6688. |
| 9. | Zhou HB, Wang H, Zhou DX, Wang H, Wang Q, Zou SS, Hu HP. Etiological and clinicopathologic characteristics of intrahepatic cholangiocarcinoma in young patients. World J Gastroenterol. 2010;16:881-885. |
| 10. | Hsing AW, Zhang M, Rashid A, McGlynn KA, Wang BS, Niwa S, Ortiz-Conde BA, Goedert JJ, Fraumeni JF Jr, O'Brien TR. Hepatitis B and C virus infection and the risk of biliary tract cancer: a population-based study in China. Int J Cancer. 2008;122:1849-1853. |
| 11. | Ahrendt SA, Nakeeb A, Pitt HA. Cholangiocarcinoma. Clin Liver Dis. 2001;5:191-218. |
| 12. | Chen RF, Li ZH, Zou SQ, Chen JS. Effect of hepatitis C virus core protein on modulation of cellular proliferation and apoptosis in hilar cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2005;4:71-74. |
| 13. | Donato F, Gelatti U, Tagger A, Favret M, Ribero ML, Callea F, Martelli C, Savio A, Trevisi P, Nardi G. Intrahepatic cholangiocarcinoma and hepatitis C and B virus infection, alcohol intake, and hepatolithiasis: a case-control study in Italy. Cancer Causes Control. 2001;12:959-964. |
| 14. | Kobayashi M, Ikeda K, Saitoh S, Suzuki F, Tsubota A, Suzuki Y, Arase Y, Murashima N, Chayama K, Kumada H. Incidence of primary cholangiocellular carcinoma of the liver in japanese patients with hepatitis C virus-related cirrhosis. Cancer. 2000;88:2471-2477. |
| 15. | Perumal V, Wang J, Thuluvath P, Choti M, Torbenson M. Hepatitis C and hepatitis B nucleic acids are present in intrahepatic cholangiocarcinomas from the United States. Hum Pathol. 2006;37:1211-1216. |
| 16. | Lee TY, Lee SS, Jung SW, Jeon SH, Yun SC, Oh HC, Kwon S, Lee SK, Seo DW, Kim MH. Hepatitis B virus infection and intrahepatic cholangiocarcinoma in Korea: a case-control study. Am J Gastroenterol. 2008;103:1716-1720. |
| 17. | Akai PS, Pungpak S, Chaicumpa W, Viroj K, Bunnag D, Befus AD. Serum antibody response to Opisthorchis viverrini antigen as a marker for opisthorchiasis-associated cholangiocarcinoma. Trans R Soc Trop Med Hyg. 1994;88:471-474. |
| 19. | Andoh H, Yasui O, Kurokawa T, Sato T. Cholangiocarcinoma coincident with schistosomiasis japonica. J Gastroenterol. 2004;39:64-68. |
| 20. | Wani NA, Chrungoo RK. Biliary ascariasis: surgical aspects. World J Surg. 1992;16:976-979. |
| 21. | Choi D, Lim JH, Lee KT, Lee JK, Choi SH, Heo JS, Choi DW, Jang KT, Lee NY, Kim S. Gallstones and Clonorchis sinensis infection: a hospital-based case-control study in Korea. J Gastroenterol Hepatol. 2008;23:e399-e404. |
| 22. | Kurathong S, Lerdverasirikul P, Wongpaitoon V, Pramoolsinsap C, Kanjanapitak A, Varavithya W, Phuapradit P, Bunyaratvej S, Upatham ES, Brockelman WY. Opisthorchis viverrini infection and cholangiocarcinoma. A prospective, case-controlled study. Gastroenterology. 1985;89:151-156. |
| 23. | Choi D, Lim JH, Lee KT, Lee JK, Choi SH, Heo JS, Jang KT, Lee NY, Kim S, Hong ST. Cholangiocarcinoma and Clonorchis sinensis infection: a case-control study in Korea. J Hepatol. 2006;44:1066-1073. |
| 24. | Iida F, Iida R, Kamijo H, Takaso K, Miyazaki Y, Funabashi W, Tsuchiya K, Matsumoto Y. Chronic Japanese schistosomiasis and hepatocellular carcinoma: ten years of follow-up in Yamanashi Prefecture, Japan. Bull World Health Organ. 1999;77:573-581. |
| 25. | Matsuda M, Fujii H. Chronic schistosomiasis japonica is an independent adverse prognostic factor for survival in hepatocellular carcinoma patients who have undergone hepatic resection: clinicopathological and prognostic analysis of 198 consecutive patients. World J Surg. 2009;33:2644-2650. |
| 27. | Haswell-Elkins MR, Sithithaworn P, Mairiang E, Elkins DB, Wongratanacheewin S, Kaewkes S, Mairiang P. Immune responsiveness and parasite-specific antibody levels in human hepatobiliary disease associated with Opisthorchis viverrini infection. Clin Exp Immunol. 1991;84:213-218. |
| 28. | Satarug S, Haswell-Elkins MR, Tsuda M, Mairiang P, Sithithaworn P, Mairiang E, Esumi H, Sukprasert S, Yongvanit P, Elkins DB. Thiocyanate-independent nitrosation in humans with carcinogenic parasite infection. Carcinogenesis. 1996;17:1075-1081. |
| 29. | Thamavit W, Pairojkul C, Tiwawech D, Shirai T, Ito N. Strong promoting effect of Opisthorchis viverrini infection on dimethylnitrosamine-initiated hamster liver. Cancer Lett. 1994;78:121-125. |
| 30. | Satarug S, Lang MA, Yongvanit P, Sithithaworn P, Mairiang E, Mairiang P, Pelkonen P, Bartsch H, Haswell-Elkins MR. Induction of cytochrome P450 2A6 expression in humans by the carcinogenic parasite infection, opisthorchiasis viverrini. Cancer Epidemiol Biomarkers Prev. 1996;5:795-800. |
| 31. | Jarnagin W, Winston C. Hilar cholangiocarcinoma: diagnosis and staging. HPB (Oxford). 2005;7:244-251. |
| 32. | Yachimski P, Pratt DS. Cholangiocarcinoma: natural history, treatment, and strategies for surveillance in high-risk patients. J Clin Gastroenterol. 2008;42:178-190. |
| 33. | Wiesner RH, LaRusso NF. Clinicopathologic features of the syndrome of primary sclerosing cholangitis. Gastroenterology. 1980;79:200-206. |
| 34. | Broomé U, Olsson R, Lööf L, Bodemar G, Hultcrantz R, Danielsson A, Prytz H, Sandberg-Gertzén H, Wallerstedt S, Lindberg G. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut. 1996;38:610-615. |
| 35. | Schrumpf E, Abdelnoor M, Fausa O, Elgjo K, Jenssen E, Kolmannskog F. Risk factors in primary sclerosing cholangitis. J Hepatol. 1994;21:1061-1066. |
| 36. | Burak K, Angulo P, Pasha TM, Egan K, Petz J, Lindor KD. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol. 2004;99:523-526. |
| 38. | Converse CF, Reagan JW, DeCosse JJ. Ulcerative colitis and carcinoma of the bile ducts. Am J Surg. 1971;121:39-45. |
| 39. | Weismüller TJ, Wedemeyer J, Kubicka S, Strassburg CP, Manns MP. The challenges in primary sclerosing cholangitis--aetiopathogenesis, autoimmunity, management and malignancy. J Hepatol. 2008;48 Suppl 1:S38-S57. |
| 40. | Chalasani N, Baluyut A, Ismail A, Zaman A, Sood G, Ghalib R, McCashland TM, Reddy KR, Zervos X, Anbari MA. Cholangiocarcinoma in patients with primary sclerosing cholangitis: a multicenter case-control study. Hepatology. 2000;31:7-11. |