Published online May 7, 2011. doi: 10.3748/wjg.v17.i17.2172
Revised: December 9, 2010
Accepted: December 16, 2010
Published online: May 7, 2011
Mallory-Denk Bodies (MDB) are important as investigators, suggesting MDB as an indicator of the histologic severity of chronic hepatitis, causes of which include hepatitis C, primary biliary cirrhosis (PBC), and nonalcoholic fatty liver disease (NAFLD). Matteoni et al scored MDB in patients with NAFLD as none, rare and many, and reported that MDB plays a prominent role in this classification scheme in an earlier classification system. In this study, we evaluated 258 patients with chronic hepatitis due to metabolic, autoimmune and viral etiologies. Liver biopsy samples were evaluated with hematoxylin and eosin, periodic acid-Schiff-diastase, Gordon and Sweet’s reticulin, Masson’s trichrome, and iron stains. Both staging and grading were performed. Additionally, MDB were evaluated and discussed for each disease. We examined patients with nonalcoholic steatohepatitis (NASH; 50 patients), alcoholic hepatitis (10 patients), PBC (50 patients), Wilson disease (WD; 20 patients), hepatitis B (50 patients), hepatitis C (50 patients) and hepatocellular carcinoma (HCC; 30 patients). Frequency of MDB was as follows; NASH: 10 patients with mild in 60% and moderate in 40% and observed in every stage of the disease and frequently seen in zone 3. PBC: 11 patients with mild in 10%, moderate in 70%, and cirrhosis in 20%, and frequently seen in zone 1. WD: 16 patients with moderate and severe in 60% and cirrhosis in 40% and frequently seen in zone 1. Hep B: 3 patients with mild in 66% and severe in 34%. Hep C: 7 patients with mild in 40% and moderate in 60% and observed in every stage. HCC: 3 patients with hep B in 2 patients. We found that there is no relationship between MDB and any form of chronic hepatitis regarding histologic severity such as alcoholic steatohepatitis and NAFLD and variable zone distribution by etiology.
- Citation: Basaranoglu M, Turhan N, Sonsuz A, Basaranoglu G. Mallory-Denk Bodies in chronic hepatitis. World J Gastroenterol 2011; 17(17): 2172-2177
- URL: https://www.wjgnet.com/1007-9327/full/v17/i17/2172.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i17.2172
Frank Burr Mallory first reported Mallory-Denk Bodies (MDB) in patients with alcoholic cirrhosis in 1911[1]. Then, this pearl of pathology was reported in various hepatic diseases such as the hepatitis B and C virus, Wilson’s disease, chronic cholestatic injuries, nonalcoholic fatty liver disease (NAFLD), drug injuries, focal nodular hyperplasia, and hepatocellular carcinoma (HCC).
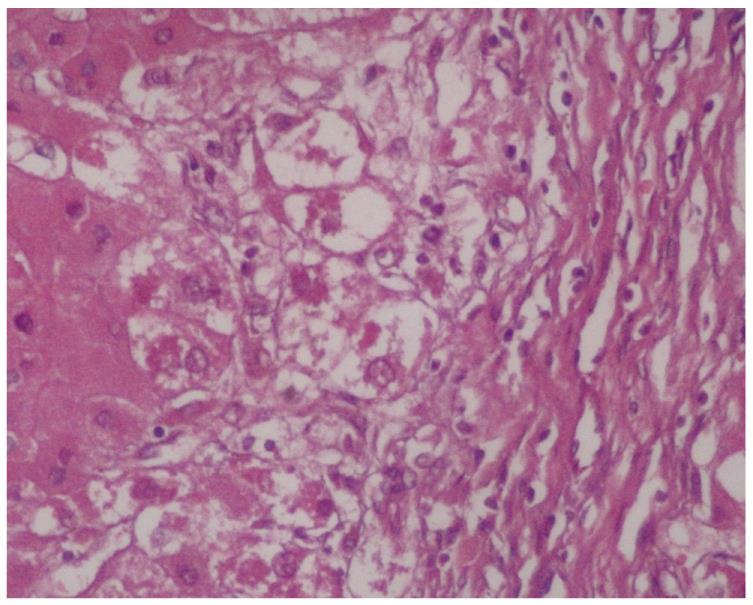
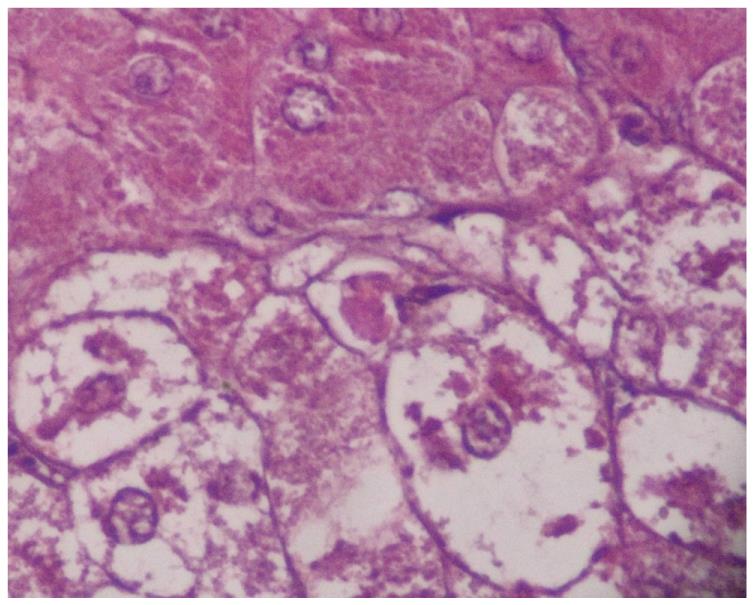
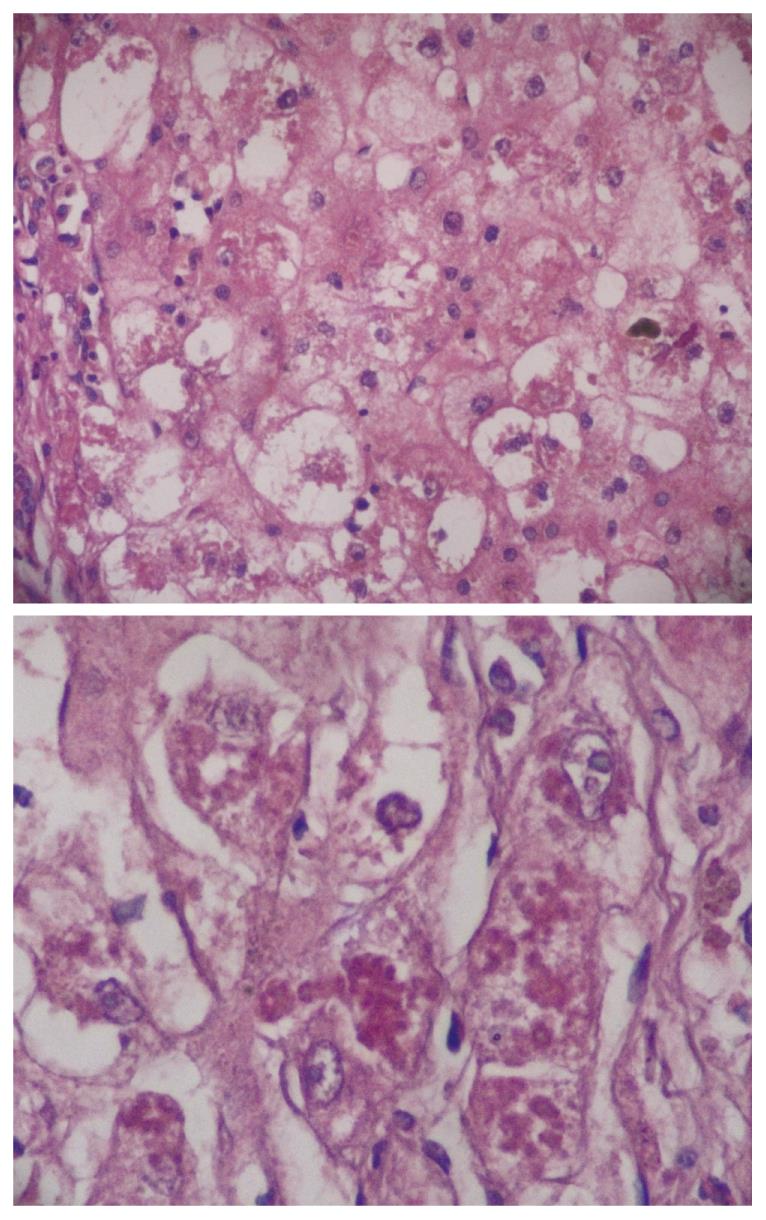
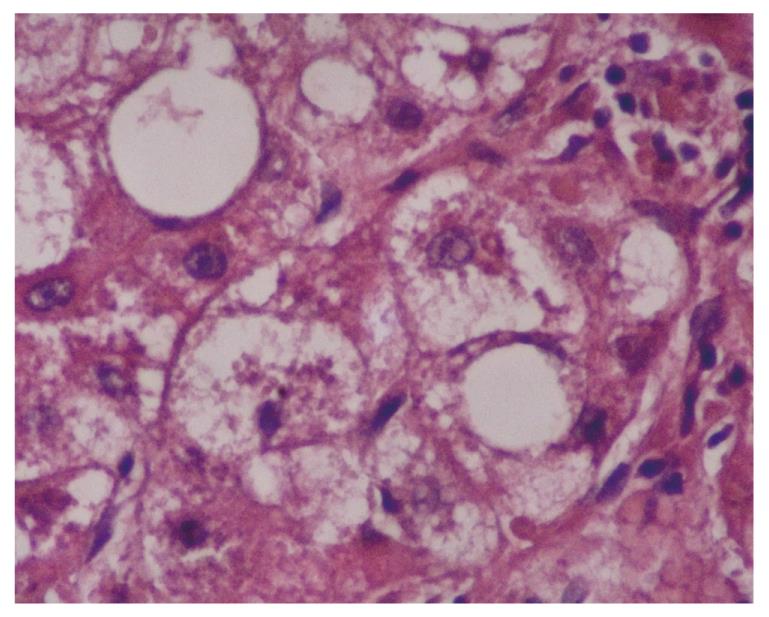
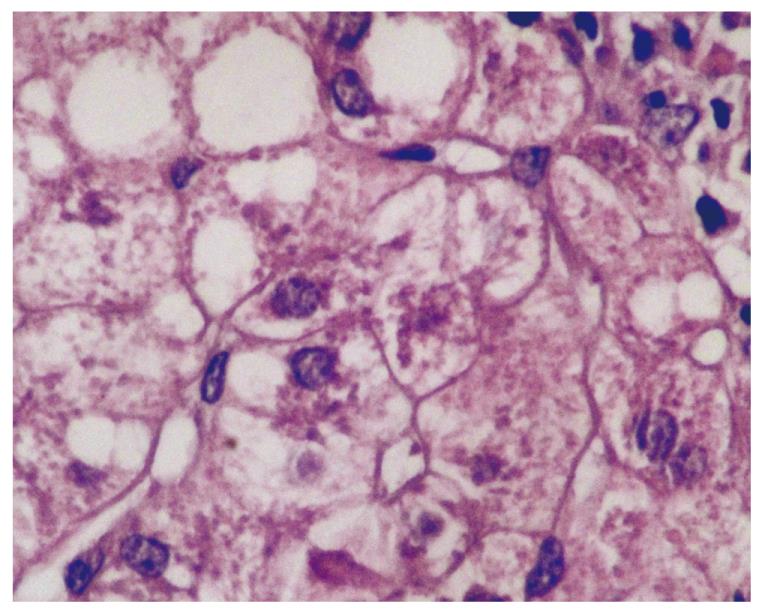
MDB are an intracellular deposition of misfolded protein aggregated into ubiquitin (Ub)-rich cytoplasmic inclusions in ballooned hepatocytes[2]. MDB formation, which consists of abnormally phosphorylated, ubiquitinated, and cross-linked keratins and non-keratin components, are not entirely interchangeable since not all ballooned hepatocytes contain MDB. Both ballooning of hepatocytes and MDB are the two hallmarks of ongoing inflammation (Figure 1). To understand the pathogenesis of MDB, we first have to know the development of ballooning of hepatocytes.
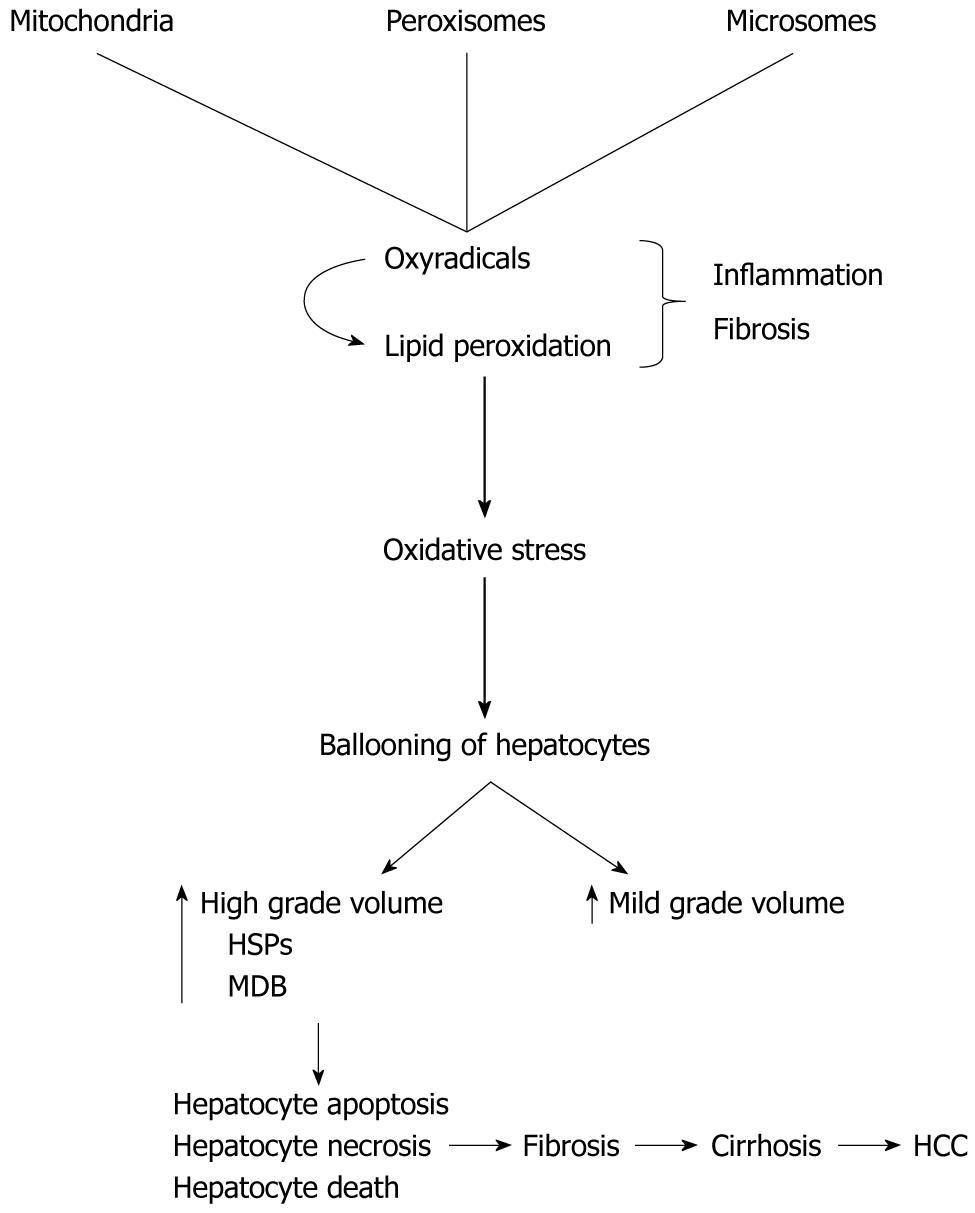
Ballooning or swelling of hepatocytes is induced by oxidative stress and its products such as oxyradicals. The swelling of hepatocytes could be explained by water accumulation in the cytoplasm as a response to accumulated stress proteins such as heat shock proteins (HSPs) or fat. HSPs are the precursor of MDB and indicate hepatocytes injury[2,3]. Currently, we do not know exactly whether ballooning are adaptive (physiological, reversible) or degenerative changes (pathological, most likely irreversible) of hepatocytes against the changed environment.
Swelling of hepatocytes represents volume increase (hydration) of the hepatocytes. It occurs against different stressors, particularly oxidative stress. It is reported that mild volume changes (up to 10% increase on the volume of the hepatocytes) without the biochemical evidence of free radicals are physiologic and adaptive. Hepatocyte damage may not be observed. However, high grade swelling (such as at least 30% increase on the volume of the hepatocytes) could be degenerative and can cause hepatocyte apoptosis, necrosis, and even death.
Most of the previous studies came from animal models. A hepatocyte includes two big compartments, namely wet (water) and cellular dry solids (fat, protein, nucleic acids, anions and cations and other solutes) which are in balance. Any intracellular solute (such as fat or abnormal proteins) accumulation into the cytoplasm may cause water movement, and water volume increase within the hepatocyte. This may change hepatocyte membrane transport system activation (such as ion channels) and may increase intracellular metabolism (increase both protein and glycogen synthesis and inhibit both proteolysis and glycogenolysis). The only purpose of these mechanisms is to maintain the functioning of both hepatocytes, and the liver as an organ. The volume ratio between these two compartments should be maintained at the same level under normal conditions and also against stressors.
It is experimentally shown in rats that the initial response of hepatocytes against iron, which is a well-known strong oxidative agent, was the accumulation of increased stress proteins, such as HSPs, within the cytoplasm. Increased stress proteins, along with the other elements of the increased metabolic process, cause macromolecular crowding and activate volume regulator mechanisms. To maintain the ratio between wet (water) and increased dry compartments (such as HSPs), volume regulator mechanisms increase the hepatocyte hydration with increasing water content of the cytoplasm (cloudy swelling). The real mission of this regulatory system is to preserve the intracellular environment and hepatocyte functioning. This is an adaptation mechanism of hepatocytes against oxidative stress (up to 10% increase on the volume of the hepatocytes). However, too much increased water within the cytoplasm of hepatocytes may cause degenerative changes and disturbances in both normal hepatocyte morphology and functioning. Then, hepatocyte apoptosis, necrosis and death may occur.
Additionally, we can discuss the toxic fatty liver animal model. Investigators used rats treated with CCl-4, which is a well-known steatogenic oxidant agent. CCl-4 is a cause of high grade hepatocyte swelling. This chemical poison was injected into the rats daily (one injection per day). Then the livers of the rats were examined for three compartments (water, fat, and fat-free dry solids) at the entry, after 1 injection, and after 6 injections. Investigators observed that both fat and water were increased significantly while fat-free dry solids were not changed or only slightly changed. The highest grade increase of water was seen after the sixth injection (25% water increase after 1 injection, and 48% water increase after 6 injections) which was related to the increased hepatocyte hydration. This means that there were many well-established ballooned hepatocytes in the liver of CCl-4 injected rats.
However, these protection mechanisms are not infinite. A strong oxidative stress may cause high amplitude ballooning of hepatocyte due to both increased hydration and dry solids. One of the increased cellular dry solids is stress protein, such as HSPs. HSPs, as examples of misfolded proteins, are the indicators of cellular dysfunction. Under normal conditions, these potentially harmful proteins are targeted by covalent attachment of multi-Ub chains. Then, a protective mechanism, the Ub-proteasome pathway eliminates these products. When this control system fails, under the strong oxidative stress, abnormal cytokeratins (CKs) may become accumulated along with HSPs (70, 90, and 25), Ub, tissue transglutaminase, proteasome subunits, tubulin, and p62. Young, tiny and then well-formed MDB were well established in ballooned hepatocytes. This mechanism has not been fully understood yet. There are two possible ways for this pathway to fail: (1) production of these misfolded proteins exceeding the capacity of this protective system or (2) inhibition of the pathway. Well-established MDB might be a degenerative rather than adaptive response of hepatocytes against stronger stressors. Then, satellite cell activation occurs. Sustained liver injury leads to fibrosis and cirrhosis.
In conclusion, there is no contrast of MDB pathogenesis in hep B, hep C, PBC, NAFLD and the others.
MDB are typical features of alcoholic steatohepatitis (ASH) and NAFLD. NAFLD exhibits slightly less prominent MDB than ASH[4]. MDBs can also be detected after intestinal bypass surgery for morbid obesity, in chronic cholestasis, PBS, Wilson disease (WD) and other types of copper toxicosis, various metabolic disturbances, and hepatocellular neoplasms. In idiopathic copper toxicosis and HCC, MDBs may coincide with another type of cytoplasmic inclusions.
MDB is not present in the majority of individuals with hep C, or even in hep C patients with NAFLD (the presence of MDB in patients with hep C is 7.1%). However, hepatocyte ballooning with MDB is much more likely to lead to advanced fibrosis in these patients.
MDB in periportal liver cells have been reported in patients with Wilson’s disease. MDBs are found in mice by feeding them the hepatotoxic substances griseofulvin and 3,5-diethoxycarbonyl- 1,4-dihydrocollidine.
MDBs have not been observed in acute cholestasis, acute viral hepatitis, acute toxic or drug-induced liver diseases.
Two hundred and fifty-eight patients with hepatitis have been examined at our pathology department. Outpatient percutaneous percussion guided needle liver biopsies were performed using Menghini soft tissue biopsy needles (1.4 or 1.6 mm, adult size, Hepafix, Braun, Melsungen, Germany). Liver biopsy samples were evaluated with hematoxylin and eosin, periodic acid-Schiff-diastase, Gordon and Sweet’s reticulin, Masson’s trichrome, and iron stains by a single pathologist, unaware of the clinical and biochemical data. We examined patients with nonalcoholic steatohepatitis (NASH; 50 patients), alcoholic hepatitis (10 patients), primary biliary cirrhosis (PBC; 50 patients), WD (20 patients), hepatitis B (50 patients) and hepatitis C (50 patients), and HCC (30 patients) for grading and staging and MDB. Results were shown at Table 1. Furthermore, the characteristics of MDB in each disease have been shown in Figures 1, 2, 3, 4, 5. MDB showed zone 1 distribution in PBC and its distribution, located perinuclear, observed in both early and later stages, such as cirrhosis. MDB, located both perinuclear and intracytoplasmic, was observed in zone 1 in early stages of WD, but in every zone at the later stages, such as cirrhosis. Hydrophobic degeneration was more exaggerated in WD and the diameter of MDB was not homogenous in patients with WD. Perinuclear localized MDB in patients with HCC depends on hepatitis B, which is responsible for MDB. Lastly, tiny and uniform distribution of MDB was seen in patients with NASH. Although patients with PBC and WD showed MDB frequently in later stages, we found that there is no relationship between MDB and any form of chronic hepatitis regarding histologic severity, such as in ASH and NAFLD. Moreover, zone distribution of MDB is very variable by etiology, such as zone 1 in PBC and WD as zone 3 in ASH and NAFLD. Of the 3 hepatitis B patients with MDB, 2 showed steatosis with mild fibrosis. Most probably, MDB in these cases was caused by steatosis due to metabolic reasons.
| No. of pts | MDB (+)pts | Staging1 in pts with MDB | Comment | |
| NASH | 50 | 10 (20) | Mild in 60% and moderate in 40% | MDB observed in every stage of NASH, predominantly in zone 3 |
| Alcoholic (ASH) | 10 | 5 (50) | Mild in 40% and moderate in 60% | MDB observed in every stage, predominantly in zone 3 |
| PBC | 50 | 11 (22) | Mild in 10%, moderate in 70%, and cirrhosis in 20% | Frequently seen in later stages with predominantly in zone 1 |
| WD | 20 | 16 (80) | Moderate and severe in 60% and cirrhosis in 40% | Frequently seen in later stages with predominantly in zone 1 |
| Hep B | 48 | 3 (6) | Mild in 66% and severe in 34% | Mild case with steatosis |
| Hep C | 50 | 7 (14) | Mild in 40% and moderate in 60% | MDB observed in every stage |
| HCC | 30 | 3 (10) | Two cases with Hep B | |
| Total | 258 | 55 (21) |
In an earlier classification system, Matteoni et al[5] scored MDB in patients with NAFLD as none, rare and many. MDB plays a prominent role in this classification scheme as well as follows; Type 1: Fatty liver alone, Type 2: Fat and inflammation, Type 3: Fat and ballooned hepatocytes, and Type 4: Fat and ballooned hepatocytes and either MDB or fibrosis.
The Kleiner system is widely accepted and, as such, has superseded the previous classification system (Matteoni et al’s system)[6]. Although this system includes several parameters, there are four main histologic features as steatosis, inflammatory infiltration, ballooning degeneration of hepatocytes, and fibrosis are more important than others. Also, significantly, presence or otherwise of MDB is of much less consequence in the Kleiner system.
Frequency of MDB is very variable, reported from 7% to 90% in adult patients with NAFLD[7-18]. Furthermore, it was not demonstrated or uncommon in pediatric patients with NAFLD[17,18].
Technical difficulties on the showing of MDB may be the most important reason for the differences. To better understand this issue and its contradictions, we must be aware of some basic knowledge about MDB. MDB are intracellular depositions in ballooned hepatocytes which reflects a peculiar morphological manifestation of liver cell injury[19,20].
Three stages are demonstrated according to the development rank of MDB in ballooned hepatocytes: (1) misfolded proteins such as CKs, Ub, protein p62, and high and low molecular weight HSPs; (2) pre-MDB (young/tiny form) positive ballooned hepatocytes; and (3) mature or well-established MDB positive ballooned hepatocytes.
Misfolded proteins present the earliest stage of MDB and induced in response to a variety of cellular injuries. They are indicators of cellular dysfunction. Normally, these potentially harmful proteins are targeted by covalent attachment of multi-Ub chains. Then, a protective mechanism, which is the Ub-proteasome pathway, eliminates these products. When this control system fails, the CKs become accumulated in MDB along with HSPs, Ub, tissue transglutaminase, and p62. Then, mature MDB in ballooned hepatocytes is well established. There are two possible ways for this pathway to fail: (1) production of these misfolded proteins exceeding the capacity of this protective system (overexpression); and (2) inhibition of this pathway.
Pathogenesis of MDB is thought to include lipid peroxidation, oxidative stress, free radicals, bile retention, defective protein synthesis and copper accumulation (Figure 6). There are many modalities to detect MDB such as immunostaining using cytokeratin and Ub, and special staining methods for other misfolded proteins. The other one, the historically important hematoxylin and eosin, is not suitable for detecting misfolded proteins or small/tiny MDB. Almost all previous studies interested in NAFLD used hematoxylin and eosin staining for showing both mature and young forms of MDB. This is a cost effective and an easily available method. Furthermore, there is more evidence and experience about its safety in the medical literature. On the other hand, the immunostaining method is relatively new and expensive. Immunostaining demonstrates not only mature form of MDB but also both its young forms and misfolded proteins such as CKs, Ub, p62 protein and HSPs in ballooned hepatocytes. The question is which one is the best to use in general practice and clinical trials? We have to look at the current medical literature to give the correct answer for this question. However, there is no growing body of evidence on this issue. A study from Denmark used immunostaining for the cell stress protein Ub in 148 fine needle liver biopsies, which included 88 biopsies from patients with clinically diagnosed or suspected alcoholic liver disease and 60 selected biopsies from non-alcoholics[20]. They showed that MDB in ballooned hepatocytes by both hematoxylin and eosin and immunostaining (Ub) in all of 33 biopsies with alcoholic hepatitis. Of the 55 biopsies from alcoholic patients without alcoholic hepatitis (without MDB or pre-MDB in hematoxylin and eosin stained sections), Ub (+) cells were found in eight (14.5%) by immunostaining. Finally, they studied 60 selected biopsies from non-alcoholic patients, and demonstrated a few Ub (+) cells in two out of ten patients with PBC, but none in biopsies with hepatitis. According to the study results, as Ub/immunostaining is a highly sensitive and specific tool in the detection of MDB and MDB precursors in alcohol-using patients, its use in other forms of hepatitis is limited.
p62 is another misfolded protein and encoded by an immediate-early response gene that rapidly responds to a variety of extracellular signals, particularly oxidative stress. Then, protein p26 binds non-covalently to Ub.
These results may also represent a sampling error of liver biopsy. The method of liver biopsy we chose (such as percutaneous or ultrasound guided or CT guided or laparoscopic), the type of biopsy needle, the average length of the biopsies performed, and how many samples are obtained or number of biopsies performed would be important.
Both environmental factors and genetic differences between the study groups may play a role in these differences[21]. These remarkable differences may be induced by different levels of alcohol consumption and an incomplete patient history of alcohol consumption in these studies. Ignored alcoholic liver disease is a significant cause of MDB in some. Incomplete examination of Wilson’s disease, such as just measuring serum ceruloplasmin levels or with no examination of hepatitis C virus infection, may play a role. This is especially relevant with Wilson’s disease and hepatitis C because both microvesicular and macrovesicular fatty changes and glycogen nuclei are shown in both diseases.
MDB is found in 70% to 75% of patients with alcoholic liver disease[22,23]. Men and women who drink more than 80 g and 40 g of ethanol/d, respectively were accepted at substantial risk for the development of liver disease in the previous studies. Recent studies showed that the risk of liver disease begins at 30 g of ethanol/d[24]. This finding has led to a general recommendation that the maximal safe level of ethanol consumption is 20 g/d or two “drinks” per day for men and 10 g/d for women. However, it should be kept in mind that disease severity does not correspond to classic dose dependency.
Although one of the major criteria for the diagnosis of NAFLD is no excessive alcohol consumption in all previous case series, there has been no certain agreement on this issue, and a wide range of alcohol consumption was generally allowed. Thus, these remarkable differences on the frequency of MDB might be explained with the generously allowed alcohol consumption in previous series. It must also be considered that patients sometimes underreport ethanol intake.
Frequency (MDB) is reported as very variable. Both environmental factors and genetic differences between the study groups, and technical difficulties for showing of MDB and its early stage components may play a role in these remarkable differences.
Peer reviewers: Wing-Kin Syn, MD, Division of Gastroenterology, GSRB-1, Suite 1073, DUMC 3256, 595 LaSalle Street, Durham, NC 27710, United States; Yuichi Yoshida, MD, PhD, Assistant Professor, Department of Gastroenterology and Hepatology, Osaka University, 2-2 Yamadaoka, Suita, Osaka 565-0871, Japan
S- Editor Sun H L- Editor Rutherford A E- Editor Zheng XM
| 1. | Strnad P, Zatloukal K, Stumptner C, Kulaksiz H, Denk H. Mallory-Denk-bodies: lessons from keratin-containing hepatic inclusion bodies. Biochim Biophys Acta. 2008;1782:764-774. |
| 2. | Caldwell S, Ikura Y, Dias D, Isomoto K, Yabu A, Moskaluk C, Pramoonjago P, Simmons W, Scruggs H, Rosenbaum N. Hepatocellular ballooning in NASH. J Hepatol. 2010;53:719-723. |
| 3. | Strnad P, Tao GZ, So P, Lau K, Schilling J, Wei Y, Liao J, Omary MB. "Toxic memory" via chaperone modification is a potential mechanism for rapid Mallory-Denk body reinduction. Hepatology. 2008;48:931-942. |
| 4. | Zatloukal K, French SW, Stumptner C, Strnad P, Harada M, Toivola DM, Cadrin M, Omary MB. From Mallory to Mallory-Denk bodies: what, how and why? Exp Cell Res. 2007;313:2033-2049. |
| 5. | Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413-1419. |
| 6. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. |
| 7. | Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434-438. |
| 8. | Itoh S, Yougel T, Kawagoe K. Comparison between nonalcoholic steatohepatitis and alcoholic hepatitis. Am J Gastroenterol. 1987;82:650-654. |
| 10. | Diehl AM, Goodman Z, Ishak KG. Alcohollike liver disease in nonalcoholics. A clinical and histologic comparison with alcohol-induced liver injury. Gastroenterology. 1988;95:1056-1062. |
| 11. | Lee RG. Nonalcoholic steatohepatitis: a study of 49 patients. Hum Pathol. 1989;20:594-598. |
| 12. | Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74-80. |
| 13. | Bacon BR, Farahvash MJ, Janney CG, Neuschwander-Tetri BA. Nonalcoholic steatohepatitis: an expanded clinical entity. Gastroenterology. 1994;107:1103-1109. |
| 14. | Pinto HC, Baptista A, Camilo ME, Valente A, Saragoça A, de Moura MC. Nonalcoholic steatohepatitis. Clinicopathological comparison with alcoholic hepatitis in ambulatory and hospitalized patients. Dig Dis Sci. 1996;41:172-179. |
| 15. | Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356-1362. |
| 16. | Ratziu V, Giral P, Charlotte F, Bruckert E, Thibault V, Theodorou I, Khalil L, Turpin G, Opolon P, Poynard T. Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117-1123. |
| 17. | Baldridge AD, Perez-Atayde AR, Graeme-Cook F, Higgins L, Lavine JE. Idiopathic steatohepatitis in childhood: a multicenter retrospective study. J Pediatr. 1995;127:700-704. |
| 18. | Rashid M, Roberts EA. Nonalcoholic steatohepatitis in children. J Pediatr Gastroenterol Nutr. 2000;30:48-53. |
| 19. | Riley NE, Li J, McPhaul LW, Bardag-Gorce F, Lue YH, French SW. Heat shock proteins are present in mallory bodies (cytokeratin aggresomes) in human liver biopsy specimens. Exp Mol Pathol. 2003;74:168-172. |
| 20. | Vyberg M, Leth P. Ubiquitin: an immunohistochemical marker of Mallory bodies and alcoholic liver disease. APMIS Suppl. 1991;23:46-52. |
| 21. | Hanada S, Strnad P, Brunt EM, Omary MB. The genetic background modulates susceptibility to mouse liver Mallory-Denk body formation and liver injury. Hepatology. 2008;48:943-952. |
| 22. | Mendenhall CL. Alcoholic hepatitis. Clin Gastroenterol. 1981;10:417-441. |
| 23. | Lelbach WK. Cirrhosis in the alcoholic and its relation to the volume of alcohol abuse. Ann N Y Acad Sci. 1975;252:85-105. |