Published online Apr 28, 2011. doi: 10.3748/wjg.v17.i16.2137
Revised: December 20, 2010
Accepted: December 27, 2010
Published online: April 28, 2011
AIM: To test the ability of penehyclidine hydrochloride (PHC) to attenuate intestinal injury in a rat cardiopulmonary bypass (CPB) model.
METHODS: Male Sprague-Dawley rats were randomly divided into six groups (eight each): sham-operated control; sham-operated low-dose PHC control (0.6 mg/kg); sham-operated high-dose PHC control (2.0 mg/kg); CPB vehicle control; CPB low-dose PHC (0.6 mg/kg); and CPB high-dose PHC (2.0 mg/kg). Blood samples were collected from the femoral artery 2 h after CPB for determination of plasma diamine oxidase (DAO), D-lactate and endotoxin levels. Spleen, liver, mesenteric lymph nodes and lung were removed for biochemical analyses. Intestinal tissue ultrastructure was examined by electron microscopy.
RESULTS: In the sham-operated groups, high- and low-dose-PHC had no significant impact on the levels of DAO, D-lactate and endotoxin, or the incidence of intestinal bacterial translocation (BT). Serum levels of DAO, D-lactate, endotoxin and the incidence of intestinal BT were significantly increased in the surgical groups, compared with the sham-operated groups (0.543 ± 0.061, 5.697 ± 0.272, 14.75 ± 2.46, and 0/40 vs 1.038 ± 0.252, 9.377 ± 0.769, 60.37 ± 5.63, and 30/40, respectively, all P < 0.05). PHC alleviated the biochemical and histopathological changes in a dose-dependent manner. Serum levels of DAO, D-lactate, and endotoxin and the incidence of intestinal BT in the high-dose PHC group were significantly lower than in the low-dose PHC group (0.637 ± 0.064, 6.972 ± 0.349, 29.64 ± 5.49, and 14/40 vs 0.998 ± 0.062, 7.835 ± 0.330, 38.56 ± 4.28, and 6/40, respectively, all P < 0.05).
CONCLUSION: PHC protects the structure and function of the intestinal mucosa from injury after CPB in rats.
- Citation: Sun YJ, Cao HJ, Jin Q, Diao YG, Zhang TZ. Effects of penehyclidine hydrochloride on rat intestinal barrier function during cardiopulmonary bypass. World J Gastroenterol 2011; 17(16): 2137-2142
- URL: https://www.wjgnet.com/1007-9327/full/v17/i16/2137.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i16.2137
Cardiopulmonary bypass (CPB) is essential during some cardiovascular surgical procedures; however, it can cause peripheral hypoperfusion as a result of non-pulsatile flow, low blood pressure, hemodilution, and other non-physiological conditions. Furthermore, an increase in intestinal permeability and bacterial translocation (BT) has been demonstrated, not only in animal models, but also in patients during CPB[1-3]. Perioperative gastrointestinal integrity is therefore now recognized as an important factor determining the outcome of cardiac surgical procedures[4]. In general, changes in mucosal permeability and morphology during CPB reflect the degree of damage to intestinal mucosal barrier function.
Previous in vitro studies have demonstrated that tropane alkaloids can stabilize the cell membrane and prevent oxidative stress. The new anticholinergic drug, penehyclidine hydrochloride (PHC), has been evaluated for its protective effects on the cardiovascular system[5-7]. Its selective blocking of M1, M3 and N receptors means that PHC has few M2 receptor-associated cardiovascular side effects. It has been shown to reduce endotoxin-stimulated acute lung injury and to attenuate liver damage during CPB in a rat model[8-10].
Based on the potential roles of PHC as an antioxidant and a cell membrane stabilizer, we hypothesized that its administration might reverse CPB-associated intestinal damage. Zhan et al[11] have suggested that PHC concentrations of 0.18-3.60 mg/kg were found to be safe, and we therefore tested this hypothesis in a rat CPB model, using high- (2.0 mg/kg) and low-dose (0.6 mg/kg) PHC.
Forty-eight male Sprague-Dawley rats (weighing 300-450 g, 18-22 wk old) were randomly assigned to one of six groups (eight each): sham-operated control; sham-operated control + low-dose PHC (0.6 mg/kg) (sham L-PHC); sham-operated control + high-dose PHC (2 mg/kg) (sham H-PHC); CPB + vehicle (control); CPB + low-dose PHC (0.6 mg/kg) (L-PHC); and CPB + high-dose PHC (2.0 mg/kg) (H-PHC). PHC (Lisite Pharmacology Co. Chendou, China, No. 080301) was dissolved in absolute ethanol and diluted in saline (final concentration of ethanol < 1.0%), and added to the priming solution for CPB. All animals received humane care in compliance with the Principles of Laboratory Animal Care. The experimental protocol was approved by the local animal use and care committee at the General Hospital of Shenyang Commend, China.
None of the sham-operated control groups underwent CPB. After PHC injection, the respiratory rate (RR), heart rate (HR), blood pressure (BP) and electrocardiography (ECG) were continually monitored. The rat CPB model was established as previously described, with some modifications[12]. In brief, rats were anesthetized by intraperitoneal administration of 10% chloral hydrate (0.3 mL/100 g body weight) to provide stable anesthesia, while maintaining spontaneous ventilation during the entire operative procedure. All subsequent procedures were performed under aseptic conditions.
After surgical-level anesthesia was achieved, the left femoral artery was cannulated using a 22-gauge Teflon heparinized catheter. Arterial pressure was monitored and blood samples were collected for gas analysis, using a blood gas analyzer (GEM Premier 3000; Mallinckrodt, Lexington, MA, USA). Following administration of heparin (250 U/kg), an 18-gauge catheter was inserted into the right jugular vein and advanced to the right atrium. A 22-gauge catheter was cannulated into the tail artery, to serve as an arterial infusion line for the CPB circuit. The mini-CPB circuit comprised a venous reservoir, a specially designed membrane oxygenator, a roller pump, and sterile tubing with inner diameters of 4 mm for the venous line and 1.6 mm for the arterial line. The CPB circuit was primed with a total volume of 15 mL of synthetic colloid solution. The perfusion flow rate was gradually adjusted to sustain a mean arterial pressure of 60-80 mmHg. The gas flow (95% O2, 5% CO2) was initiated at around 50-75 mL/kg per minute and adjusted to maintain blood gas analysis parameters within the physiological range. When the flow rate reached 80-100 mL/kg per minute, it was maintained for 60 min. At the end of CPB, the flow rate was reduced stepwise to achieve hemodynamic stabilization. Throughout the experiment, central body temperature was monitored with a rectal probe and kept at 37.5 ± 1.0°C using a heat lamp placed above the animal and CPB equipment. The mean arterial pressure was maintained at 60-80 mmHg.
Rats from each group were sacrificed by decapitation, and arterial blood (2.0 mL) and terminal ileums were sampled at 2 h after CPB. Plasma was prepared by centrifugation at 3000 g for 5-10 min at 4°C and stored at -70°C for determination of serum diamine oxidase (DAO), D-lactate, and endotoxin. Intestinal (ileum) tissue samples were obtained for electron microscopy.
The permeability of the intestinal mucosa was assayed by measuring D-lactate and DAO levels in plasma. Plasma D-lactate levels were measured by enzymatic spectrophotometric assay using a centrifugal analyzer at 30°C, as described previously[13]. Plasma DAO activities were also determined by enzymatic spectrophotometry, as described previously[14]. D-lactate, D-lactate dehydrogenase, NAD+, O-dianisidine, cadaverine dihydrochloride and DAO were purchased from Sigma Chemical Company (Milan, Italy).
The endotoxin content of the plasma sample was assayed using the Limulus amebocyte lysate test using the Endochrome K test kit (CoaChrom, Vienna, Austria). In brief, heparinized plasma was diluted 1:10 in pyrogen-free water and kept heated at 75°C for 10 min to remove non-specific inhibitors. A quantitative chromogenic kinetic method was used, as specified by the manufacturer, using a Thermo microplate reader (Tecan Spectra, Salzburg, Austria). The method had a detection limit of 0.75 pg/mL at a 1:10 plasma dilution.
A midline incision was made using a sterile technique. Mesenteric lymph nodes (MLNs), portal vein, and samples from the liver, spleen, and lung were harvested and weighed prior to determination of bacterial growth. The samples were homogenized in test tubes containing 3 mL Brain Heart Infusion Broth (Difco, Detroit, IL, USA). The supernatant (0.2 mL) was cultured for growth of aerobic, microaerophilic and anaerobic bacteria. All media for aerobic cultures were incubated at 37°C for at least 3-5 d, while organ samples for anaerobic bacteria were cultured at 37°C for 7 d. Enteric Gram-negative bacteria were identified using the API 20 system (BioMérieux SA, Marcy-l’Etoile, France) and Lactobacillus acidophilus by API 50 CH (Analytab Products Inc., Plainview, NY, USA). All other aerobic, microaerophilic and anaerobic microbes isolated were identified by standard procedures. The numbers of living bacteria were calculated and expressed as the numbers of living organisms per gram of organ tissue.
For transmission electron microscopy, ileum tissues were removed immediately from anesthetized rats 2 h after CPB, and then fixed with 2% paraformaldehyde and 2.5% glutaraldehyde in PBS (pH 7.3) for 2 h at room temperature (25°C). The tissues were washed with PBS, fixed with 1% osmium tetroxide for 2 h, washed again, and then embedded in Araldite 6005. Tissue sections were cut with a Leica EM FCS (Vienna, Austria) ultramicrotome. Tissue sections (1 μm) were initially stained with toluidine blue-Azur II to select the region of interest for subsequent procedures. Thin sections (60-70 nm) were stained with uranyl acetate and lead citrate and examined and photographed using an H-7200 transmission electron microscope (80 kV; Oberkochen, Germany). Electron microscopy pictures were evaluated twice by two independent histologists with at least 10 years of experience, who were blinded to our study.
All experimental data were expressed as mean ± SD and analyzed using a SPSS for Windows v. 13.0 (Chicago, IL, USA). One-way ANOVA was used for comparisons among various treatment groups. Post-hoc comparisons were analyzed using least significant difference test or Dunnett’s T3 test. P < 0.05 was considered to be statistically significant.
There were no obvious changes in the RR, HR, BP or ECG at any time points (0, 30, 60, or 120 min) after anesthesia in the sham-operated groups (Table 1). Hemodynamic changes in the vehicle control, L-PHC and H-PHC groups are shown in Table 1. As shown in Table 2, DAO and D-lactate levels increased significantly in vehicle-treated CPB rats, compared with the sham group (P < 0.05), which effect was largely reversed by treatment with PHC in a dose-dependent manner (P < 0.05). H-PHC had significantly greater effects on the values of DAO and D-lactate than L-PHC (Table 2, P < 0.05).
| Groups | Pre-CPB | CPB 30 min | CPB 60 min | Post-CPB 2 h | |
| MAP (mmHg) | Controls | 86.33 ± 16.82 | 84.26 ± 14.55 | 85.45 ± 10.36 | 83.63 ± 11.24 |
| CPB + vehicle | 84.50 ± 7.05 | 62.14 ± 15.23ac | 67.08 ± 19.12ac | 72.18 ± 17.39 | |
| CPB + L-PHC | 85.45 ± 9.36 | 66.58 ± 11.26ac | 69.78 ± 14.05ac | 76.15 ± 13.65 | |
| CPB + H-PHC | 87.54 ± 10.43 | 65.73 ± 9.85ac | 70.85 ± 12.36ac | 77.84 ± 12.36 | |
| HR (beats/min) | Controls | 325 ± 34 | 320 ± 25 | 315 ± 20 | 324 ± 15 |
| CPB + vehicle | 315 ± 30 | 286 ± 28 | 305 ± 42 | 310 ± 37 | |
| CPB + L-PHC | 305 ± 26 | 315 ± 34 | 302 ± 38 | 304 ± 27 | |
| CPB + H-PH | 310 ± 20 | 296 ± 25 | 301 ± 35 | 305 ± 30 | |
| PH | Controls | 7.40 ± 0.02 | 7.41 ± 0.03 | 7.39 ± 0.02 | 7.40 ± 0.02 |
| CPB + vehicle | 7.41 ± 0.03 | 7.38 ± 0.05 | 7.35 ± 0.06 | 7.39 ± 0.02 | |
| CPB + L-PHC | 7.38 ± 0.04 | 7.43 ± 0.02 | 7.41 ± 0.03 | 7.36 ± 0.04 | |
| CPB + H-PHC | 7.42 ± 0.01 | 7.42 ± 0.03 | 7.38 ± 0.04 | 7.43 ± 0.02 | |
| BE (mmol/L) | Controls | -1.96 ± 0.45 | -2.36 ± 0.75 | -1.54 ± 0.85 | -1.95 ± 0.54 |
| CPB + vehicle | -1.86 ± 0.35 | -1.36 ± 0.26 | -1.60 ± 0.84 | -2.30 ± 1.50 | |
| CPB + L-PHC | -1.75 ± 0.26 | -1.55 ± 0.96 | -1.95 ± 0.63 | -2.04 ± 0.72 | |
| CPB + H-PHC | -1.85 ± 0.36 | -2.05 ± 0.90 | -1.74 ± 0.42 | -2.45 ± 0.14 | |
| HCT (%) | Controls | 41.10 ± 1.85 | 40.20 ± 1.53 | 40.60 ± 1.46 | 39.80 ± 1.35 |
| CPB + vehicle | 41.80 ± 3.73 | 26.45 ± 4.24ac | 21.54 ± 3.71ac | 27.82 ± 3.66ac | |
| CPB + L-PHC | 42.20 ± 2.45 | 27.65 ± 3.65ac | 23.05 ± 5.30ac | 29.53 ± 5.45ac | |
| CPB + H-PHC | 42.45 ± 2.50 | 28.76 ± 5.38ac | 22.46 ± 4.25ac | 28.75 ± 4.56ac |
Bacteriological cultures from all sham-operated animals were negative. The incidence of Escherichia coli-positive cultures was significantly increased in the CPB-vehicle group, while pretreatment with PHC seemed to prevent systemic dissemination. The incidence of intestinal BT to the MLNs, spleen, liver, lung and blood was significantly higher in the CPB-vehicle group, compared with that in the sham groups (Table 3, P < 0.05), which was largely reversed by treatment with PHC in a dose-dependent manner (Table 3, P < 0.05). The plasma endotoxin level was increased in the CPB-vehicle group compared with the sham groups (Table 2, P < 0.05) and PHC decreased the plasma endotoxin levels in a dose-dependent manner (Table 2, P < 0.05).
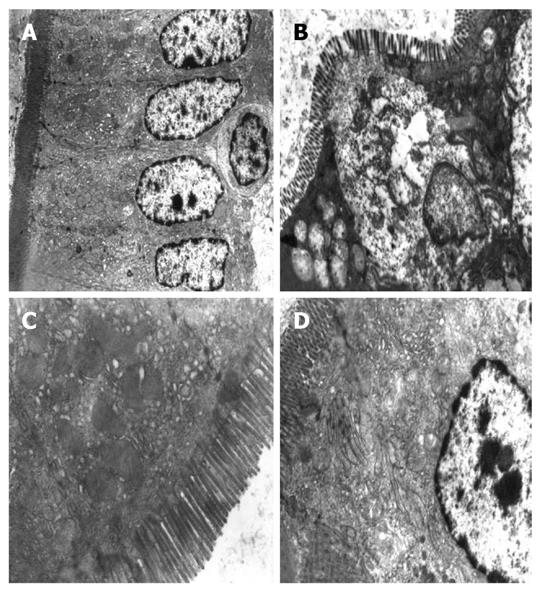
Transmission electronic microscopy demonstrated normal intestinal ultrastructure in the sham-operated group, including regularly aligned microvilli in the intestinal epithelium, integral mitochondria and rough endoplasmic reticulum (RER) and distinct junction complexes (Figure 1A). In the vehicle control group, the microvilli were reduced in number, and showed irregular lengths and arrangements. The mitochondria were swollen with cracked and vacuolated cristae. Some RER structures were destroyed, and the intercellular spaces between epithelial cells were widened. The structure of the tight junctions became shortened (Figure 1B). Mitochondrial swelling with damage to mitochondrial cristae and vacuolar degeneration was present in the L-PHC group. The nuclear structure was incomplete (Figure 1C). In the H-PHC group, microvilli in the intestinal epithelium were regularly aligned, and the tight junction structure became tight. However, mild swelling of the mitochondria and RER were seen (Figure 1D). These results indicated dose-dependent effects of PHC on the reduction of cellular damage after CPB.
The present study focused on intestinal barrier injury after CPB and the potential of PHC as a therapeutic agent. We demonstrated that application of PHC during CPB preserved intestinal barrier function in a dose-dependent manner, and histological evidence is provided to support these biochemical results.
DAO reduces the concentration of polyamines required for cell proliferation. DAO is localized to the small intestine and placenta, which are both organs with rapid cell turnover rates. In humans, DAO activity is especially high in the upper portion of the small intestinal villi, and has therefore been used as an index of small intestinal mucosal mass and integrity. Serum DAO levels have been found to increase markedly when the small intestine is strangulated, and elevations are thus believed to reflect small intestine mucosal ischemia[15]. Tsunooka et al[4,16] have demonstrated simultaneous increases in serum DAO activity and peptidoglycan concentrations during clinical CPB, suggesting the occurrence of small intestinal mucosal ischemia and BT.
D-Lactate is habitually tested for in the intensive care unit. Mammals only have one type of enzyme: L-lactate dehydrogenase. L-Lactate is a marker of cell hypoxemia, and its levels correlate with survival in patients with septic shock[17,18]. Microorganisms however, particularly bacteria, are equipped with D-lactate dehydrogenase and produce D-lactate during fermentation, and D-lactate therefore acts as a marker of bacterial infection. D-Lactate has also recently been proposed as a sensitive, specific and early marker of translocation in gut ischemia[19].
In the current study, all the rats survived the CPB procedures. DAO and D-lactate activities remained low in the sham-operated animals (based on previously reported normal DAO value of 0.46 ± 0.087 U/L, and D-lactate value of 5.245 ± 0.653 mg/L[13,14]). However, significant increases in DAO, D-lactate and endotoxin levels were found in the vehicle-treated CPB group. The incidence of intestinal BT to the MLNs, spleen, liver, lung and blood was also significantly higher in the vehicle-treated CPB group, compared with that in the sham groups. These results indicate the occurrence of severe intestinal barrier injury after CPB. Transmission electron microscopic examination of intestinal tissues further confirmed the intestinal barrier injury in this rat CPB model.
There is increasing interest in developing PHC as a novel therapeutic agent. PHC is a new anticholinergic drug derived from hyoscyamine, which has few M2 receptor-associated cardiovascular side effects, because of its selective blocking of M1, M3 and N receptors. Recent clinical results have demonstrated that PHC has curative effects in soman poisoning and pulmonary dysfunction associated with chronic obstructive pulmonary disease[6,9,20]. In addition to improving microcirculation, PHC can inhibit lipid peroxidation, attenuate the release of lysosomes, and depress microvascular permeability[9,20]. Moreover, it can significantly decrease brain nuclear factor (NF)-κB expression in cerebral ischemia/reperfusion (I/R) injury. Furthermore, PHC can improve acute lung injury stimulated by endotoxin and attenuate liver damage during CPB in a rat model[11].
In the present study, PHC lowered DAO, D-lactate and endotoxin levels in PHC-treated rats in a dose-dependent manner, suggesting its potential clinical application. The incidences of intestinal BT to MLNs, spleen, liver, lung and blood were lower in PHC-treated CPB rats than in untreated CPB ones, suggesting that the use of PHC resulted in an overall decrease in bacteria. In intestinal mucosal injury, PHC can efficiently inhibit NF-κB expression in intestinal mucosal I/R injury. More importantly, PHC can improve the microcirculation, inhibit lipid peroxidation, attenuate the release of lysosomes, and decrease microvascular permeability, leading to the inhibition of inflammation[21].
However, our research was subject to some limitations. PHC treatment was only performed prior to CPB; although this pretreatment was effective, this study did not establish the efficacy of PHC given after CPB. Future studies are required to assess the effects of postoperative treatment with PHC. Additional research is also needed to establish the optimal time of PHC administration.
In conclusion, the present study demonstrated that PHC protected rat intestine from morphological and functional mucosal injury after CPB. These results suggest that PHC could be clinically useful for the treatment of intestinal injury induced by CPB.
An increase in intestinal permeability and bacterial translocation has been demonstrated not only in animal models, but also in patients during cardiopulmonary bypass (CPB). Previous in vitro studies have demonstrated that tropane alkaloids could stabilize the cell membrane and prevent oxidative stress. The new anticholinergic drug penehyclidine hydrochloride (PHC) has been evaluated for its protective effects on the cardiovascular system.
PHC can improve microcirculation, inhibit lipid peroxidation, attenuate the release of lysosomes, and decrease microvascular permeability, leading to the inhibition of inflammation. The effects of PHC on intestinal barrier function during CPB have not been unequivocally addressed. We hypothesized that the administration of PHC could reverse CPB-associated intestinal damage.
The selectivity of PHC in blocking M1, M3 and N receptors means that it has few M2 receptor-associated cardiovascular side effects. It can reduce endotoxin-stimulated acute lung injury and attenuate liver damage during CPB in a rat model. The present study demonstrated that the application of PHC during CPB preserved intestinal barrier function in a dose-dependent manner, and provided histological findings to support these biochemical results.
The present study demonstrated the ability of PHC to protect rat intestine from morphological and functional mucosal injury after CPB. These results suggest that PHC could be clinically useful in the treatment of intestinal injury induced by CPB.
PHC is a new anticholinergic drug with antioxidant and cell membrane-stabilizing activities.
The authors have demonstrated a protective role for PHC in preventing intestinal breakdown during CPB. The conclusion was reached that the administration of PHC could prevent intestinal damage and its sequelae following CPB surgery. This manuscript describes an interesting and well-performed study with convincing results.
Peer reviewer: Dr. Richard A Rippe, Department of Medicine, The University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-7038, United States
S- Editor Sun H L- Editor Kerr C E- Editor Zheng XM
| 1. | Aydin NB, Gercekoglu H, Aksu B, Ozkul V, Sener T, Kiygil I, Turkoglu T, Cimen S, Babacan F, Demirtas M. Endotoxemia in coronary artery bypass surgery: a comparison of the off-pump technique and conventional cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2003;125:843-848. |
| 2. | Riddington DW, Venkatesh B, Boivin CM, Bonser RS, Elliott TS, Marshall T, Mountford PJ, Bion JF. Intestinal permeability, gastric intramucosal pH, and systemic endotoxemia in patients undergoing cardiopulmonary bypass. JAMA. 1996;275:1007-1012. |
| 3. | Watarida S, Mori A, Onoe M, Tabata R, Shiraishi S, Sugita T, Nojima T, Nakajima Y, Matsuno S. A clinical study on the effects of pulsatile cardiopulmonary bypass on the blood endotoxin levels. J Thorac Cardiovasc Surg. 1994;108:620-625. |
| 4. | Tsunooka N, Hamada Y, Imagawa H, Nakamura Y, Shiozaki T, Suzuki H, Kikkawa H, Miyauchi K, Watanabe Y, Kawachi K. Ischemia of the intestinal mucosa during cardiopulmonary bypass. J Artif Organs. 2003;6:149-151. |
| 5. | Shoji T, Omasa M, Nakamura T, Yoshimura T, Yoshida H, Ikeyama K, Fukuse T, Wada H. Mild hypothermia ameliorates lung ischemia reperfusion injury in an ex vivo rat lung model. Eur Surg Res. 2005;37:348-353. |
| 6. | Liang SW, Chen YM. [Effects of penehyclidine hydrochloride or atropine combined with neostigmine for antagonizing residual neuromuscular block on patient's hemodynamics]. Diyi Junyi Daxue Xuebao. 2005;25:1581-1582. |
| 7. | Han XY, Liu H, Liu CH, Wu B, Chen LF, Zhong BH, Liu KL. Synthesis of the optical isomers of a new anticholinergic drug, penehyclidine hydrochloride (8018). Bioorg Med Chem Lett. 2005;15:1979-1982. |
| 8. | Yin L, Li K, Lü L. [Clinical observation of penehyclidine hydrochloride as the preanesthetic medication before operation for patients with cleft lip/palate]. Huaxi Kouqiang Yixue Zazhi. 2008;26:413-415. |
| 9. | Gong P, Zhang Y, Liu H, Zhao GK, Jiang H. [Effects of penehyclidine hydrochloride on the splanchnic perfusion of patients with septic shock]. Zhongguo Weizhongbing Jijiu Yixue. 2008;20:183-186. |
| 10. | Cai DS, Jin BB, Pei L, Jin Z. Protective effects of penehyclidine hydrochloride on liver injury in a rat cardiopulmonary bypass model. Eur J Anaesthesiol. 2010;27:824-828. |
| 11. | Zhan J, Wang Y, Wang C, Li J, Zhang Z, Jia B. Protective effects of penehyclidine hydrochloride on septic mice and its mechanism. Shock. 2007;28:727-732. |
| 12. | Gourlay T, Ballaux PK, Draper ER, Taylor KM. Early experience with a new technique and technology designed for the study of pulsatile cardiopulmonary bypass in the rat. Perfusion. 2002;17:191-198. |
| 13. | Fürst W, Schiesser A. Test for stereospecifity of an automated Dd-lactate assay based on selective removal of Ll-lactate. Anal Biochem. 1999;269:214-215. |
| 14. | Li JY, Lu Y, Hu S, Sun D, Yao YM. Preventive effect of glutamine on intestinal barrier dysfunction induced by severe trauma. World J Gastroenterol. 2002;8:168-171. |
| 15. | Bounous G, Echavé V, Vobecky SJ, Navert H, Wollin A. Acute necrosis of the intestinal mucosa with high serum levels of diamine oxidase. Dig Dis Sci. 1984;29:872-874. |
| 16. | Tsunooka N, Maeyama K, Hamada Y, Imagawa H, Takano S, Watanabe Y, Kawachi K. Bacterial translocation secondary to small intestinal mucosal ischemia during cardiopulmonary bypass. Measurement by diamine oxidase and peptidoglycan. Eur J Cardiothorac Surg. 2004;25:275-280. |
| 17. | Bakker J, Gris P, Coffernils M, Kahn RJ, Vincent JL. Serial blood lactate levels can predict the development of multiple organ failure following septic shock. Am J Surg. 1996;171:221-226. |
| 18. | Dell'Aglio DM, Perino LJ, Kazzi Z, Abramson J, Schwartz MD, Morgan BW. Acute metformin overdose: examining serum pH, lactate level, and metformin concentrations in survivors versus nonsurvivors: a systematic review of the literature. Ann Emerg Med. 2009;54:818-823. |
| 19. | Isbir CS, Ergen A, Tekeli A, Zeybek U, Gormus U, Arsan S. The effect of NQO1 polymorphism on the inflammatory response in cardiopulmonary bypass. Cell Biochem Funct. 2008;26:534-538. |
| 20. | Lei LR, Wang YL, Jia BH. [Protective effect of penehyclidine hydrochloride on lung injury in mice with sepsis and its mechanism]. Zhongguo Weizhongbing Jijiu Yixue. 2007;19:623-624. |
| 21. | Shi H, Dong CM. [The effect of penehyclidine hydrochloride on the expression of inflammatory factor in rat with sepsis-associated lung injury]. Zhongguo Weizhongbing Jijiu Yixue. 2009;21:685-687. |