Published online Apr 7, 2011. doi: 10.3748/wjg.v17.i13.1694
Revised: December 22, 2010
Accepted: December 29, 2010
Published online: April 7, 2011
AIM: To determine the effect of Legalon-SIL (LS) on hepatitis C virus (HCV) core and NS5A expression and on heme oxygenase-1 (HMOX-1) and its transcriptional regulators in human hepatoma cells expressing full length HCV genotype 1b.
METHODS: CON1 cells were treated with 50 μmol/L or 200 μmol/L LS. Cells were harvested after 2, 6 and 24 h. HCV RNA and protein levels were determined by quantitative real-time polymerase chain reaction and Western blotting, respectively.
RESULTS: HCV RNA (core and NS5A regions) was decreased after 6 h with LS 200 μmol/L (P < 0.05). Both 50 and 200 μmol/L LS decreased HCV RNA levels [core region (by 55% and 88%, respectively) and NS5A region (by 62% and 87%, respectively) after 24 h compared with vehicle (dimethyl sulphoxide) control (P < 0.01). Similarly HCV core and NS5A protein were decreased (by 85%, P < 0.01 and by 65%, P < 0.05, respectively) by LS 200 μmol/L. Bach1 and HMOX-1 RNA were also downregulated by LS treatment (P < 0.01), while Nrf2 protein was increased (P < 0.05).
CONCLUSION: Our results demonstrate that treatment with LS downregulates HCV core and NS5A expression in CON1 cells which express full length HCV genotype 1b, and suggests that LS may prove to be a valuable alternative or adjunctive therapy for the treatment of HCV infection.
- Citation: Mehrab-Mohseni M, Sendi H, Steuerwald N, Ghosh S, Schrum LW, Bonkovsky HL. Legalon-SIL downregulates HCV core and NS5A in human hepatocytes expressing full-length HCV. World J Gastroenterol 2011; 17(13): 1694-1700
- URL: https://www.wjgnet.com/1007-9327/full/v17/i13/1694.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i13.1694
Over 170 million people are infected by hepatitis C virus (HCV) worldwide, and of these, approximately 85% will develop chronic hepatitis C (CHC). This could potentially lead to fibrosis, cirrhosis, end-stage liver disease and hepatocellular carcinoma[1]. The ultimate goal of antiviral treatment for hepatitis C is the sustained elimination of HCV. Currently, the standard of care for individuals with CHC is pegylated interferon (IFN) α-2a or IFN α-2b plus ribavirin. However, this protocol is far from ideal. Even under the best conditions of sponsored clinical trials, sustained virologic responses have been achieved in only 40%-50% of those with HCV genotype 1 infection[2,3]. Furthermore, serious side effects are associated with this therapy. The paucity of effective and affordable treatments for HCV-infected patients has led scientists to seek alternative therapies. At present, novel therapeutic agents with various mechanisms of action are under development or in clinical trials.
Although the precise mechanisms underlying hepatocellular injury associated with HCV has yet to be determined, there is compelling evidence that HCV produces increased oxidative stress in human liver cells that is linked to the production of reactive oxygen species (ROS), and consequent increases in cellular lipid peroxidation and other oxidative damage. Oxidative stress appears to be an important aspect of HCV-induced hepatocellular injury[4]. Microsomal heme oxygenase-1 (HMOX-1) is an inducible cytoprotective enzyme that catalyzes the initial and rate-limiting reaction in heme catabolism to release free iron and equimolar amounts of carbon monoxide and biliverdin[5,6]. A variety of DNA-binding proteins interact with regions that contain multiple antioxidant response elements (ARE). Among these are nuclear factor erythroid 2-related factor 2 (Nrf2) and Bach1, a leucine b-zipper transcription protein, which form heterodimers with the small Maf proteins[7,8]. Nrf2 is known to be associated with activation of HMOX-1 and numerous other antioxidant genes in response to multiple agents[9], while Bach1 is a negative regulator of HMOX-1[10].
Milk thistle (Silybum marianum) has been used since ancient times as a liver tonic. Silymarin (SI), a purified extract of polyphenolic flavonoids isolated from milk thistle, is composed mainly of silychristin, silydianin, silybin A, silybin B, isosilybin A and isosilybin B. After oral administration, the SI flavonolignans are rapidly metabolized[11]. Silybin (SBN) constitutes approximately 50% of SI and is the most biologically active component[12]. A number of studies have shown that SI has potent antioxidant and immunomodulatory effects in addition to numerous metabolic actions that may contribute to its purported hepatoprotective actions[13-15].
We recently showed that SI downregulates HCV RNA (core region) and protein in CNS3 cells that stably express HCV RNA core to the amino terminal of NS3 proteins[16]. Another recent study in vitro showed that SI exerts antiviral and antiinflammatory effects in hepatoma cell lines expressing the HCV full length genome of genotype 2a[17]. On the other hand, a randomized, double-blind, placebo-controlled study administering oral SI to CHC patients failed to show a significant effect on either serum aminotransferase levels or quality-of-life measures[18].
Legalon-SIL (LS) is a form of SBN which is a water-soluble formulation of the dihydro-succinate sodium salt of SBN A and SBN B in equal proportion. Recent results from a pilot study in patients with chronic HCV using LS indicate that some SI flavonolignans may have antiviral activity[19]. In this study we assessed the effects of LS on HCV RNA and protein levels in cell lines expressing the full length genome of HCV genotype 1b. We also determined the effects of LS on HMOX-1, Bach1, and Nrf2 expression in these cells.
LS was obtained from Rottapharm-Madaus (Italy). Dimethyl sulphoxide (DMSO) was purchased from Thermo Fisher Scientific Inc (Rock ford, IL, USA). A 100 mmol/L LS stock solution (molecular weight = 726) was prepared in DMSO and filtered through a 0.2 μmol/L nylon filter. LS was prepared fresh just prior to use in each experiment. Mouse monoclonal antibody against HCV core protein was purchased from Abcam (Cambridge, MA, USA). Mouse monoclonal antibody against HCV NS5A was purchased from Virogen Corporation (Watertown, MA, USA). Rabbit polyclonal antibody against HMOX-1 was purchased from Stress Gene (Ann Arbor, MI, USA). Goat monoclonal antibody against Bach1, rabbit polyclonal antibody against Nrf2, and mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) monoclonal antibody were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Enhanced chemiluminescence (ECL)-Plus Western blotting detection reagent was obtained from Amersham Biosciences (Piscataway, NJ, USA).
Huh-7.5 and CON1 subgenomic genotype 1b HCV cell lines were from Apath LLC (St. Louis, MO, USA). Huh-7.5 is a highly permissive, IFN-cured Huh-7 human hepatocellular carcinoma cell line derivative. The CON1 cell line is a Huh-7.5 cell population containing the full-length HCV genotype 1b replicon.
Huh-7.5 and CON1 cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, and selection antibiotic for CON1 cells (750 μg/mL G418).
Cellular proliferation of treated CON1 cells was assessed by measuring the conversion of MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide] to MTT formazan (Sigma-Aldrich). The absorbance was measured on a Synergy HT microtiter plate reader (Biotek Instruments, Winooski, VT, USA), at a wavelength of 570 nm with background subtraction at 690 nm. Decreases in absorption were taken as an index of decreased cellular proliferation.
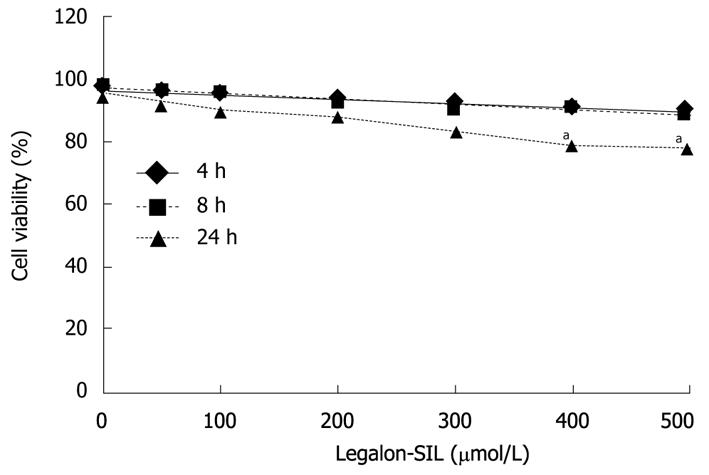
The viability of CON1 cells treated with LS was also confirmed by the standard propidium iodide [(PI); Invitrogen, USA)] assay. The experiment was performed according to the manufacturer’s recommended protocol. CON1 cells were plated in 12-well plates 24 h before treatment and incubated at 37°C. LS was dissolved in DMSO and added to the cell culture medium. The effects of LS on cell viability were studied at various concentrations (0, 50, 100, 200, 300, 400 and 500) μmol/L and at different time points (4, 8, and 24 h). Percent cell viability was determined by counting cell density in drug-treated cells and in DMSO-treated cells as control in the same incubation period [percentage of cellular viability = (total cell count-PI positive cell count)/total cell count*100]. All experiments were repeated 3 times.
RNA from treated cells was isolated by TRIZOL reagent (Invitrogen). The RNA concentration and purity were determined by measuring absorbance at 260/280 nm. Reverse transcription was performed on 1 μg of total RNA to generate cDNA using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA, USA). Quantitative real time reverse transcriptase (RT)-polymerase chain reaction (PCR) was performed using a CFX-96 Real-Time PCR Detection System (Bio-Rad Laboratories) and iQTM SYBR Green Supermix (Bio-Rad Laboratories). Sequence specific primers for HMOX-1, Bach1, and GAPDH were designed as described[20]. Nucleotide sequences of other primers are as follows: NS5A: Forward primer, 5'-CGGACGTAGCAGTGCTCACTTC-3' and reverse primer, 5-'TGATGAGCTGGCCAAGGAGG-3'; Nrf2: Forward primer, 5'-CCTTTCTCGCCTAGGCATCA-3', reverse primer, 5'-CCCTTCAGCTCTCCCTACCG-3'. Fold change values were calculated by comparative cycle threshold (Ct) value analysis after normalizing for the quantity of GAPDH mRNA in samples.
Cells were grown to near confluence and washed with phosphate-buffered saline (PBS), lysed in a buffer containing 1% Triton X-100 with PBS and Halt Protease Inhibitor Cocktail (Pierce Chemicals, Rockford, IL, USA). Protein concentrations were measured using the bicinchoninic acid method. Total proteins (10 μg) were separated on 4%-12% gradient sodium dodecyl sulphate-polyacrylamide gel (Invitrogen Laboratories) and electrophoretically transferred onto an Immun-Blot PVDF (Invitrogen Laboratories). The membranes were blocked for 1 h in PBS containing 5% nonfat dry milk, and then incubated for 1 h with the primary antibody at room temperature. The dilutions of the primary antibodies were as follows: 1:1000 for anti-HCV core antibody; 1:1000 for anti-HCV NS5A antibody; 1:500 for anti-HMOX-1, and 1:1000 for anti-Bach1, anti-Nrf2, and anti-GAPDH antibody. After 4 washes with 0.1% Tween 20 in PBS (PBS-T), the membranes were incubated for 1 h with a secondary antibody (anti-rabbit, anti-goat or anti-mouse immunoglobulin G; dilution 1:10 000). Finally, the membranes were washed 4 times with PBS-T, and the bound antibodies were visualized with the ECL-Plus chemiluminescence system. A computer based imaging system, LAS 3000 (Fuji Film, USA) was used to measure the relative optical density of each specific band obtained after Western blotting.
Data are expressed as mean ± SE of the mean. Statistical differences between groups were analyzed by analysis of variance followed by Dunnett’s test. P < 0.05 was considered significant.
Appropriate doses of LS in the cell lines were determined. Effects of different doses of LS on cell viability in CON1 cells were assessed by PI staining (Figure 1). LS concentrations of 50-300 μmol/L had no significant effect on cell viability, whereas concentrations equal to or more than 400 μmol/L caused significant cytotoxicity in the cells (P < 0.05). Similar results were demonstrated with the MTT proliferation assay (data not shown).
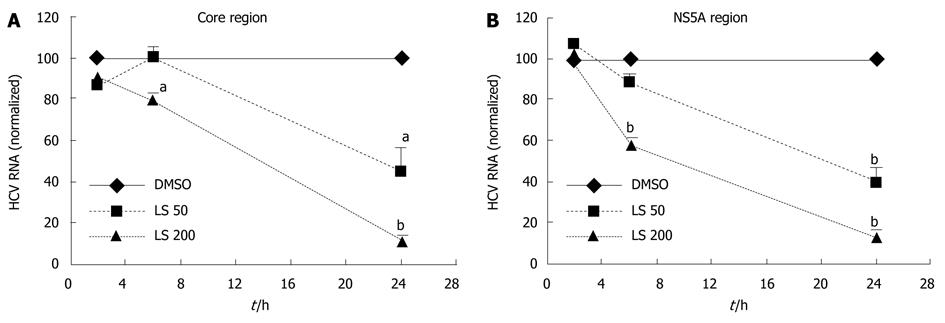
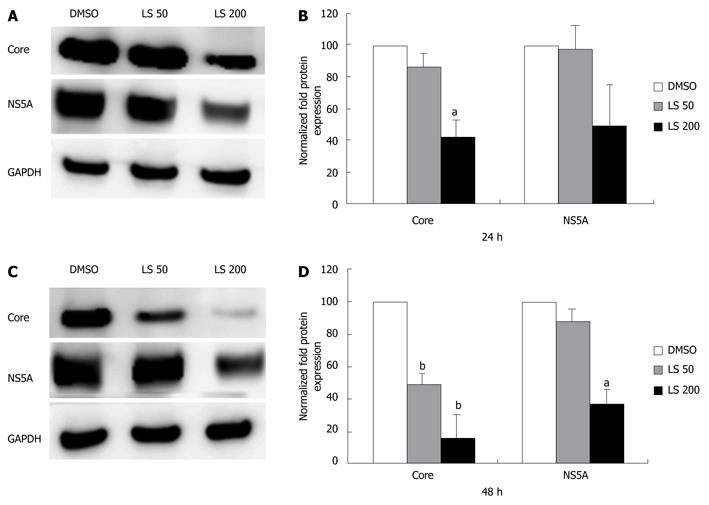
LS downregulated HCV RNA (core region) in a dose-dependent and also a time-dependent manner in CON1 cells. The HCV RNA (core region) level was decreased 21% following 6 h treatment with LS 200 μmol/L compared with the DMSO control (P < 0.05, Figure 2A). HCV RNA (core region) levels were further decreased after 24 h treatment by both LS 50 μmol/L (55% decrease, P < 0.05) and 200 μmol/L (88% decrease, P < 0.01) when compared with vehicle (DMSO) control (Figure 2A). The HCV RNA (NS5A region) level was also decreased 43% following 6 h treatment with LS 200 μmol/L compared with DMSO control (P < 0.01 Figure 2B), and was also further decreased after 24 h treatment by both LS 50 μmol/L (62% decrease, P < 0.01) and 200 μmol/L (87% decrease, P < 0.01) (Figure 2B). LS 200 μmol/L also downregulated HCV core (by 57%) and NS5A protein (by 49%) after 24 h of treatment although this was statistically significant only for HCV core protein, P < 0.05). This effect was more pronounced following 48 h of treatment: LS 200 μmol/L decreased HCV NS5A protein expression by 65% (P < 0.05), while LS significantly decreased HCV core protein expression in a dose-dependent manner (52% reduction at 50 μmol/L and 85% reduction at 200 μmol/L P < 0.01) (Figure 3).
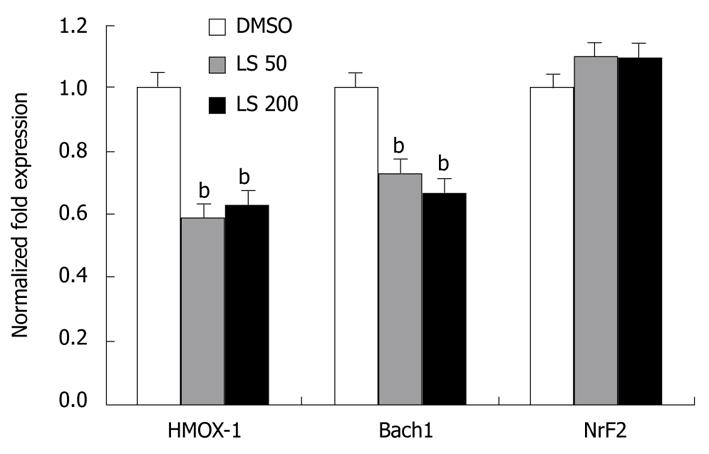
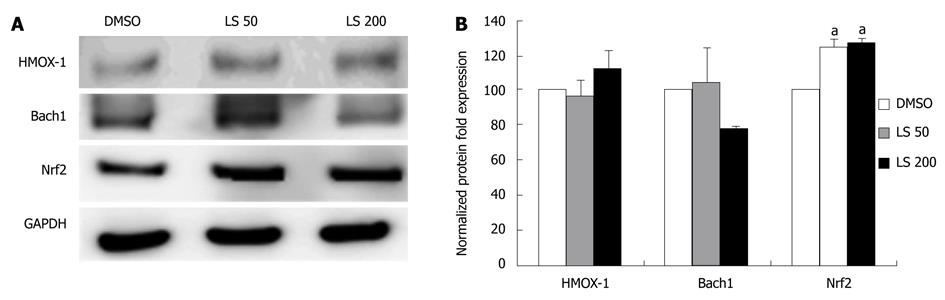
HMOX-1 and Bach1 mRNA levels were significantly decreased following 24 h treatment by both LS 50 μmol/L and 200 μmol/L when compared with the DMSO control (HMOX-1 decreased by 40%, P < 0.01; Bach1 decreased by 35%, P < 0.01; Figure 4). LS treatment decreased Bach1 protein level, although not significantly, while it significantly increased Nrf2 protein expression (P < 0.05) (Figure 5).
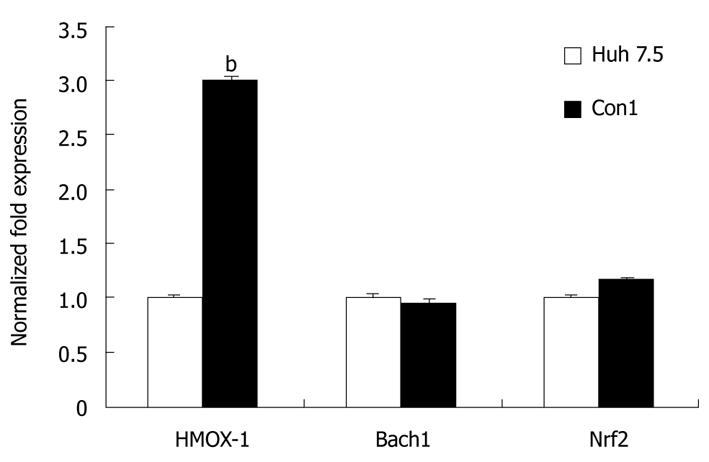
For investigation of possible effects of stable transfection of the HCV genome in CON1 replicon system, we compared HMOX-1, Bach1, and Nrf2 mRNA levels between CON1 cells and Huh7.5 cells, the ‘parental’ cell line. In untreated CON1 cells, the HMOX-1 mRNA level was 3-fold higher than in untreated Huh7.5 cells (P < 0.01), while there was no significant difference in the level of Bach1 or Nrf2 mRNA between CON1 and Huh7.5 cells (Figure 6).
The current treatment for CHC is a combination of pegylated IFN-α and ribavirin which is effective only in 40%-50% of treated patients infected with genotype-1, by far the most frequent HCV genotype worldwide. This therapeutic regimen is expensive, prolonged (usually at least 48 wk) and causes serious side effects. Therefore investigations continue to search for alternative treatments for hepatitis C. SI is an herbal remedy that has been used to treat acute and chronic liver diseases for millennia[12-22]. Despite this broad use, the exact molecular mechanism by which SI confers hepatoprotection is yet to be elucidated. Ferenci et al[19] recently showed that LS, as used in our studies, significantly reduced serum HCV RNA level in patients who had not responded to combination therapy with full dose pegylated IFN and ribavirin. They showed that SBN was effective only when administered intravenously, as LS, and that the antiviral effect was dose-dependent. They reported that HCV RNA was undetectable in 7 (out of 14) patients receiving 15 and 20 mg/kg SBN as LS. In a recent study in vitro[23], SBN A, SBN B, a mixture of SBN A and SBN B, and LS were shown to inhibit HCV RNA-dependent RNA polymerase (RdRP) function and inhibited HCV genotype 1b sub-genomic replicon replication and HCV genotype 2a strain JFH1 replication in cell culture. Our results extend these findings showing that LS inhibits HCV replication in human hepatoma cells expressing full-length HCV genotype 1b. In this study, we also showed that LS doses equal to 400 μmol/L and higher are cytotoxic for human hepatoma cells similar to other recent results[23]. The exact pharmacokinetics of SI and LS remain to be determined. However, it is suggested that oral doses of SI up to 2.1 g/d are safe and well-tolerated[24]. In the present study, we showed that LS started to downregulate HCV RNA in a dose-dependent manner after 2 h, but the significant effect was observed after 6 h of treatment. Although LS 200 μmol/L was found to downregulate HCV RNA and proteins significantly in CON1 cells, LS 50 μmol/L was also found to be effective in downregulation of HCV RNA.
In addition to a direct effect of LS on HCV replication, for instance inhibition of RdRP, LS might downregulate HCV through interaction with signaling molecules or other as yet unknown host factors. For example, Polyak et al[25] reported that SI suppresses TNF-α activation of nuclear factor-κB-dependent transcription, without affecting binding of p50 and p65 to DNA. They also reported that all SI-related compounds which they studied blocked JFH-1 virus-induced oxidative stress, including compounds that lacked antiviral activity.
In the present study, we found that LS decreased HMOX-1 mRNA in CON1 cells as well as the mRNA level of Bach1, which is its transcriptional repressor. However, no effect was observed on HMOX-1 and Bach1 protein levels, despite modest upregulation of Nrf2 protein. Bach1 and Nrf2 compete for binding to the ARE and exert their Bach1-induced repressive, or Nrf2-induced activating effects on HMOX-1 transcription. It is noteworthy to mention that for Nrf2 to bind to ARE, Bach1 needs to be dislocated from the binding sites[26]. Therefore, a lower level of Bach1 is necessary for the maximal effect of Nrf2 on HMOX-1 transcription. As our study did not show downregulation of Bach1 protein, it is possible to hypothesize that Bach1 does not dissociate from ARE to allow Nrf2 to bind. Therefore HMOX-1 is not upregulated despite modest elevation of Nrf2 protein. Additionally, other HMOX-1 transcriptional factors may be affected by LS (e.g. changes in Keap1 or Kelch, glutathione, other thiol-containing compounds, levels of heme, other metalloporphyrins, etc.) which were not all examined in this study[27].
Oxidative stress plays an important role in various diseases, including viral infection and chronic inflammation. HCV gene expression, in particular HCV RNA (core region) expression, can increase the levels of ROS[28], and therefore upregulate HMOX-1[20,29]. We also compared HMOX-1 mRNA levels between CON1 cells and its parental cell line (Huh7.5), and we identified a 3-fold upregulation of HMOX-1 in the HCV replicon system compared with the parental cell line. Although SBN is a well-known antioxidant and cytoprotective enzyme, and upregulation of Nrf2 protein could be attributed to this antioxidant effect of LS, lack of HMOX-1 upregulation could be explained by anti-HCV activity of LS. Since HMOX-1 is already increased by HCV infection in CON1 cells, its level is decreased toward normal levels as LS attenuates HCV infection. This downregulatory effect of LS on HMOX-1 seems to be more pronounced at the RNA level, and is compensated by an Nrf2 increase at the protein level.
In conclusion, LS downregulates HCV in hepatoma cell lines expressing full length HCV genotype 1b. LS treatment is also associated with downregulation of HMOX-1 mRNA and upregulation of Nrf2 protein. Further research is needed to further delineate mechanisms of the LS effect on HCV replication and on CHC.
Chronic hepatitis C virus (HCV) infection is a global health problem, and one causal factor for development of liver cirrhosis and hepatocellular carcinoma. The current therapy for HCV is interferon (IFN) α-2a or IFNα-2b plus ribavirin which is effective in only 40%-50% of those with HCV genotype 1 infection. Furthermore, serious side effects are associated with this therapy. The paucity of effective and affordable treatments for HCV-infected patients has led scientists to seek alternative therapies.
Silymarin, also known as milk thistle extract, inhibits HCV infection and also displays antioxidant, antiinflammatory, and immunomodulatory actions that contribute to its hepatoprotective effects. In this study the authors demonstrated that Legalon-SIL (LS), a water-soluble formulation of silybin A and silybin B downregulates expression of HCV mRNA and protein in replicon CON1 cells which are human hepatoma cell lines stably transfected with full-length HCV genotype 1b.
This report highlighted that LS decreases HCV core and NS5A expression. Additionally, the authors demonstrated that LS modulates expression of heme oxygenase-1 and its transcriptional regulators, Bach1 and Nrf2. This is the first study examining the effects of LS using a human hepatoma cell line expressing full-length HCV genotype 1b.
Our results suggest that LS may prove to be a valuable alternative or adjunctive therapy for the treatment of HCV infection.
LS is a form of SBN which is a water-soluble formulation of the dihydro-succinate sodium salt of silybin A and silybin B in equal proportions.
The paper will appeal to clinicians as well as researchers involved in the field of elucidating the mechanisms of silymarin in HCV positive patients.
Peer reviewer: Dr. Jeff Butterworth, MB, FRCP, Department of Gastroenterology, Shrewsbury and Telford Hospital NHS Trust, Mytton Oak Road, Shrewsbury, Shropshire, SY3 8XQ, United Kingdom
S- Editor Tian L L- Editor Cant MR E- Editor Ma WH
| 1. | Hoofnagle JH. Course and outcome of hepatitis C. Hepatology. 2002;36:S21-S29. |
| 2. | Hadziyannis SJ, Sette H Jr, Morgan TR, Balan V, Diago M, Marcellin P, Ramadori G, Bodenheimer H Jr, Bernstein D, Rizzetto M. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346-355. |
| 3. | McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, Goodman ZD, Ling MH, Cort S, Albrecht JK. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485-1492. |
| 4. | Abdalla MY, Ahmad IM, Spitz DR, Schmidt WN, Britigan BE. Hepatitis C virus-core and non structural proteins lead to different effects on cellular antioxidant defenses. J Med Virol. 2005;76:489-497. |
| 5. | Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA. 1968;61:748-755. |
| 6. | Tenhunen R, Marver HS, Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969;244:6388-6394. |
| 7. | Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313-322. |
| 8. | Motohashi H, Shavit JA, Igarashi K, Yamamoto M, Engel JD. The world according to Maf. Nucleic Acids Res. 1997;25:2953-2959. |
| 9. | Shan Y, Lambrecht RW, Donohue SE, Bonkovsky HL. Role of Bach1 and Nrf2 in up-regulation of the heme oxygenase-1 gene by cobalt protoporphyrin. FASEB J. 2006;20:2651-2653. |
| 10. | Shan Y, Lambrecht RW, Ghaziani T, Donohue SE, Bonkovsky HL. Role of Bach-1 in regulation of heme oxygenase-1 in human liver cells: insights from studies with small interfering RNAS. J Biol Chem. 2004;279:51769-51774. |
| 11. | Wen Z, Dumas TE, Schrieber SJ, Hawke RL, Fried MW, Smith PC. Pharmacokinetics and metabolic profile of free, conjugated, and total silymarin flavonolignans in human plasma after oral administration of milk thistle extract. Drug Metab Dispos. 2008;36:65-72. |
| 12. | Crocenzi FA, Roma MG. Silymarin as a new hepatoprotective agent in experimental cholestasis: new possibilities for an ancient medication. Curr Med Chem. 2006;13:1055-1074. |
| 13. | Dehmlow C, Murawski N, de Groot H. Scavenging of reactive oxygen species and inhibition of arachidonic acid metabolism by silibinin in human cells. Life Sci. 1996;58:1591-1600. |
| 14. | Lee JS, Kim SG, Kim HK, Lee TH, Jeong YI, Lee CM, Yoon MS, Na YJ, Suh DS, Park NC. Silibinin polarizes Th1/Th2 immune responses through the inhibition of immunostimulatory function of dendritic cells. J Cell Physiol. 2007;210:385-397. |
| 15. | Manna SK, Mukhopadhyay A, Van NT, Aggarwal BB. Silymarin suppresses TNF-induced activation of NF-kappa B, c-Jun N-terminal kinase, and apoptosis. J Immunol. 1999;163:6800-6809. |
| 16. | Bonifaz V, Shan Y, Lambrecht RW, Donohue SE, Moschenross D, Bonkovsky HL. Effects of silymarin on hepatitis C virus and haem oxygenase-1 gene expression in human hepatoma cells. Liver Int. 2009;29:366-373. |
| 17. | Polyak SJ, Morishima C, Shuhart MC, Wang CC, Liu Y, Lee DY. Inhibition of T-cell inflammatory cytokines, hepatocyte NF-kappaB signaling, and HCV infection by standardized Silymarin. Gastroenterology. 2007;132:1925-1936. |
| 18. | Gordon A, Hobbs DA, Bowden DS, Bailey MJ, Mitchell J, Francis AJ, Roberts SK. Effects of Silybum marianum on serum hepatitis C virus RNA, alanine aminotransferase levels and well-being in patients with chronic hepatitis C. J Gastroenterol Hepatol. 2006;21:275-280. |
| 19. | Ferenci P, Scherzer TM, Kerschner H, Rutter K, Beinhardt S, Hofer H, Schöniger-Hekele M, Holzmann H, Steindl-Munda P. Silibinin is a potent antiviral agent in patients with chronic hepatitis C not responding to pegylated interferon/ribavirin therapy. Gastroenterology. 2008;135:1561-1567. |
| 20. | Ghaziani T, Shan Y, Lambrecht RW, Donohue SE, Pietschmann T, Bartenschlager R, Bonkovsky HL. HCV proteins increase expression of heme oxygenase-1 (HO-1) and decrease expression of Bach1 in human hepatoma cells. J Hepatol. 2006;45:5-12. |
| 21. | Seeff LB, Curto TM, Szabo G, Everson GT, Bonkovsky HL, Dienstag JL, Shiffman ML, Lindsay KL, Lok AS, Di Bisceglie AM. Herbal product use by persons enrolled in the hepatitis C Antiviral Long-Term Treatment Against Cirrhosis (HALT-C) Trial. Hepatology. 2008;47:605-612. |
| 22. | Zhang L, Wang G, Hou W, Li P, Dulin A, Bonkovsky HL. Contemporary clinical research of traditional Chinese medicines for chronic hepatitis B in China: an analytical review. Hepatology. 2010;51:690-698. |
| 23. | Ahmed-Belkacem A, Ahnou N, Barbotte L, Wychowski C, Pallier C, Brillet R, Pohl RT, Pawlotsky JM. Silibinin and related compounds are direct inhibitors of hepatitis C virus RNA-dependent RNA polymerase. Gastroenterology. 2010;138:1112-1122. |
| 24. | Hawke RL, Schrieber SJ, Soule TA, Wen Z, Smith PC, Reddy KR, Wahed AS, Belle SH, Afdhal NH, Navarro VJ. Silymarin ascending multiple oral dosing phase I study in noncirrhotic patients with chronic hepatitis C. J Clin Pharmacol. 2010;50:434-449. |
| 25. | Polyak SJ, Morishima C, Lohmann V, Pal S, Lee DY, Liu Y, Graf TN, Oberlies NH. Identification of hepatoprotective flavonolignans from silymarin. Proc Natl Acad Sci USA. 2010;107:5995-5999. |
| 26. | Reichard JF, Motz GT, Puga A. Heme oxygenase-1 induction by NRF2 requires inactivation of the transcriptional repressor BACH1. Nucleic Acids Res. 2007;35:7074-7086. |
| 27. | Gozzelino R, Jeney V, Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323-354. |
| 28. | Gong G, Waris G, Tanveer R, Siddiqui A. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-kappa B. Proc Natl Acad Sci USA. 2001;98:9599-9604. |
| 29. | Sendi H, Mehrab-Mohseni M, Steuerwald N, Bonkovsky HL. Heme oxygenase 1 [HMOX1] expression is up-regulated by HCV in human hepatoma cells and is normalized through IFN treatment. Hepatology. 2009;50:S936A. |