Published online Mar 28, 2011. doi: 10.3748/wjg.v17.i12.1584
Revised: February 15, 2011
Accepted: February 22, 2011
Published online: March 28, 2011
AIM: To study whether over-starvation aggravates intestinal mucosal injury and promotes bacterial and endotoxin translocation in a high-altitude hypoxic environment.
METHODS: Sprague-Dawley rats were exposed to hypobaric hypoxia at a simulated altitude of 7000 m for 72 h. Lanthanum nitrate was used as a tracer to detect intestinal injury. Epithelial apoptosis was observed with terminal deoxynucleotidyl transferase dUTP nick end labeling staining. Serum levels of diamino oxidase (DAO), malondialdehyde (MDA), glutamine (Gln), superoxide dismutase (SOD) and endotoxin were measured in intestinal mucosa. Bacterial translocation was detected in blood culture and intestinal homogenates. In addition, rats were given Gln intragastrically to observe its protective effect on intestinal injury.
RESULTS: Apoptotic epithelial cells, exfoliated villi and inflammatory cells in intestine were increased with edema in the lamina propria accompanying effusion of red blood cells. Lanthanum particles were found in the intercellular space and intracellular compartment. Bacterial translocation to mesenteric lymph nodes (MLN) and spleen was evident. The serum endotoxin, DAO and MDA levels were significantly higher while the serum SOD, DAO and Gln levels were lower in intestine (P < 0.05). The bacterial translocation number was lower in the high altitude hypoxic group than in the high altitude starvation group (0.47 ± 0.83 vs 2.38 ± 1.45, P < 0.05). The bacterial translocation was found in each organ, especially in MLN and spleen but not in peripheral blood. The bacterial and endotoxin translocations were both markedly improved in rats after treatment with Gln.
CONCLUSION: High-altitude hypoxia and starvation cause severe intestinal mucosal injury and increase bacterial and endotoxin translocation, which can be treated with Gln.
- Citation: Zhou QQ, Yang DZ, Luo YJ, Li SZ, Liu FY, Wang GS. Over-starvation aggravates intestinal injury and promotes bacterial and endotoxin translocation under high-altitude hypoxic environment. World J Gastroenterol 2011; 17(12): 1584-1593
- URL: https://www.wjgnet.com/1007-9327/full/v17/i12/1584.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i12.1584
Multiple organs can be damaged by rapid ascent to an altitude above 3000 m. High altitude cerebral edema (HACE) and high altitude pulmonary edema (HAPE) are the clinical manifestations of acute mountain sickness (AMS)[1-3]. AMS is a threat to those who live at or ascend to a high altitude. Our previous study showed that multiple organ dysfunction syndrome (MODS) can occur if HAPE and HACE are not treated timely and properly, and the condition of such patients can deteriorate, thus complicating their treatment[4]. However, the mechanism underlying acute severe mountain sickness (ASMS) accompanying MODS is still poorly understood.
The gastrointestinal tract is an important organ, and its primary function is to digest and absorb nutrients. However, in addition to nutrient absorption, the gastrointestinal tract functions as a barrier to prevent the translocation of intraluminal bacteria and endotoxin to systemic organs and tissues. It has been confirmed that intestinal mucosal injury caused by a variety of factors may decrease the intestinal barrier function, thus leading to the translocation of intraluminal bacteria and endotoxin to systemic organs and tissues, which is a principal cause of systemic inflammatory response syndrome (SIRS), MODS and multiple organ failure[5-7]. However, little is known about the relation between intestinal barrier dysfunction and ASMS accompanying MODS, and whether the intestinal barrier dysfunction caused by hypobaric hypoxia promotes bacterial translocation and spread of endotoxin. In addition, the degree of dysfunction caused by hypobaric hypoxia and the role of acute intestinal barrier dysfunction in the occurrence and development of ASMS are still unclear.
To explore the detrimental effect of hypobaric hypoxia on intestinal barrier function and the role of acute intestinal barrier dysfunction in the occurrence and development of ASMS, we examined the microstructure and ultra-structure of intestinal mucosa from rats exposed to hypobaric hypoxia, under light and electron microscopes. The activity of serum and intestinal diamine oxidase (DAO), malondialdehyde (MDA), superoxide dismutase (SOD) and NO, as well as glutamine (Gln) was assayed. In addition, rats were given Gln intragastrically to explore its protective effect on intestinal mucosa. The results of this study may provide the functional and morphological information about the effect of ASMS on the spread of endotoxin and bacterial translocation, as well as important information about the pathogenesis, prevention and treatment of MOSD caused by hypobaric hypoxia.
Instruments used in the present study were animal decompression chamber (Guizhou Aviation Industry, China), electronic balance (Shanghai Balance, China), ultralow freezer (Heraneus, Germany), microplate reader (Bio-tek, USA), spectrophotometer (Changsha Persee, China), endotoxin detection system (Tianjin Wireless Electronics, China), scanning electron microscope (Hitachi, Japan) and transmission electron microscope (Philips, Netherlands).
Reagents used in this study include compound glutamine granules (Heilongjiang Aolida, China), horseradish peroxidase (Shanghai Guoyuan Biotech, China), 3,3’-dimethoxybenzidine (Sigma, USA), cadaverine dihydrochloride (Sigma, USA), lysine (Sigma, USA), protein kinase (Amresco, USA), levamisole (Sigma, USA), sodium dimethyl arsenite (Shanghai Genebase, China), limulus test kit (Zhanjiang Bokang, China), control standard endotoxin (Zhanjiang Bokang, China), terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) kit (Roche, Switzerland), MDA kit (Nanjing Jiancheng, China), NO kit (Nanjing Jiancheng, China), SOD kit (Nanjing Jiancheng, China), glutamine kit (Nanjing Jiancheng, China) and lanthanum nitrate (Chongqi Boyi, China).
A total of 40 male Sprague-Dawley rats weighing 200 ± 20 g were purchased from Animal Center of Third Military Medical University (Chongqing, China) and housed in dedicated cages. The rats were randomly divided into normal control group (C), hypobaric hypoxia group (H), hypobaric hypoxia plus starvation group (HH), and hypobaric hypoxia plus Gln group (HG), 10 rats in each group. All procedures were performed in accordance with the Animal Care Guidelines of Third Military Medical University, conforming to the Health Guide for the Care and Use of Laboratory Animals of Third Military Medical University. This study was approved by the Ethics Committee of Third Military Medical University.
Rats in the control group were housed under a normal atmospheric pressure and weighed daily, with free access to food. Rats in groups H, HH and HG were exposed to a simulated altitude of 7000 m for 72 h in an animal decompression chamber, to established rat model of hypoxia exposure. Rats in group H had free access to food and water and weighed daily, rats in group HH were fasted with free access to water, and rats in group HG had free access to food and water in addition to intragastric Gln (0.5 g/d per 100 g body weight) at 09:00, am, daily. Gln was prepared in warm water at a dilution of 1:4. The animals were taken out of the chamber for feeding, medication, weighing and cleaning for 30 min, and returned to the chamber for continuous hypoxia exposure.
Three days after exposed to hypobaric hypoxia, the rats were sacrificed by decapitation with 5 mL blood collected into a pyrogen-free tube. The blood was centrifuged at 2500 r/min at -4°C, and serum was stored at -20°C before use. Additionally, the heart, liver, spleen, lungs and mesenteric lymph nodes (MLN) were removed from rats, weighed, and subjected to bacterial culture. About 5 cm of ileum, approximately 5 cm from the ileocecal junction, was obtained for light and electron microscopy. About 3 cm of intestine was put into a 3-fold volume of PBS (0.1 mol/L, pH 7.2), homogenized and centrifuged at 1000 r/min for 30 min. Then, the supernatant was collected.
The spontaneous activity, mental state, eating status and weight of rats were observed.
About 2 cm of intestine at 5 cm from the ileocecal valve was obtained and cut open longitudinally. The intestine was washed with normal saline, fixed in 10% formaldehyde at 4°C for 24 h, rinsed with PBS, embedded in paraffin and consecutively cut into 4-μm thick sections which were stained with hematoxylin and eosin (HE). The structure of intestinal mucosal epithelium was observed and the thickness of mucosa was measured[8]. The height and area of 15 randomly selected intestinal villi were measured and averaged[9] according to the following equation: Area = 2πrh, where r represents the radius of villus and h is the height of villus. The number of villi was counted in each field of vision.
About 2 cm of intestine at 5 cm from the ileocecal valve was obtained and cut open longitudinally. The intestine was washed with normal saline, fixed in 10% formaldehyde at 4°C for 24 h, rinsed with PBS, embedded in paraffin, and consecutively cut into 4-μm thick sections which were mounted onto 0.05% lysine-treated slides. The slides were dried at room temperature for 20 min, dry-heated at 60°C for 30 min and stored at room temperature before use. The sections were stained with TUNEL as previously described[10]. The cells with blue nuclei were considered positive cells. Two slides of each sample were selected, and 4 randomly selected fields were used to calculate the apoptotic intestinal mucosal epithelial cells at a magnification × 400. The counts were averaged. The apoptotic index (AI) was calculated as follows: number of TUNEL positive cells/number of total cells[11].
Part of the ileum was obtained, rinsed with cold normal saline, and cut into 2 mm × 2 mm sections which were fixed in 2.5% glutaraldehyde and 10% osmium, dehydrated in sucrose solution containing PBS. Subsequently, the cells were labeled with gold and observed under a scanning electron microscope[12]. The arrangement of microvilli in intestinal mucosa and organelles in columnar epithelial cells were observed. Special attention was paid to the deformed and exfoliated villi and the intercellular space between epithelia.
Jejunal tissue was cut into 0.7 cm × 0.7 cm sections and adherent feces were removed from the colon mucosa with saline. The sections were immediately placed into a 4°C fixative (4 g paraformaldehyde, 20 mL glutaraldehyde, 100 mL 0.2 mol/L phosphate buffer solution, and 80 mL distilled water, pH 7.4) for 2 h, and then cut into 1 mm × 1 mm ×1 mm pieces. The specimens were washed three times (10 min each) with 10% sucrose phosphate buffer, fixed in 1% osmium tetroxide at 4°C for 1 h, dehydrated, embedded in resin, then cut into ultra-thin sections which were stained with uranyl acetate and lead citrate, and examined by transmission electron microscopy and photographed. The tight junctions between intestinal mucosal epithelia, arrangement of microvilli, integrity of columnar epithelial cells, organelles and nuclei, and structure of glands in lamina propria were observed.
Two rats in each group were anesthetized through intraperitoneal administration of 1% sodium pentobarbital (1.0 mL/100 g body weight). Transcardial perfusion was performed with a mixture containing 3% glutaraldehyde, 4% paraformaldehyde and 2% lanthanum nitrate. The mixture was prepared with 0.1 mol/L sodium dimethyl arsenite in PBS. Part of the intestine was obtained and fixed in the same mixture followed by 1% osmium. The tissues were rinsed with 0.1 mol/L sodium dimethyl arsenite solution, and slides were prepared as indicated for transmission electron microscopy[13].
The blood, heart, liver, spleen, lungs and MLN were independently placed into a 9-fold volume of normal saline, and the mixture was homogenized. Then, 0.5 mL homogenates was loaded onto the MacConkey solid medium to culture bacteria for 24 h, and the bacteria were analyzed biochemically.
The serum endotoxin level was measured by limulus test as previously described[14]. The serum was prepared with warm water, and 0.1 mL serum was mixed with 0.9 mL processing solution and incubated at 70°C for 15 min. Subsequently, 0.2 mL serum was added into the enzyme reaction solution and reacted in the endotoxin detection system EDS-99 for 1 h. The serum endotoxin level was automatically outputted from the system.
About 0.5 mL serum was mixed with a solution containing 0.1 mol/L PBS (3 mL, pH 7.2), horseradish peroxidase (4 g, 0.1 mL), 3,3´-dimethoxybenzidine (500 g, 0.1 mL) and cadaverine dihydrochloride (175 g, 0.1 mL) and incubated at 37°C for 30 min. The solution was replaced by PBS as a blank control. The optical density (OD) was detected at 436 nm, and the DAO content was measured. The standard curve was delineated with DAO[15]. About 0.5 mL intestinal homogenates was used to measure the DAO content with the same method. Approximately 0.1 mL serum was used to detect the SOD activity and measure the NO and MDA content as previously described[16]. The SOD and NO contents were measured using the nitrate reductase activity, the MDA content was measured using the thiobarbituric acid method, and the OD values of Gln were calculated using the Gln synthetic enzymatic method. In addition, the content of proteins in homogenates was measured with Coomassie brilliant blue, and the OD value of Gln was obtained and the Gln content was measured.
Statistical analysis was performed using the SPSS version 13.0, and quantitative data were presented as mean ± SD. One-way ANOVA was used for comparison between groups. Qualitative data were expressed as percentages, and t test was used for comparison of means between groups. P < 0.05 was considered statistically significant.
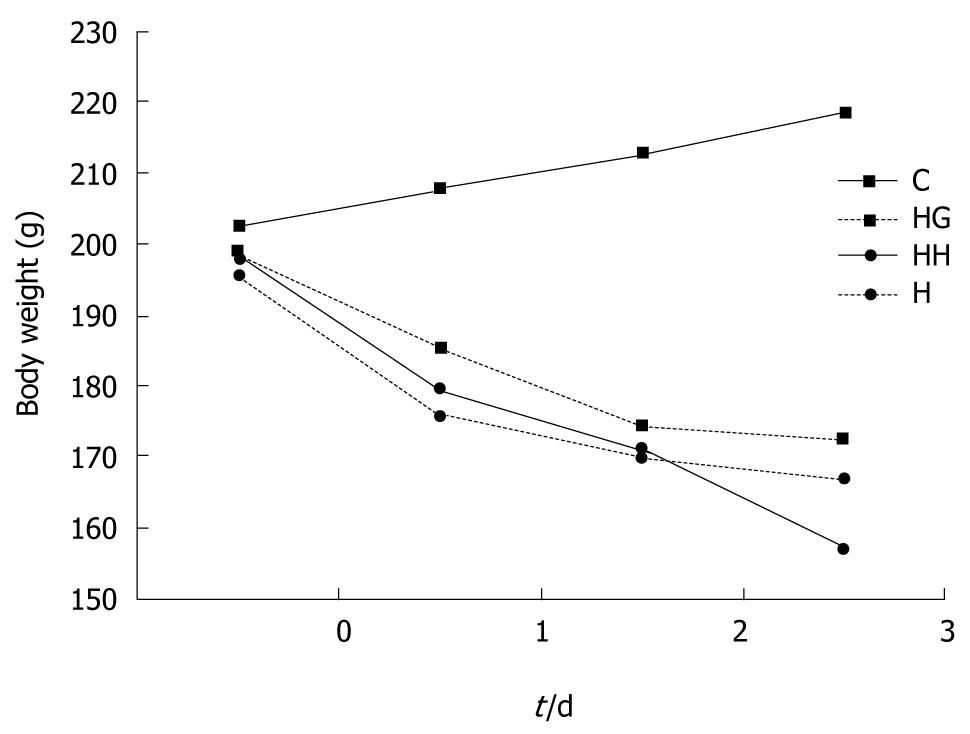
No rats died during the experiment. The activity of rats was markedly lower in groups H, HH and HG with a poor mental state than in control group. The food-intake was significantly higher in group C than in group H (50 g/d vs 23 g/d, P < 0.05). The food intake was slight decreased in group HG (38 g/d). The body weight in group C increased stably, and decreased most evidently in group HH, followed by groups H and HG (Figure 1).
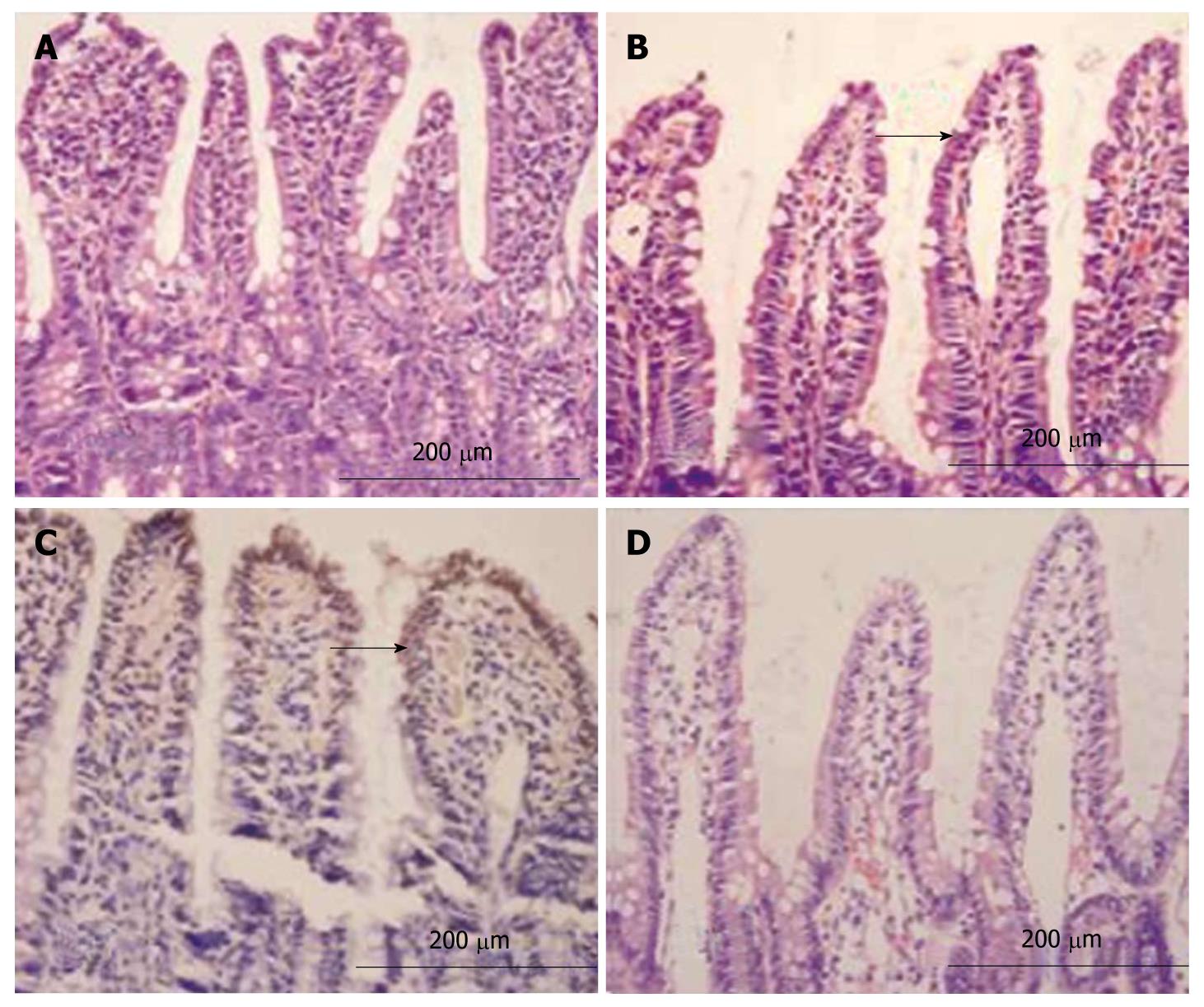
The intestinal mucosa was smooth with intact epithelia and ordered arrangement of villi in group C with no defects detected in villi (Figure 2A). The intestinal mucosa was exfoliated and the villi became thinner in group H. In addition, the number of mucosal villi was reduced, and the villi were irregular and disorganized (Figure 2B). In group HH, atrophic and thinned of villi accompanying a loose and disordered arrangement were observed with edema and infiltration of inflammatory cells in the mastoid lamina of villi, lodged and exfoliated villi with loss of goblet cells and effusion of red blood cells around the capillaries (Figure 2C). In group HG, the intestinal mucosal villi were relatively intact with ordered arrangement with alleviated edema in the mastoid lamina of villi accompanying a few infiltrated inflammatory cells (Figure 2D). The height of intestinal villi and the thickness of mucosa accompanying a decreased villous area were lower in groups H and HH than group C (P < 0.05). The height and area of intestinal villi and the thickness of mucosa were significantly higher in group HG than in group H (P < 0.05, Table 1).
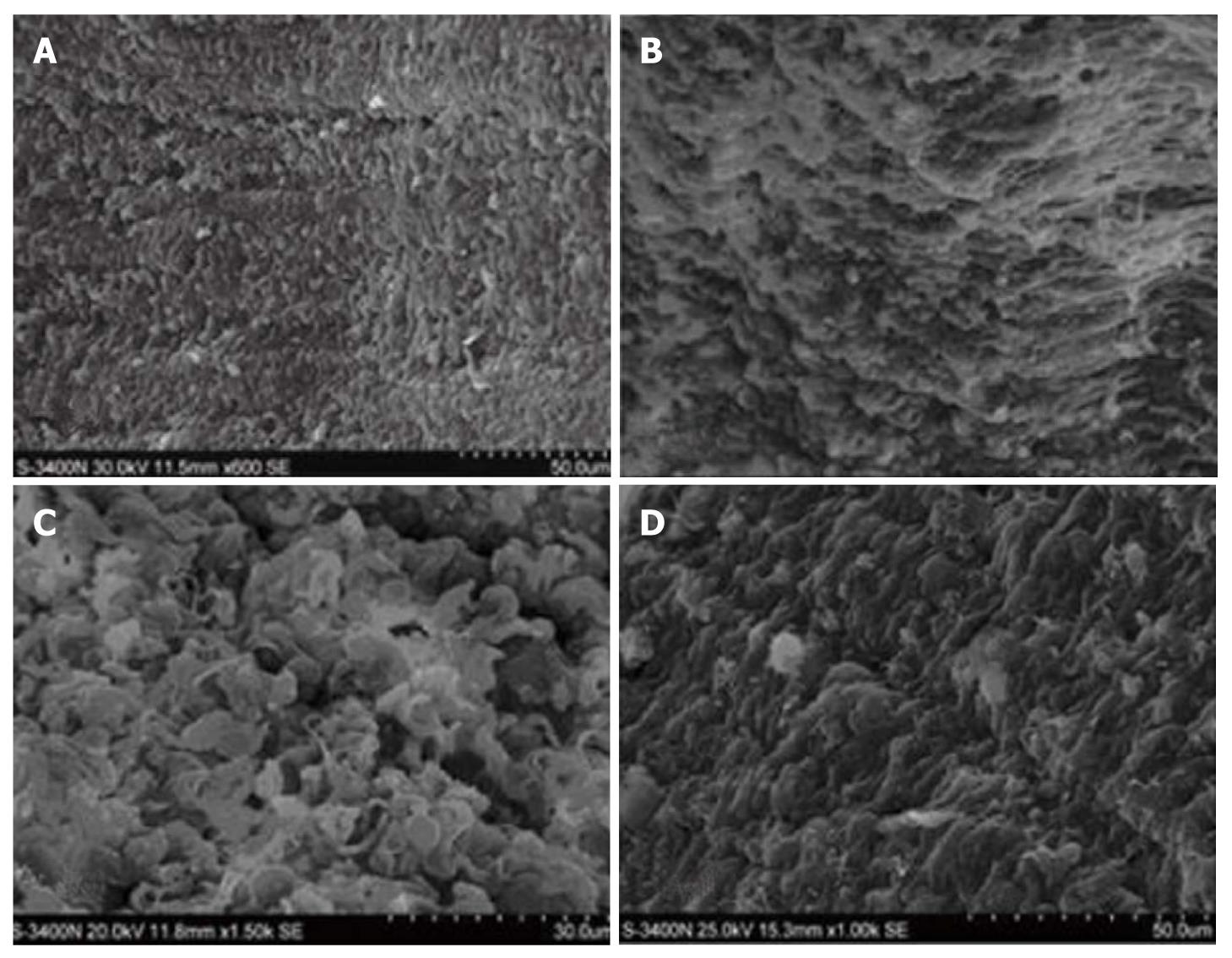
Orderly intestinal villi with a smooth surface and fullness were observed in group C (Figure 3A). The epithelia were atrophic with disordered arrangement of the villi and widened villous spaces in group H (Figure 3B). Villous atrophy and disordered villi with widened villous spaces and exfoliated microvilli were observed in group HH (Figure 3C). The intestinal mucosa was almost intact with orderly villi with few lodged villi, less effusion of red blood cells and no disc-shaped cells and cellulose in group HG (Figure 3D).
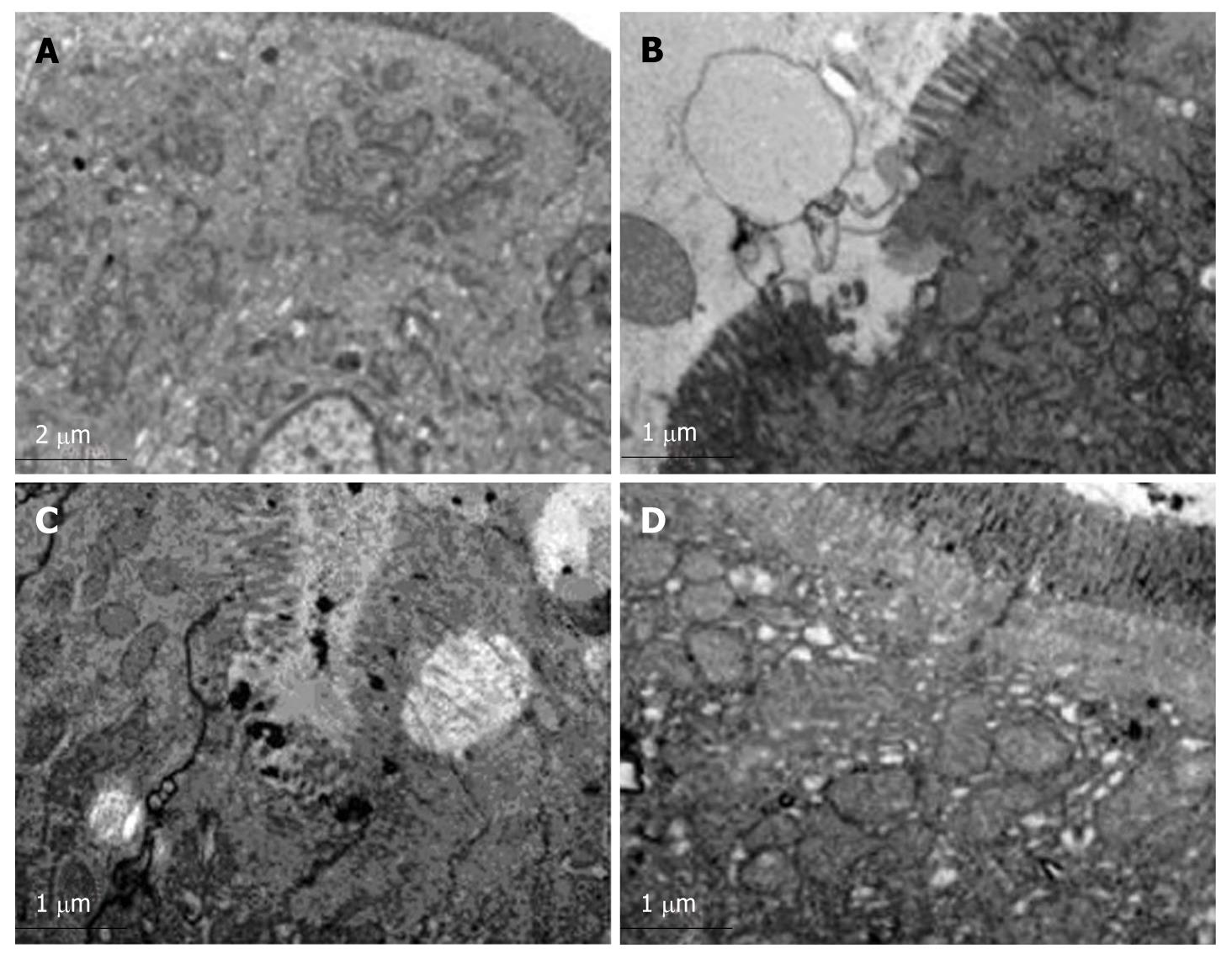
Orderly mucosal villi and integrated tight junctions as well as intact organelles with regular nuclei were observed with no edge aggregation in chromatin and no lanthanum granules in tissue space and cells in group C (Figure 4A). The microvilli were exfoliated and incomplete with widened cellular spaces, swollen endoplasmic reticulum and mitochondria as well as a small number of lanthanum granules in group H (Figure 4B). The Golgi complex was dilated with irregular nuclei with edge aggregation in chromatin and a large number of lanthanum granules in the tight junction gap and cells in group HH (Figure 4C). Mildly deformed microvilli and gland proliferation in the lamina propria and a small number of lanthanum granules were confined to vessels and epithelial surface in group HG (Figure 4D). The number of blue particles was 3.5 ± 1.5 unit/cell/gap in group C, 17.5 ± 2.5 unit/cell/gap in group H, 36 ± 2.7 unit/cell/gap in group HH, and 12 ± 2.1 unit/cell/gap in group HG, respectively.
Negative bacterial cultures were obtained in group C. Bacterial translocation occurred in MLN and spleen of group H and group HH but not in peripheral blood (P < 0.05). The translocation organ number of bacteria was the greatest in group HH. The incidence of bacterial translocation was markedly lower in group HG than in group H (P < 0.05, Table 2).
The serum DAO level was higher in groups H and HH than in group C (P < 0.05), and lower in group HG than in groups H and HH (P < 0.05). The serum Gln level was lower in groups H and HH than in group C (P < 0.05), and higher in group HG than in groups H and HH (P < 0.05, Table 3). The intestinal DAO and Gln levels were lower in groups H and HH than in group C (P < 0.05) and higher in group HG than in groups H and HH (P < 0.05, Table 4).
The serum MDA and endotoxin levels were higher in groups H and HH than in group C (P < 0.05) and lower in group HG than in groups H and HH (P < 0.05, Table 5). The serum SOD and NO levels were lower in groups H and HH than in group C (P < 0.05) and higher in group HG than in groups H and HH (P < 0.05, Table 5).
| Group | Rats(n) | endotoxin (kEU/L) | MDA(mol/L) | SOD(kU/L) | NO(mol/L) |
| C | 10 | 0.032 ± 0.003 | 6.332 ± 0.649 | 146.659 ± 3.554 | 31.097 ± 1.491 |
| H | 10 | 0.277 ± 0.053b | 9.732 ± 0.675b | 66.550 ± 6.144b | 23.397 ± 1.909b |
| HH | 10 | 0.582 ± 0.061b | 13.157 ± 0.848b | 46.851 ± 3.183b | 19.586 ± 1.203b |
| HG | 10 | 0.113 ± 0.015a | 7.183 ± 0.497a | 119.682 ± 5.481a | 27.584 ± 1.168a |
The normal intestinal barrier function depends on the intact intestinal mechanical, biological, immunological and chemical barriers. The mechanical barrier is the most important. Complete intestinal mucosal epithelium is the dominant component of the mechanical barrier, and the integrity of mucosal epithelium plays a critical role in protection against the spread of endotoxin and bacterial translocation[17]. The tight junction between adjacent cells, composed of proteins with various functions, can be found between villous and duct epithelia, and plays a crucial role in preventing molecules and ions from passing between cells[18,19].
Gastrointestinal motility is another component of the intestinal mechanical barrier. The swing of intestinal villi reduces the adhesion of pathogens to mucosal epithelia. Furthermore, intestinal peristalsis pushes the food residue to the distal end and reduces the stay time of bacteria in intestinal mucosa and the chance of bacteria reaching epithelia through the mucous layer, which results in intestinal self-cleaning. The normal flora in intestine forms a biological layer with multiple levels, which comprises the immunological barrier of intestine with no specific immune functions[20].
The balance between bacterial location, amount and type is critical for the maintenance of intestinal homeostasis. Numerous environmental changes may lead to an imbalance between humans and bacteria, and between different types of bacteria, thus resulting in injury to the intestinal biological barrier[21]. The immunological barrier is composed of secretory IgA, which is secreted by plasma cells in the lamina propria and lymphoid tissues in intestine. A few Paneth cells consume necrotic cells and secrete several immune substances, which function as an immunological barrier[22]. Additionally, gastric acid, bile, lysozymes, mucopolysaccharide and proteolytic enzymes form a gastrointestinal chemical barrier that exerts bactericidal effects. Normally, a viscoelastic layer on intestinal mucosa constitutes the chemical barrier, with no specific immunological function. The mucus secreted by goblet cells mainly consists of mucin and its main function is to lubricate the intestinal mucosa, thus protecting the mucosa against mechanical and chemical injury. In addition, the non-specific and specific adhesions between oligosaccharides in mucin and cells interfere with the colonization of opportunistic pathogens.
It has been shown that a variety of factors cause intestinal barrier functional injury, and that hypobaric hypoxia may directly damage mucosal epithelia[23]. As a result of energy deficiency, the swing of mucosal villi is also compromised, accompanying suppressed intestinal peristalsis, which enhances intestinal absorption. Furthermore, hypoxia may damage aerobic metabolism and increase glycolysis, resulting in intracellular acidosis. Subsequently, the mucosal permeability increases, leading to intestinal barrier functional injury. Intestinal mucosal injury, cytoclasis of goblet cells, and reduced amount of mucus caused by hypobaric hypoxia may attenuate the ability of intestinal mucosa to combat gastric acid and pepsin. At the same time, the gastrointestinal vagus nerve is in an excitatory state, which increases the secretion of gastrin, and the gastric acid and pepsin deteriorate the intestinal injury. Hypobaric hypoxia may also reduce secretion of secretory IgG, which compromises the specific immune function of intestine. Moreover, the expressed adhesion molecules of white blood cells and endothelia enhance the phagocytosis of neutrophils, which release several proteolytic enzymes, thus resulting in intestinal mucosal injury[24,25]. There is evidence that hypobaric hypoxia can reduce bile secretion and cause disorderliness of enterohepatic circulation, leading to gastrointestinal dysfunction and overpopulation of intestinal bacteria, and further intestinal biological barrier damage. The damaged biological barrier, together with the injured mechanical barrier and increased mucosal permeability, increases the possibility of overproduced bacteria and endotoxin entering parenteral organs through injured mucosa, thus resulting in the spread of endotoxin and bacterial translocation[26], which is also the basic cause of SIRS.
In the present study, after the rats were exposed to a simulated altitude of 7000 m for 72 h, their food intake was significantly reduced, accompanying weight loss. Light microscopy showed that the intestinal mucosa was exfoliated, and the height of mucosa decreased. The number of villi was reduced, along with their height, accompanying an irregular morphology. The epithelia had different sizes and disordered arrangement. The number of goblet cells was decreased, and a few of them showed signs of degeneration. Electron microscopy revealed atrophic and thinned intestinal villi and disordered epithelia. The villi were incomplete and exfoliated, accompanying widened intercellular spaces. Swollen endoplasmic reticulum and mitochondria and dilated Golgi complex were observed with irregular nuclei and edge aggregation of chromatin. In addition, lanthanum granules were found in intercellular spaces, basement membrane, tissue spaces, and intracellular compartment. At the same time, TUNEL staining showed that the number of apoptotic epithelial cells was significantly elevated after the rats were exposed to hypobaric hypoxia. Under a hypobaric hypoxia environment, starvation might markedly enhance the mucosal injury that leads to exfoliation, atrophy and decreased height of mucosal villi. Vacuolar degeneration was noted in a few epithelia with effusion of red blood cells around capillaries, accompanying infiltration of inflammatory cells. The intestinal mucosal injury was dramatically improved after treatment with Gln. These findings demonstrate that acute hypobaric hypoxia can severely damage intestinal mucosa, thus resulting in intestinal barrier functional injury. In this study, Gln exerted its protective effects on the injured intestinal mucosa to a certain extent.
Damage to the intestinal barrier function may increase mucosal permeability, which leads to bacterial translocation and SIRS. Therefore, detection of bacterial translocation and spread of endotoxin, as well as measurement of some parameters (DAO, MDA, SOD, NO and Gln) related to intestinal function and systemic inflammatory reaction, may directly reflect the intestinal barrier function. The spread of endotoxin and bacterial translocation represent an increased mucosal permeability, which occurs after intestinal mucosal injury. The activity of DAO in mucosal villi may reflect the structure and function of intestine[27,28]. When mucosal cells are injured and necrotized, DAO is released into the blood or enters the intestinal tract together with necrotic mucosal cells, thus increasing the serum and intestinal tract DAO level and decreasing the DAO levels in intestinal mucosa. The activity of SOD represents its ability to scavenge free radicals. MDA is the end product of lipid oxidation and indirectly represents lipid peroxidation. NO is an antioxidant. When the intestinal mucosa is damaged, the activity of SOD decreases, accompanying decreased NO and increased MDA contents. It was reported that the serum Gln level is decreased in intestinal mucosa of patients with some critical illnesses. In the present study, the activity of serum DAO and MDA and endotoxin levels were markedly higher in rats exposed to hypobaric hypoxia than in those not exposed to hypobaric hypoxia. However, the activity of DAO and the content of Gln, and the serum NO and Gln levels in intestinal mucosa were significantly lower in rats exposed to hypobaric hypoxia than in those not exposed to hypobaric hypoxia. These changes were more evident in rats after exposed to hypobaric hypoxia and starvation. The serum activity of DAO, MDA and endotoxin was dramatically decreased after treatment with Gln. Moreover, the activity of DAO in intestinal mucosa and the serum NO and Gln levels in intestine were markedly increased. These results suggest that Gln, as an intestinal nutrient, can confer protective effects against intestinal mucosal injury caused by hypobaric hypoxia.
The results of this study show that hypobaric hypoxia can severely injure the intestinal barrier function and increase the intestinal mucosal permeability. In addition, the release of factors involved in SIRS was enhanced accompanying reduced production of protective factors after exposed to hypobaric hypoxia. At the same time, the ability of hypobaric hypoxia to combat lipid peroxidation was reduced. These changes finally resulted in the spread of endotoxin and bacterial translocation. The spread of endotoxin and bacterial translocation, on one hand, activates Kupffer cells in the liver, resulting in the release of numerous cytokines, and on the other hand, leads to endotoxemia, which activates monocytes, macrophages, T and B lymphocytes, and promotes the release of a large number of cytokines, thus resulting in a cytokine cascade[24]. Additionally, the release of numerous inflammatory mediators, including metabolites of arachidonic acid (prostaglandin E2, prostacyclin, NO, platelet activating factor, leukotriene and bradykinin), may induce SIRS[29]. SIRS further promotes the release of inflammatory mediators, which result in inflammatory-mediator-related cascade effects, exacerbation of intestinal mucosal injury[30], and suppression of intestinal immune function, thus leading to aggravation of bacterial translocation and spread of endotoxin, which are the basis of the later stage of SIRS[31]. Therefore, the intestinal tract is not only the target organ of SIRS but also the initiator of SIRS[32], which forms a vicious cycle that results in an endogenous and uncontrollable systemic inflammatory reaction, deterioration of tissue, organ injury, and finally, MODS. Therefore, the damaged intestinal barrier function resulting from hypobaric hypoxia may be one of the important causes of high-altitude MODS.
Both high-altitude hypoxia and starvation may cause severe intestinal barrier function injury, and increased bacterial and endotoxin translocation, but high-altitude starvation causes more sever intestinal mucosal injury, and bacterial and endotoxin translocation than simple hypoxic exposure. High-altitude over-starvation can aggravate intestinal mucosal injury and promote bacterial and endotoxin translocation, which can be markedly alleviated after intragastric administration of Gln.
Rapid access to 3000 m above sea level can lead to body function change, and even acute severe mountain sickness (ASMS), which can be life-threatening. High altitude pulmonary edema and high altitude cerebral edema are normal in ASMS.If they are not treated effectively, many people can develop multiple organ dysfunction syndrome (MODS). However, the mechanism for complication of ASMS by MODS is still unclear. It has been demonstrated that gastrointestinal mucosal barrier dysfunction plays an important role in translocation of intestinal bacteria and endotoxin, systemic inflammatory response syndrome (SIRS), and MODS. However, whether high altitude hypoxia can cause gastrointestinal mucosal barrier dysfunction promoting bacterial and endotoxin translocation is currently unknown.
Studies have shown that high altitude hypoxia can directly cause pathological damage to the intestinal mucosa, and increase intestinal permeability. High altitude hypoxia can reduce secretion of IgG from the gastrointestinal mucosa, decrease the mucosal immune barrier, reduce bile secretion, cause enterohepatic circulation disorders, and destroy the intestinal biological barrier. Intestinal barrier damage can increase intestinal permeability, which results in bacterial translocation and occurrence of SIRS and MODS. Therefore, observation of intestinal translocation of bacteria and endotoxins can indirectly reflect intestinal mucosal barrier function.
In this study, the authors found that high altitude hypoxia altered intestinal barrier function, and increased permeability and bacterial translocation. High altitude hypoxia complicated by excessive hunger can increase damage to the intestinal barrier function and translocation of intestinal bacteria and endotoxins, and induce high altitude MODS. Glutamine has a protective effect on gastrointestinal mucosal injury in the hypoxic environment, reduces intestinal bacterial and endotoxin translocation, and promotes repair of intestinal injury.
This study has high clinical significance and practical value. First, it reminds people to improve monitoring of gastrointestinal mucosal injury in ASMS. Second, when gastrointestinal mucosal injury is found, glutamine should be administered early.
Intestinal mucosal barrier function: It included mechanical barriers, biological barrier, immune barriers and chemical barriers. Mechanical barrier is complete gastrointestinal mucosa to prevent bacterial translocation; biological barrier is the normal intestinal bacteria group in the intestine to form a multi-level special biological layer, a non-specific immune intestinal biological barrier; immune barrier is the intestinal immune barrier lamina propria plasma cells by the secretion of secretory immunoglobulin A (SIgA) and together constitute the gut-associated lymphoid tissue; chemical barrier is the gastrointestinal tract such as gastric acid, bile, lysozyme, mucopolysaccharide and proteolytic enzymes have a certain material form the bactericidal effect of the chemical barrier. Bacterial and endotoxin translocation: Intestinal bacteria and endotoxin from the intestine into other organs or blood when the intestinal mucosal barrier is broken, the body can be an "intestinal" sepsis-like. Systemic inflammatory response syndrome (SIRS): When subject was suffered a variety of damage, and show a high metabolic response. As the body in a high metabolic state, it can increase oxygen consumption; on the other hand, metabolic hyperactivity can enhance the body break down protein, negative nitrogen balance; sugar enhanced anaerobic glycolysis, lactate accumulation, acidosis, eventually leading to tissue failure. Multiple organ dysfunction syndrome: MODS is defined as severe trauma, infection and shock, the original organ dysfunction in patients with no more than two successive system and organ dysfunction.
This is a study of the effects of hypobaric hypoxia on intestinal integrity in rats (n = 40). Animals exposed to hypobaric hypoxia for 72 h demonstrated histological damage to the small intestine, translocation of lanthanum particles, and increased serum levels of DAO, MDA and endotoxin. This was accompanied by increased translocation of bacteria into lymph nodes and the spleen. Concomitant treatment of rats with glucosamine reduced the severity of intestinal injury.
Peer reviewers: Sam B Ho, MD, Gastroenterology Section 111D, VA San Diego Healthcare System, 3350 La Jolla Village Drive, San Diego, CA 92161, United States; Dr. Sherif M Karam, Professor, Department of Anatomy, Faculty of Medicine and Health Sciences, United Arab Emirates University, PO Box 17666, Al-Ain, United Arab Emirates
S- Editor Sun H L- Editor Wang XL E- Editor Ma WH
| 1. | Bailey DM, Bärtsch P, Knauth M, Baumgartner RW. Emerging concepts in acute mountain sickness and high-altitude cerebral edema: from the molecular to the morphological. Cell Mol Life Sci. 2009;66:3583-3594. |
| 2. | Hanaoka M, Droma Y, Ota M, Ito M, Katsuyama Y, Kubo K. Polymorphisms of human vascular endothelial growth factor gene in high-altitude pulmonary oedema susceptible subjects. Respirology. 2009;14:46-52. |
| 3. | Burtscher M. High-altitude cerebral effects: risks and mechanisms. Lancet Neurol. 2009;8:604-605; author reply 605. |
| 4. | Yang DZ, Zhou QQ, Li SZ, Zheng BH, Gao YQ, Jiang SH, Huang XW. Gastrointestinal dysfunction in acute severe mountain sickness and its relation with multiple organ dysfunction syndrome. Zhongguo Weizhongbing Jijiu Yixue. 2009;21:95-98. |
| 5. | Suliburk J, Helmer K, Moore F, Mercer D. The gut in systemic inflammatory response syndrome and sepsis. Enzyme systems fighting multiple organ failure. Eur Surg Res. 2008;40:184-189. |
| 6. | Urban Z, Hucthagowder V, Schürmann N, Todorovic V, Zilberberg L, Choi J, Sens C, Brown CW, Clark RD, Holland KE. Mutations in LTBP4 cause a syndrome of impaired pulmonary, gastrointestinal, genitourinary, musculoskeletal, and dermal development. Am J Hum Genet. 2009;85:593-605. |
| 7. | Swank GM, Deitch EA. Role of the gut in multiple organ failure: bacterial translocation and permeability changes. World J Surg. 1996;20:411-417. |
| 8. | Murata H, Yagi M, Kubota M, Ushiki T. Embryonic development of the myenteric nerve plexus in the rat small intestine as revealed by light and scanning electron microscopy. Arch Histol Cytol. 2003;66:453-468. |
| 9. | Ogiolda L, Wanke R, Rottmann O, Hermanns W, Wolf E. Intestinal dimensions of mice divergently selected for body weight. Anat Rec. 1998;250:292-299. |
| 10. | Groos S, Reale E, Luciano L. General suitability of techniques for in situ detection of apoptosis in small intestinal epithelium. Anat Rec A Discov Mol Cell Evol Biol. 2003;272:503-513. |
| 11. | Vyas D, Robertson CM, Stromberg PE, Martin JR, Dunne WM, Houchen CW, Barrett TA, Ayala A, Perl M, Buchman TG. Epithelial apoptosis in mechanistically distinct methods of injury in the murine small intestine. Histol Histopathol. 2007;22:623-630. |
| 12. | Habold C, Dunel-Erb S, Chevalier C, Laurent P, Le Maho Y, Lignot JH. Observations of the intestinal mucosa using environmental scanning electron microscopy (ESEM); comparison with conventional scanning electron microscopy (CSEM). Micron. 2003;34:373-379. |
| 13. | Morroni M, Cangiotti AM, Cinti S. Brush cells in the human duodenojejunal junction: an ultrastructural study. J Anat. 2007;211:125-131. |
| 14. | Endo S, Aikawa N, Fujishima S, Sekine I, Kogawa K, Yamamoto Y, Kushimoto S, Yukioka H, Kato N, Totsuka K. Usefulness of procalcitonin serum level for the discrimination of severe sepsis from sepsis: a multicenter prospective study. J Infect Chemother. 2008;14:244-249. |
| 15. | Su Y, Wu S, Fan Y, Jin J, Zhang Z. The preliminary experimental and clinical study of the relationship between the pigment gallstone and intestinal mucosal barrier. J Gastroenterol Hepatol. 2009;24:1451-1456. |
| 16. | Prasad K, Lee P, Mantha SV, Kalra J, Prasad M, Gupta JB. Detection of ischemia-reperfusion cardiac injury by cardiac muscle chemiluminescence. Mol Cell Biochem. 1992;115:49-58. |
| 17. | Shen L. Functional morphology of the gastrointestinal tract. Curr Top Microbiol Immunol. 2009;337:1-35. |
| 18. | Al-Sadi R, Boivin M, Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci. 2009;14:2765-2778. |
| 19. | Visser J, Rozing J, Sapone A, Lammers K, Fasano A. Tight junctions, intestinal permeability, and autoimmunity: celiac disease and type 1 diabetes paradigms. Ann N Y Acad Sci. 2009;1165:195-205. |
| 20. | Yang HY, Liu SL, Ibrahim SA, Zhao L, Jiang JL, Sun WF, Ren FZ. Oral administration of live Bifidobacterium substrains isolated from healthy centenarians enhanced immune function in BALB/c mice. Nutr Res. 2009;29:281-289. |
| 21. | Kleessen B, Schroedl W, Stueck M, Richter A, Rieck O, Krueger M. Microbial and immunological responses relative to high-altitude exposure in mountaineers. Med Sci Sports Exerc. 2005;37:1313-1318. |
| 22. | Selsted ME, Miller SI, Henschen AH, Ouellette AJ. Enteric defensins: antibiotic peptide components of intestinal host defense. J Cell Biol. 1992;118:929-936. |
| 23. | Recavarren-Arce S, Ramirez-Ramos A, Gilman RH, Chinga-Alayo E, Watanabe-Yamamoto J, Rodriguez-Ulloa C, Miyagui J, Passaro DJ, Eza D. Severe gastritis in the Peruvian Andes. Histopathology. 2005;46:374-379. |
| 24. | Wang ZT, Yao YM, Xiao GX, Sheng ZY. Risk factors of development of gut-derived bacterial translocation in thermally injured rats. World J Gastroenterol. 2004;10:1619-1624. |
| 25. | Diebel LN, Liberati DM, Taub JS, Diglio CA, Brown WJ. Intestinal epithelial cells modulate PMN activation and apoptosis following bacterial and hypoxic challenges. J Trauma. 2005;58:1126-1133. |
| 26. | Wang XD, Soltesz V, Andersson R. Cisapride prevents enteric bacterial overgrowth and translocation by improvement of intestinal motility in rats with acute liver failure. Eur Surg Res. 1996;28:402-412. |
| 27. | Luk GD, Bayless TM, Baylin SB. Plasma postheparin diamine oxidase. Sensitive provocative test for quantitating length of acute intestinal mucosal injury in the rat. J Clin Invest. 1983;71:1308-1315. |
| 28. | Moriyama K, Kouchi Y, Morinaga H, Irimura K, Hayashi T, Ohuchida A, Goto T, Yoshizawa Y. Diamine oxidase, a plasma biomarker in rats to GI tract toxicity of oral fluorouracil anti-cancer drugs. Toxicology. 2006;217:233-239. |
| 29. | Senthil M, Brown M, Xu DZ, Lu Q, Feketeova E, Deitch EA. Gut-lymph hypothesis of systemic inflammatory response syndrome/multiple-organ dysfunction syndrome: validating studies in a porcine model. J Trauma. 2006;60:958-965; discussion 965-967. |
| 30. | Deitch EA, Xu D, Kaise VL. Role of the gut in the development of injury- and shock induced SIRS and MODS: the gut-lymph hypothesis, a review. Front Biosci. 2006;11:520-528. |
| 31. | Shi C, Zhao X, Lagergren A, Sigvardsson M, Wang X, Andersson R. Immune status and inflammatory response differ locally and systemically in severe acute pancreatitis. Scand J Gastroenterol. 2006;41:472-480. |
| 32. | Velasco N. Gut barrier in the critically ill patient: facts and trends. Rev Med Chil. 2006;134:1033-1039. |