Published online Mar 21, 2011. doi: 10.3748/wjg.v17.i11.1442
Revised: December 30, 2010
Accepted: January 6, 2011
Published online: March 21, 2011
AIM: To establish a gastric cancer nude-mouse model with improved orthotopic implantation and investigate its biological characteristics at different time points.
METHODS: Human gastric cancer SGC-7901 cell suspensions were injected subcutaneously into a nude mouse to develop solid tumors, and the tumor tissue pieces were implanted under the serous coat. The nude mice were then euthanized in group every two weeks to observe the primary tumor growth and metastases.
RESULTS: Within 2-4 wk, there were no obvious changes about the primary tumor in stomach. At the sixth week, the primary tumor began to grow fast, resulting in incrassation of the gastric wall and stenosis of the gastric cavity, and metastases into the liver and lymph nodes were detected. The tumor, which compressed the adjacent organs, gradually became bigger and bigger followed by stenosis or vanishment of the gastric cavity from 8 to 12 wk. There were massive metastases, and the rate of metastasis was 58% in lymph nodes, 78% in liver, 39% in kidney, and 81% in peritoneum or septum.
CONCLUSION: A gastric cancer model is established, which can simulate the clinical tumor behavior and provide experimental carrier for clinical trials of gastric cancer treatment.
- Citation: Li Y, Li B, Zhang Y, Xiang CP, Li YY, Wu XL. Serial observations on an orthotopic gastric cancer model constructed using improved implantation technique. World J Gastroenterol 2011; 17(11): 1442-1447
- URL: https://www.wjgnet.com/1007-9327/full/v17/i11/1442.htm
- DOI: https://dx.doi.org/10.3748/wjg.v17.i11.1442
Metastasis is not only still the barrier to tumor therapy, but also linked with the prognosis of patients. Patients with gastric cancer hardly have symptoms in their early stage so that they are always found with metastases after clinical examinations. Therefore, novel tumor models are needed to study the metastasis mechanism as well as therapeutic approaches. It is well known that orthotopic transplantation technique has gain popularity in the field of the animal model[1-12]. Orthotopic tumor models not only mimic clinical cancer course, but also promote metastasis[13]. In recent years, the procedure of orthotopic implantation has been improved from the“sewing”method to the“adhering”one[2-4,14]. The progress greatly shortens the course of surgical operation and decreases the mortality of animals. To our knowledge, gastric cancer models of orthotopic implantation with intact tumor tissue have been well established[1-3,5-8]. However, serial examination of tumor development and metastasis needs to be made in order to tailor the treatment strategies. Based on the previous researches, we intend to establish an orthotopic model of gastric cancer using tissue glue and observe tumor progress and metastasis consecutively to detect an appropriate therapeutic target for clinical trials.
Human gastric cancer cell lines SGC-7901 (poorly differentiated) was used for this study. The cell was obtained from the Centre of Cell Cultures of Chinese Academy of Medical Sciences, Shanghai, China, and cultured at 37°C in a humidified air with 5% CO2, in RPMI-1640 medium (Gibco, Grand Island, NY) supplemented with 10% fetal calf serum (FCS; Gibco, Grand Island, NY), 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mmol/L glutamine, and 1 mmol/L sodium pyruvate.
Five to six-week-old male Balb/c nu-nu mice weighing 18-20 g were obtained from the Shanghai Experimental Animal Center of the Chinese Academy of Medical Sciences, China. All mice were maintained in a pathogen-free environment (temperature 25-27°C, humidity 45%-50%) and supplied with food and water ad libitum. All experimental procedures were conducted in conformity with institutional guidelines for the care and use of laboratory animals in Chongqing Medical University, China, and the National Institutes of Health Guide for Care and Use of Laboratory Animals.
SGC-7901 cells were collected at the log phase and injected subcutaneously into the mice at 107/0.2 mL. Two weeks later, tumors (about 2.0 cm × 2.0 cm × 1.0 cm) were harvested from the mice under anesthesia and minced into small pieces (1 mm3) in RPMI-1640 basal medium. All procedures were then performed under anesthesia with Sumianxin II (0.02 mL per animal; China Academy of Military Medical Sciences). For implantation, the mouse stomach was gently exteriorized via a left-side upper abdominal incision, and one small tissue pocket was formed in the middle wall of the greater curvature using a microscissor. One tumor fragment was placed into the pocket and fixed with a drop of medical tissue glue (gifts from Shunkang Corporation of Biological Adhesive, Beijing, China). The quantity of the tissue adhesive should be strictly controlled to avoid adhering to adjacent normal tissues. The stomach was then relocated into the abdominal cavity followed by the abdominal closure with 4-0 absorbable sutures.
All mice were divided into 6 groups of 6 animals each after orthotopic implantation. One group was sacrificed every two weeks. At autopsy, the primary tumor, lymph nodes, and other organs were examined in detail. The samples were fixed in 10% formalin for paraffin sections, and stained with hematoxylin and eosin for microscopic examination.
Paraffin sections were examined histologically with the Cytokeratin 20 (CK20) mAb KS20.8 and Epithelial Membrane Antigen (EMA) mAb GP1.4 (MAB-0057, MAB-0061; Maixin Inc., Fuzhou, China), which are usually combined to diagnose the gastrointestinal adenocarcinoma. Ultrasensitive streptavidin-peroxidase (SP) kit (KIT-9710; Maixin Inc., Fuzhou, China) and DAB kit (DAB-0031; Maixin Inc., Fuzhou, China) were used in the immunohistochemical stain according to the manufacture’s instructions. CK20 and EMA expression was defined as positive if the stained region of tumor cells was in the cytoplasm.
The primary tumor volume was calculated by the following formula: V = 0.4 × ab2 (a: maximum diameter; b: minimum diameter)[15]. Then the tumor growth curve was depicted reflecting the tumor growth trend.
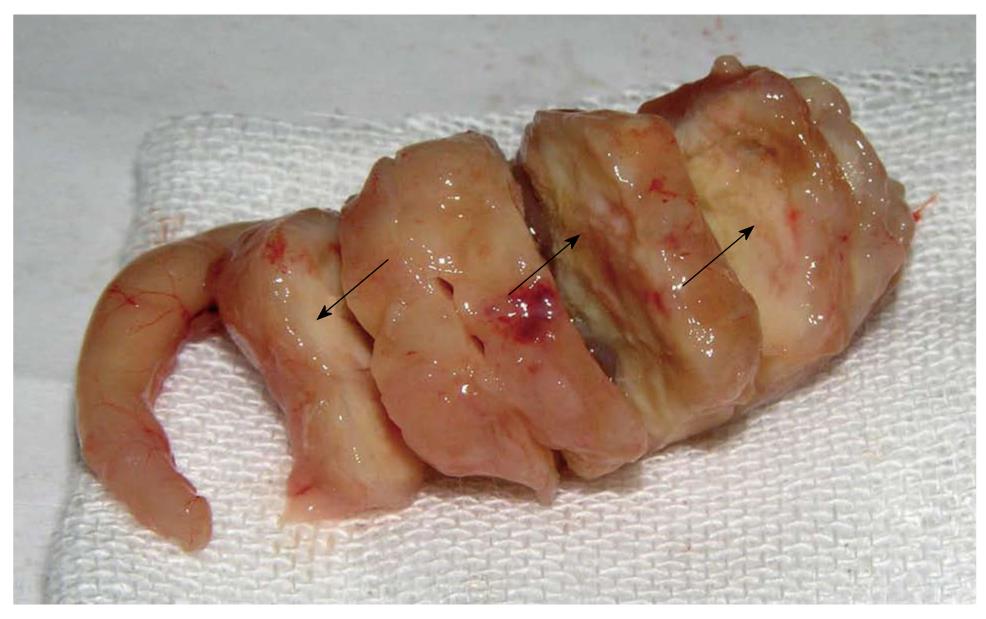
From 2 to 4 wk, the primary tumor developed without obvious volume change, and showed insufficient blood supply. The gastric cavity of tumor-bearing mice appeared almost normal in size. At the 2nd wk after orthotopic implantation, metastatic infiltration was not found in all detected organs. However, tumor invasion into the liver was found at the 4th wk. Six weeks after transplantation, the tumor in situ grew increasingly in size, with sparse blood vessels. The stomach wall of mice was thickened and gastric cavity decreased, companied with enlargement of lymph nodes from gastric area and hilus pulmonis. The tumor invaded into the liver, resulting in metastatic nodules formation. At the 8th wk, the stomach tumor was characterized by large-size, irregular volume and rich blood supply. The resulting tumor squeezed the adjacent organs and deformed the stomach contributing to distal stenosis. The lymph nodes of the gastric area, hilus pulmonis and mesenterium presented enlargement caused by tumor metastasis. Metastatic lesions were found in the organs such as liver, kidney, peritoneum and diaphragm. From 10 to 12 wk, the tumor grew fast and huge, occupying almost the upper abdomen and squeezing severely the surrounding organs. The necrosis from central tumor areas was macroscopically visible, indicating that the tumor outgrew the sufficient blood supply. In most mice, the gastric cavity became narrow, even vanished, and pylorus was obstructed partly or totally (Figure 1). The stomach of some mice was so severely disfigured that the greater and lesser curvature failed to be distinguished. Enlargement of many lymph nodes was visible in the gastric area, hilus pulmonis and mesenterium. The metastatic infiltration into the liver and kidney was always detected, with characteristics of grey-white neoplastic nodules. At autopsy, a little clear and yellowish ascites was found (Table 1).
| Items | 2 wk | 4 wk | 6 wk | 8 wk | 10 wk/12 wk |
| Primary tumor | |||||
| Shape | Oval | Oval | Globular | Irregularly lobular | Irregularly lobular |
| Color | Gray-white | Gray-white | Gray-white | Grayish yellow | Grayish yellow |
| Texture | Stiff | Stiff | Stiff | Stiff | Stiff |
| Vascularity | Sparse | Sparse | Rich | Richer | Richer |
| Section of stomach tumor | |||||
| Gastric cavity | No change | Mildly stenosis | Obvious stenosis | Obvious stenosis | Narrow or vanished |
| Greater and lesser curvature | Distinguishable | Distinguishable | Distinguishable | Distinguishable | Undistinguishable |
| Pylorochesis | No | No | Partly | Partly | Totally |
| Effects on adjacent organs | |||||
| Abdominal cavity | No effects | No effects | No effects | Occupying upper abdomen | Occupying whole abdomen |
| Adhering extent | Adhering to liver lobes | Adhering to liver lobes | Adhering to liver lobes | Adhering to liver lobes or posterior abdominal wall | Adhering to liver lobes or posterior abdominal wall |
| Compressed status | No compression | No compression | No compression | Partly oppressed | Partly oppressed |
| Ascites | |||||
| Liquid quantity | Zero | Zero | Zero | Little | Little |
| Property | - | - | - | Clear and yellowish | Clear and yellowish |
| Metastasis | |||||
| Lymph nodes | No | No | Yes | Yes | Yes |
| Other organs | No | Yes | Yes | Yes | Yes |
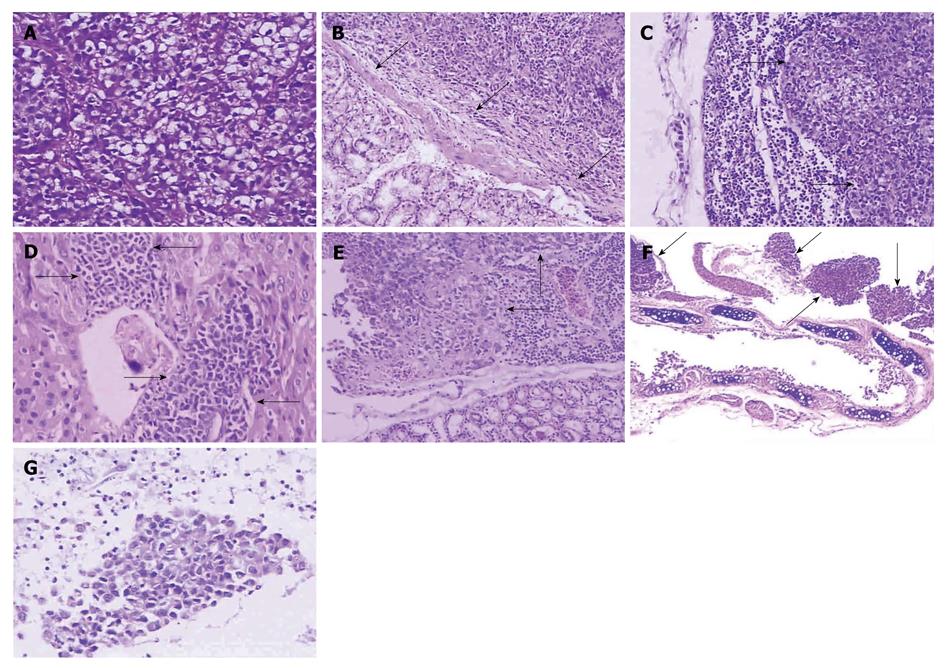
Tumor cells spread with schistose, nest-like, or cable-like structure, with characteristics of nuclear polymorphism, nuclear hyperchromatism, much red nucleolus, and rich pathological karyokinesis. Mucus secreted in the cytoplasm, which resulted in mucus lake, was visible in part of tumor cells (Figure 2A). Glandular differentiation and rich vascularity were present in tumor areas. The stomach tumor invaded the gastric wall following the disruption of the integrity of the mucous layer or muscularis mucosae (Figure 2B). The lymph nodes were infiltrated and destroyed by metastatic tumor, characterized by the narrowness or diminishment of the lymph sinus (Figure 2C) Metastases were also detected in the liver (Figure 2D) and the kidney (Figure 2E), occasionally in the paranephros or pancreas (not shown). Metastatic lesions were always separated from adjacent normal tissues by fibrous integument, and lymphoid infiltration was found in the peripheral tumor areas. Tumor metastasized to the lung and surrounded bronchia or bronchiole (Figure 2F). Smear of cast-off cells from ascites confirmed the malignant cells from the primary adenocarcinoma (Figure 2G).
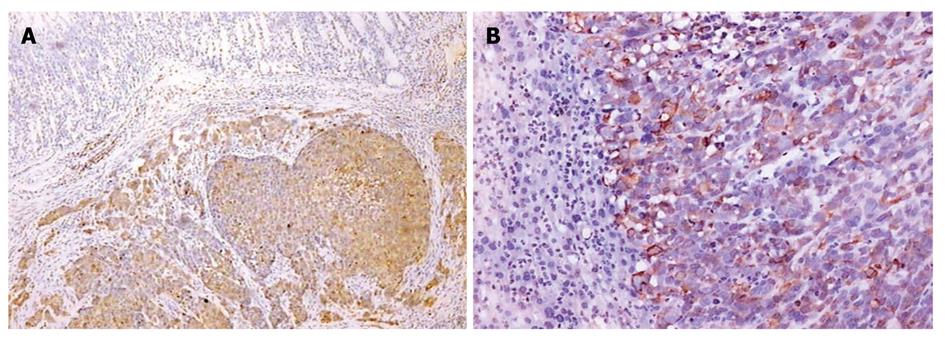
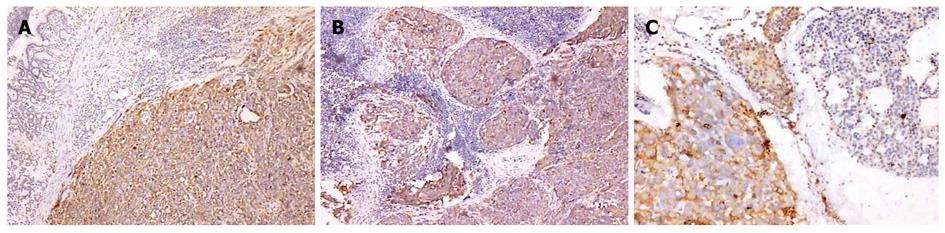
All primary tumors and metastases from other organs by immunohistochemical staining showed the positive expression of CK-20 (Figure 3A and B) and EMA (Figure 4A-C) protein.
The tumor volume gradually increased from the 6th wk after implantation, and reached a peak when nude mice died at the 12th wk (Figure 5). From 10 to 12 wk, the model mice declined in their general conditions, accompanied by cachexia and ascites.
After all mice were sacrificed, the metastasis incidence of involved organs was analyzed and the following results were obtained: lymph nodes 58% (21/36), liver 78% (28/36), kidney 39% (14/36), and peritoneum and diaphragm 81% (29/36).
There are little proofs supporting serial observations on gastric cancer models although many researchers have constructed orthotopic models using intact tumor tissues. This makes it difficult to learn the whole process of tumor growth and metastasis occurrence. In this study, we presented in detail such evidences as the process of primary tumor growth, the time point of metastasis, and the sites and incidence of metastasis.
The patients with gastric cancer hardly have symptoms in their early stage, whereas they are always tortured by gastrointestinal symptom and metastasis in their terminal stage. In the present study, an animal model of stomach cancer was established to replicate the tumor behavior of clinical patients. It showed that the primary tumor grew into the log phrase from the 6th wk after implantation and thereafter the tumor volume gradually increased. From the 6th wk, lymph node metastases and distant metastases were detected, accompanied by the increase of involved organs with the tumor progress. The tested mice revealed obvious cachexia from the 10th wk, and systemic failure at the 12th wk or so.
There are several pathways such as direct infiltration, lymphatic metastasis, vascular spread, and implantation dissemination which play important roles in metastasis of gastric cancer. Previous studies recapitulated the metastatic incidence of systematic organs, but did not mention the metastatic pathways of gastric cancer models. Moreover, there are uneven metastasis rates among different laboratories resulting from different experimental conditions, animal species and cell lines[1,2,3,10,11,14]. The SGC-7901 cell line used in our study was derived from metastatic lymph node of poorly differentiated human gastric carcinoma. We could detect the metastatic lesions in the lymph nodes from gastroepiploic plexus, hilus pulmonis and mesenterium, which accorded with the feature of this cell line. It is well known that liver is the most sensitive organ to vascular metastasis. In the present study, we found that the liver was not only invaded by the neoplastic cells from blood pathway but also directly infiltrated by the primary tumor. In addition, the fringe of kidney also showed metastatic spread, yet only under renal capsule rather than in parenchyma. Hereby, we consider that metastatic tumor may float in the ascites by breaking through the stomach serous coat and then invade straightly the kidney, or metastasize to the lymphatic system of renal capsule via lymphatic route, instead of invading kidney through blood vessel.
The model constructed in our study could simulate the clinical tumor behavior and provide basic theory on gastric cancer models. However, orthotopic implantation models still have some limitations such as failure in clinical staging. It is well known that gastric cancer stage (early, median, late) is judged by tumor growth and infiltration and metastasis rather than disease course, i.e. TNM staging published by Union for International Cancer Control (UICC). The morphological changes of gastric carcinogenesis are as follows: diffuse hyperplasia → focal proliferation and metaplasia → benign tumor → grade I , II or III dysplasia → carcinoma in situ → invasive carcinoma, which reveals that the pathological changes of gastric cancer originate from mucous layer and progress layer-by-layer towards serous coat[16]. However, orthotopic implantation was performed with tumor fragments under the stomach serous coat, which is unable to mimic the histopathological mechanism of gastric cancer. In fact, the procedure of orthotopic implantation mimics the artificial advanced stage which is characterized by tumor growth from serous coat to mucous layer or outside membrane serosa. The histological origin of primary tumor was not consistent with the principle of TMN stage although lymph node metastasis or distant dissemination was also detected. So the orthotopic model of gastric cancer is not suitable for clinical staging.
Some researchers had established the models of stomach cancer with chemical carcinogens which could simulate the histological origin of gastric carcinoma[16-20]. But there are some disadvantages such as long period and accidental animal death. Some authors employed transgenic techniques in attempt to replicate the mechanism of gastric cancer[18,21,22]. However, transgenic method is limited due to expensive cost, long period and advanced technical requirements for molecular biology. Compared with other methods for constructing gastric cancer model, the surgical orthotopic implantation is still a desired technique characteristic of short period, low cost and simple practice.
Some experimental skills for this animal model should be noticed. For example, necrotic area should be removed from tumor tissues used for orthotopic implantation; the quantity of medical glue should be strictly controlled to avoid adhering to adjacent organs; and the middle part of the greater gastric curvature is the first choice for orthotopic implantation because of the operational convenience and rich blood supply.
In conclusion, we can succeed in establishing a gastric cancer model if only we master the experimental skills above and avoid the adverse factors.
Metastasis is still the barrier to the treatment of patients with gastric cancer. Gastric cancer models of orthotopic implantation with intact tumor tissues have been well established. Moreover, the procedure of orthotopic implantation has been improved from the “sewing” method to the “adhering” one. However, there are little evidences regarding serial observations on gastric cancer models to tailor treatment strategies.
It is well known that orthotopic transplantation technique plays an important role in establishing animal models of various tumors. Gastric cancer model of orthotopic implantation could mimic clinical cancer process and contribute to metastasis occurrence. The procedure greatly facilitates surgical operation course and decreases the mortality of animals.
Orthotopic implantation with “glue paste technique” is a popular and new method in recent years which is characterized by easy performance, low cost and short operation course. This study makes it possible to help researchers learn the whole process of tumor development and metastasis, thus choosing the appropriate therapy target for clinical trials.
Serial observations on the models can provide evidences for researchers to treat stomach cancer and test the therapeutic effects.
The study is interesting and well done. The model used by the Authors well mimics the dynamics of local growth and spread of gastric cancer cells at both lymph-nodes and distant organs.
Peer reviewers: Fabio Grizzi, PhD, Laboratories of Quantitative Medicine, Istituto Clinico Humanitas IRCCS, Via Manzoni 56, 20089 Rozzano, Milan,Italy; Dr. Jianyuan Chai, PhD, MS, BS, Assistant Professor, Research (09-151), VA Long Beach Healthcare System, 5901 E. 7th St, Long Beach, CA 90822, United States
S- Editor Tian L L- Editor Ma JY E- Editor Ma WH
| 1. | Shi J, Wei PK, Zhang S, Qin ZF, Li J, Sun DZ, Xiao Y, Yu ZH, Lin HM, Zheng GJ. OB glue paste technique for establishing nude mouse human gastric cancer orthotopic transplantation models. World J Gastroenterol. 2008;14:4800-4804. |
| 2. | Chen YL, Wei PK, Xu L, Su XM. [Nude mouse model of human gastric carcinoma metastasis constructed by orthotopic transplantation using organism glue paste technique]. Ai Zheng. 2005;24:246-248. |
| 3. | Bhargava S, Hotz B, Buhr HJ, Hotz HG. An orthotopic nude mouse model for preclinical research of gastric cardia cancer. Int J Colorectal Dis. 2009;24:31-39. |
| 4. | Yang B, Tuo S, Tuo CW, Zhang N, Liu QZ. A liver-metastatic model of human primary gastric lymphoma in nude mice orthotopically constructed by using histologically intact patient specimens. Chin J Cancer. 2010;29:579-584. |
| 5. | Illert B, Otto C, Thiede A, Timmermann W. Detection of disseminated tumor cells in nude mice with human gastric cancer. Clin Exp Metastasis. 2003;20:549-554. |
| 6. | Takemura S, Yashiro M, Sunami T, Tendo M, Hirakawa K. Novel models for human scirrhous gastric carcinoma in vivo. Cancer Sci. 2004;95:893-900. |
| 7. | Furukawa T, Fu X, Kubota T, Watanabe M, Kitajima M, Hoffman RM. Nude mouse metastatic models of human stomach cancer constructed using orthotopic implantation of histologically intact tissue. Cancer Res. 1993;53:1204-1208. |
| 8. | Koyama T, Tsubota A, Nariai K, Yoshikawa T, Mitsunaga M, Sumi M, Nimura H, Yanaga K, Yumoto Y, Mabashi Y. Detection of sentinel nodes by a novel red-fluorescent dye, ATX-S10Na (II), in an orthotopic xenograft rat model of human gastric carcinoma. Lasers Surg Med. 2007;39:76-82. |
| 9. | Hoffman RM. Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: a bridge to the clinic. Invest New Drugs. 1999;17:343-359. |
| 10. | An Z, Jiang P, Wang X, Moossa AR, Hoffman RM. Development of a high metastatic orthotopic model of human renal cell carcinoma in nude mice: benefits of fragment implantation compared to cell-suspension injection. Clin Exp Metastasis. 1999;17:265-270. |
| 11. | Rashidi B, Gamagami R, Sasson A, Sun FX, Geller J, Moossa AR, Hoffman RM. An orthotopic mouse model of remetastasis of human colon cancer liver metastasis. Clin Cancer Res. 2000;6:2556-2561. |
| 12. | Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc Natl Acad Sci USA. 1992;89:5645-5649. |
| 13. | Kozlowski JM, Fidler IJ, Campbell D, Xu ZL, Kaighn ME, Hart IR. Metastatic behavior of human tumor cell lines grown in the nude mouse. Cancer Res. 1984;44:3522-3529. |
| 14. | Fujihara T, Sawada T, Hirakawa K, Chung YS, Yashiro M, Inoue T, Sowa M. Establishment of lymph node metastatic model for human gastric cancer in nude mice and analysis of factors associated with metastasis. Clin Exp Metastasis. 1998;16:389-398. |
| 15. | Kyriazis AP, Kyriazis AA, McCombs WB 3rd, Kereiakes JA. Biological behavior of human malignant tumors grown in the nude mouse. Cancer Res. 1981;41:3995-4000. |
| 16. | Kossoy G, Ben-Hur H, Elhayany A, Schneider DF, Zusman I. The morphological pathway for mouse forestomach cancer. Oncol Rep. 2006;15:479-483. |
| 17. | Zaidi NH, O'Connor PJ, Butler WH. N-methyl-N'-nitro-N-nitrosoguanidine-induced carcinogenesis: differential pattern of upper gastrointestinal tract tumours in Wistar rats after single or chronic oral doses. Carcinogenesis. 1993;14:1561-1567. |
| 18. | Pritchard DM, Przemeck SM. Review article: How useful are the rodent animal models of gastric adenocarcinoma? Aliment Pharmacol Ther. 2004;19:841-859. |
| 19. | Tsuzuki T, Egashira A, Igarashi H, Iwakuma T, Nakatsuru Y, Tominaga Y, Kawate H, Nakao K, Nakamura K, Ide F. Spontaneous tumorigenesis in mice defective in the MTH1 gene encoding 8-oxo-dGTPase. Proc Natl Acad Sci USA. 2001;98:11456-11461. |
| 20. | Xu X, Brodie SG, Yang X, Im YH, Parks WT, Chen L, Zhou YX, Weinstein M, Kim SJ, Deng CX. Haploid loss of the tumor suppressor Smad4/Dpc4 initiates gastric polyposis and cancer in mice. Oncogene. 2000;19:1868-1874. |
| 21. | Koike K, Hinrichs SH, Isselbacher KJ, Jay G. Transgenic mouse model for human gastric carcinoma. Proc Natl Acad Sci USA. 1989;86:5615-5619. |
| 22. | Maruta F, Sugiyama A, Ishida K, Ikeno T, Murakami M, Kawasaki S, Ota H, Tatematsu M, Katsuyama T. Timing of N-methyl-N-nitrosourea administration affects gastric carcinogenesis in Mongolian gerbils infected with Helicobacter pylori. Cancer Lett. 2000;160:99-105. |