Published online Feb 28, 2010. doi: 10.3748/wjg.v16.i8.987
Revised: December 9, 2009
Accepted: December 16, 2009
Published online: February 28, 2010
AIM: To clarify the effects of anti-hypertensive drugs on esophageal contraction and determine their possible relationship with gastro-esophageal reflux disease.
METHODS: Thirteen healthy male volunteers were enrolled. Esophageal body peristaltic contractions and lower esophageal sphincter (LES) pressure were measured using high resolution manometry. All subjects were randomly examined on four separate occasions following administrations of nifedipine, losartan, and atenolol, as well as without any drug administration.
RESULTS: Peristaltic contractions by the esophageal body were separated into three segments by two troughs. The peak peristaltic pressures in the mid and lower segments of the esophageal body under atenolol administration were significantly higher than those without medication in a supine position. On the other hand, peristaltic pressures under nifedipine administration were lower than those observed without drug administration. Losartan did not change esophageal body peristalsis. Atenolol elevated LES pressure and slowed peristaltic wave transition, while the effects of nifedipine were the opposite.
CONCLUSION: Among the anti-hypertensive drugs tested, atenolol enhanced esophageal motor activity, which was in contrast to nifedipine.
- Citation: Yoshida K, Furuta K, Adachi K, Ohara S, Morita T, Tanimura T, Nakata S, Miki M, Koshino K, Kinoshita Y. Effects of anti-hypertensive drugs on esophageal body contraction. World J Gastroenterol 2010; 16(8): 987-991
- URL: https://www.wjgnet.com/1007-9327/full/v16/i8/987.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i8.987
Impaired esophageal motor functions, such as decreased lower esophageal sphincter (LES) pressure and weak esophageal body peristalsis, are the main causes of gastro-esophageal reflux disease (GERD)[1,2]. Conventional manometry has demonstrated that several different drugs impair esophageal motor function. It has also been speculated that they increase esophageal acid exposure[3]. Nifedipine, a calcium-channel blocker, was shown to reduce LES pressure, as well as the amplitude and duration of esophageal peristaltic contractions, in healthy subjects. It also increased esophageal acid exposure time[4-6]. In addition, candesartan, an angiotensin II (AngII) receptor antagonist, was reported to reduce the amplitude of swallow-induced peristaltic contractions and LES pressure[7]. In contrast, atenolol, a catecholamine β receptor antagonist, was found to inhibit the relaxation of esophageal smooth muscle induced by catecholamine β stimulation[8].
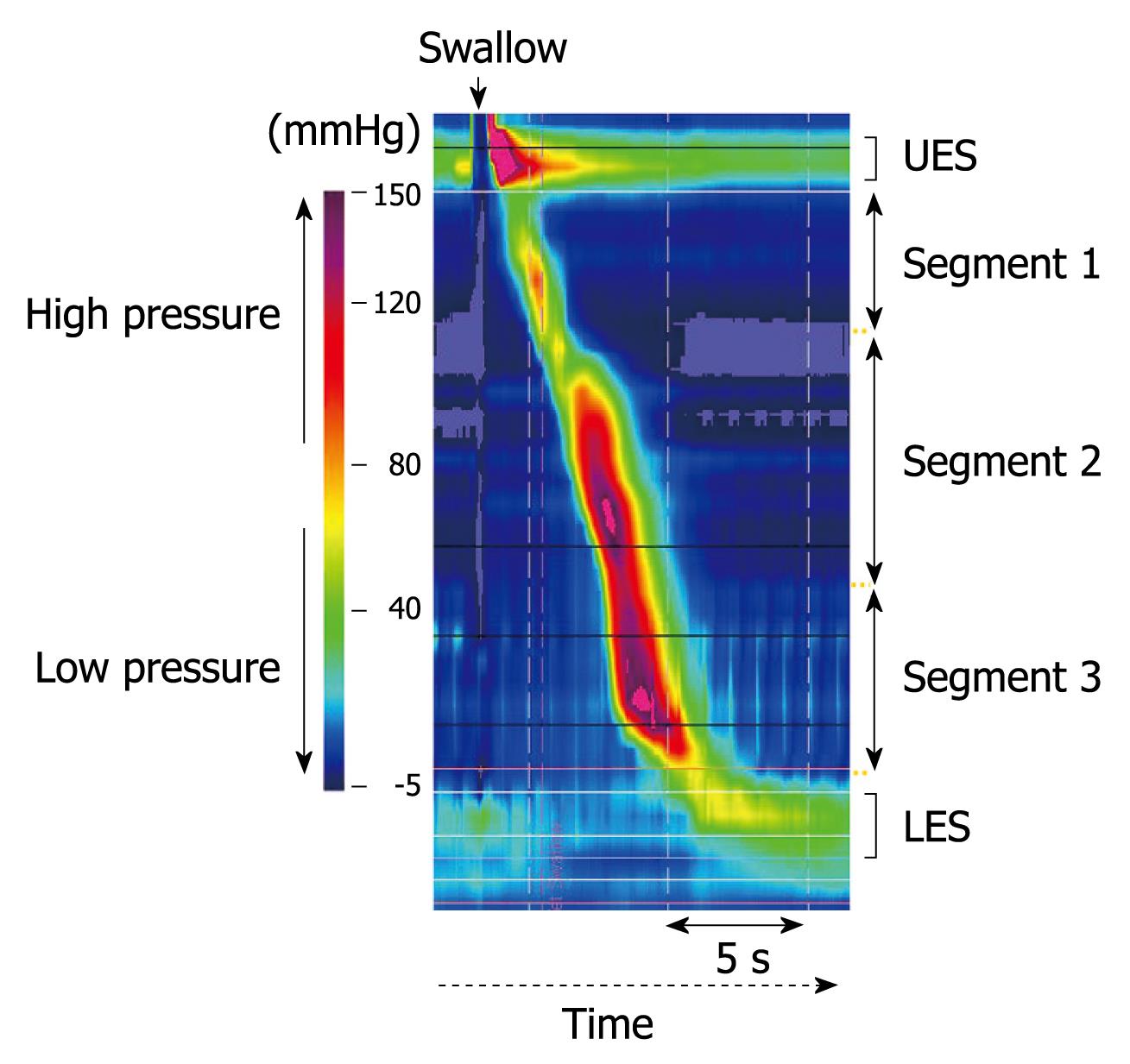
Recent studies using high-resolution manometry revealed that esophageal body peristalsis was composed of a chain of three contraction segments separated by two troughs[9-11]. Conventional esophageal manometry techniques are not sensitive enough to detect those three separate contraction segments[12-14]. The uppermost (first) peristaltic segment represents the skeletal muscle component of the esophageal body, while the lowest (third) segment represents the smooth muscle component. In the middle (second) segment, the muscle tissue is considered to shift from skeletal to smooth type[9-13]. Therefore, neuromuscular contraction control might be different between the three segments. In addition, the second segment was reported to be more responsive to cholinergic stimulation, whereas the third has been demonstrated to be under stronger control of non-cholinergic and non-adrenergic neurons[15-17]. Therefore, the various drugs reported to have an influence on esophageal motor function might have different effects on each of the segments of the esophageal body, though the effects of anti-hypertensive drugs on these segments have not been investigated.
The aim of this study was to clarify the effects of three different types of anti-hypertensive drugs on the three different segments of esophageal body contractions using a recently developed high-resolution manometric system.
Thirteen male volunteers were recruited for this study (mean age: 34.7 years). None of the subjects had upper gastrointestinal symptoms, a history of upper gastrointestinal surgery, or were taking medications known to influence esophageal motor function. Written informed consent was obtained from each volunteer before starting the study, which was carried out in accordance with the Declaration of Helsinki. This study was approved by the Ethics Committee of Shimane University.
We evaluated esophageal motor function under separate administrations of nifedipine, a calcium-channel blocker, atenolol, a catecholamine β receptor antagonist, and losartan, an angiotensin II receptor antagonist. All subjects were examined four separate times after an overnight fast, with the following administrations; (1) without medication, (2) 10 mg of nifedipine, (3) 50 mg of losartan, and (4) 50 mg of atenolol, which were performed randomly in each case. The administrations of nifedipine, losartan, and atenolol were given at 1, 4, and 4 h, respectively, before performing the esophageal motor function test, to match the maximal blood concentration of each drug with the examination period. In addition, all subjects were instructed to drink 200 mL of water at 4 h and 1 h before each esophageal motor function test.
We performed high-resolution manometric tests using a ManoScan 360™ (Sierra Scientific Instruments, Inc., California)[18]. The manometric catheter used with this system is 4.2 mm in diameter and has 36 intraluminal pressure transducers at 1-cm intervals, which are used to measure peristaltic pressure in the upper esophageal sphincter (UES) to LES simultaneously and continuously. Before performing esophageal pressure measurements, transducers were calibrated at 0 and 100 mmHg using externally applied pressure, according to the manufacturer’s instructions. The manometric catheter was inserted in a transnasal manner using 2% lidocaine jelly (Xylocaine jelly; AstraZeneka Co., Osaka, Japan), LES pressure (LESP) was then measured in a sitting position during a 5-min resting period. Next, esophageal body peristaltic function in the sitting position was examined by swallowing 5 mL of room temperature water, which was repeated at 2-min intervals until five recordings of complete esophageal peristalsis were obtained. After finishing the tests in the sitting position, they were repeated in a supine position. The peristaltic contractions in the esophageal body were divided into three different segments (segments 1, 2, and 3 from oral to anal) separated by two troughs, as shown in Figure 1. LESP and peak intraesophageal contraction pressure in the three segments of the esophageal body were analyzed using ManoView™ analysis software (Sierra Scientific Instruments, Los Angeles, CA). Peristaltic contraction velocity between 5 and 15 cm above LES was also determined.
Statistical analysis of paired data was performed using a Wilcoxon signed rank test. All calculations were done using the Stat View 5.0 software package (Abacus Concepts Inc., Berkeley, CA, USA) for Macintosh. Differences at P < 0.05 were considered to be statistically significant.
All 13 subjects completed the study protocol without any adverse events. LESP values in the supine position were significantly higher than those in the sitting position, both with and without administration of the drugs investigated in this study. Furthermore, LESP with the administration of nifedipine tended to be lower, while that under the administration of atenolol tended to be higher, as compared to the control (Table 1).
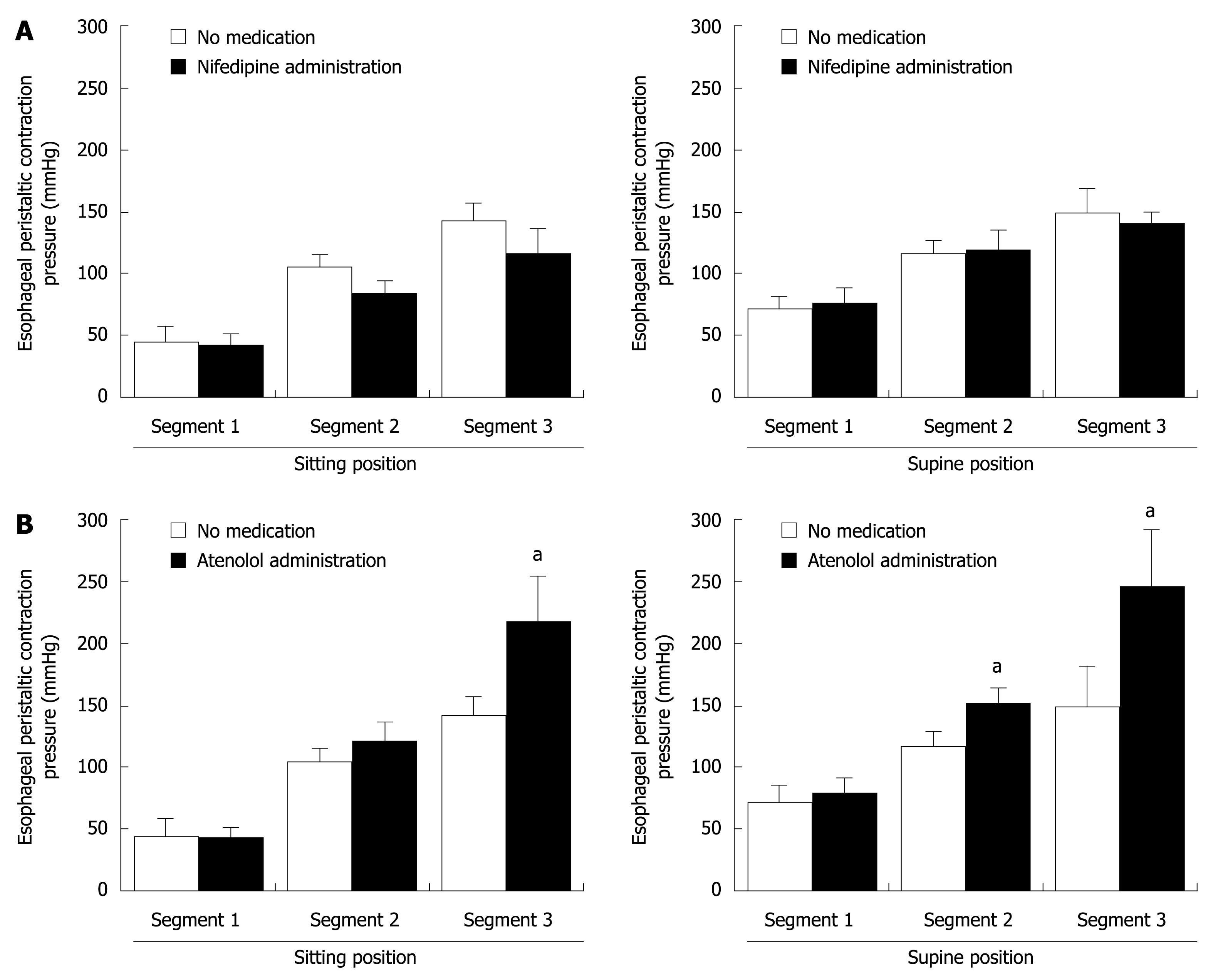
The peak peristaltic pressures in the three segments of the esophageal body in the supine position were significantly higher than those in the sitting position, and also significantly increased from segment 1 to 3 (Table 2). However, there was no difference in peak contraction pressure in segment 1 of the esophageal body with and without administration of the drugs. In contrast, peak contraction pressure in segments 2 and 3 with nifedipine administration tended to be lower than those without medication (Table 2 and Figure 2). On the other hand, peak pressures in those segments under atenolol administration were higher than without medication (Table 2 and Figure 2). Losartan did not significantly affect peak contraction pressures in the second and third segments (Table 2).
| No medication | Nifedipine | Losartan | Atenolol | |||||
| Sitting | Supine | Sitting | Supine | Sitting | Supine | Sitting | Supine | |
| Segment 1 (mmHg) | 45.3 ± 12.9 | 72.4 ± 14.4 | 42.1 ± 10.1 | 77.4 ± 12.6 | 40.5 ± 10.5 | 77.5 ± 12.0 | 42.8 ± 8.2 | 79.3 ± 12.2 |
| Segment 2 (mmHg) | 105.5 ± 10.7 | 117.0 ± 12.3 | 83.7 ± 9.7 | 109.5 ± 15.4 | 96.4 ± 17.3 | 120.5 ± 34.9 | 122.0 ± 15.6 | 153.8 ± 12.4a |
| Segment 3 (mmHg) | 141.7 ± 15.2 | 148.9 ± 31.6 | 115.5 ± 20.6 | 140.4 ± 9.8 | 152.2 ± 27.9 | 181.1 ± 31.9 | 216.9 ± 39.4a | 246.5 ± 45.1a |
Esophageal peristaltic velocity in the supine position tended to be lower than that in the sitting position. Furthermore, under the administration of nifedipine, it tended to be faster than that without medication. On the other hand, velocity with atenolol was significantly slower than that without medication (Table 3).
Calcium-channel blockers, such as nifedipine, inhibit the entry of calcium into smooth muscle cells of the arterial wall, and are widely used for treatment of patients with ischemic heart disease and hypertension[5]. Orally administered nifedipine was shown not only to decrease LESP in healthy subjects and patients with achalasia, but also reduced the amplitude and duration of esophageal peristaltic contractions in healthy subjects[4-6]. Ang II is the key mediator of the rennin-angiotensin system, which maintains extracellular fluid volume and electrolyte homeostasis, and also regulates vascular tone and blood pressure[19]. The physiological functions of Ang II are mediated specifically by the Ang II type 1 (AT1) and type 2 (AT2) receptors. Losartan, an AT1 receptor antagonist, was previously reported to reduce the amplitude of swallow-induced peristaltic esophageal contractions and LESP[7]. The catecholamine β-adrenoceptor is currently classified into β1, β2, and β3 subtypes, all of which are expressed in smooth muscle cells. Smooth muscle in the gastrointestinal tract is known to have the β1-adrenoceptor subtype and it has been shown that smooth muscle relaxes in response to β-adrenoceptor stimulation. In addition, atenolol, a β1 blocker, was reported to inhibit the relaxation of esophageal smooth muscle induced by β1[8]. These observations of the effects of nifedipine, losartan, and atenolol were obtained in studies that used conventional intraesophageal pressure monitoring systems. Those are able to determine intraesophageal pressure at some sites, but they are not sensitive enough to measure peristaltic contractions in the three different esophageal body segments[8,9].
Peristaltic contraction in segment 3 of the esophageal body is the strongest in amplitude and the most important factor for volume clearance of acidic refluxant from the stomach[20]. Therefore, to clarify the effects of the three anti-hypertensive drugs on the development of GERD, their effects on the three different segments, especially the lowest segment (segment 3) were investigated in the present study.
We used high-resolution manometry to measure intraesophageal pressure in 36 different sites at the same time, while contractions in each of the three esophageal segments were separately measured[14]. As reported by other investigators using conventional manometry, nifedipine tended to decrease LESP, while atenolol increased it. Similarly, nifedipine tended to decrease the amplitude of peristaltic contractions in segment 3, while atenolol significantly increased it in both the sitting and supine positions. These observations fit well with previous reports that a regular administration of calcium antagonists for treatment of hypertension is a risk factor for the future occurrence of GERD. Interestingly, atenolol significantly elevated LESP and the peristaltic amplitude in the lower esophageal body (segment 3). Such atenolol-induced alterations of esophageal motor activity may prevent the development of GERD.
An interesting finding in our study was the reciprocal relationship between contraction amplitude and peristaltic velocity of the esophageal body. Factors that induce augmentation of peristaltic amplitude, such as atenolol administration and the supine position, were found to delay peristaltic velocity[21,22]. On the other hand, factors that caused a decrease in contraction amplitude, such as nifedipine administration and the sitting position, accelerated peristaltic contraction velocity[21,22]. Although the mechanisms by which these reciprocal phenomena occur are not clear, slowly progressing high amplitude peristalsis might be a more efficient peristaltic wave for propelling esophageal contents down to the stomach.
Another interesting finding was the lack of significant effect by losartan on esophageal motor activity, which differs from previous reports, though the reason is not clear. We used modern high-resolution measurements in the present study. Therefore, at least in healthy volunteers, we believe that the inhibiting effect of losartan on esophageal motor function is not clinically important.
In conclusion, of the anti-hypertensive drugs tested, atenolol enhanced esophageal motor activity, which was in contrast to a calcium antagonist.
Nifedipine, a calcium-channel blocker, was shown to decrease lower esophageal sphincter pressure and increase esophageal acid exposure time, while atenolol, a β1 blocker, was shown to inhibit relaxation of the smooth muscle of the esophagus. However, the influence of these anti-hypertensive drugs on the segment of esophageal body contraction using high-resolution manometry was not fully investigated.
Several reports have demonstrated that anti-hypertensive drugs affect the esophageal motor function and might facilitate gastroesophageal reflux. However, the details of the drug-induced impairment of esophageal motor function are not clear. This is the first published data concerning the use of high-resolution manometry to study the effects of anti-hypertensive drugs on the motor activity of the different segments of the esophageal body.
Previous data on the effect of anti-hypertensive drugs were obtained in studies that used conventional intraesophageal pressure monitoring systems. In this study, the authors observed esophageal body contraction using high-resolution manometry with 36 intraruminal transducers. The esophageal body was divided into three segments manometrically. Nifedipine and atenolol were found to affect motor activity only in the middle and lower segments of the esophagus. Anatomical differences in the contraction of the esophageal body during the administration of anti-hypertensive drugs were clarified for the first time.
By understanding the anatomical responses of the esophageal body motor activity to the administration of anti-hypertensive drugs, more appropriate selection of anti-hypertensive drugs for patients with gastro-esophageal reflux disease (GERD) will be facilitated.
Nifedipine atenolol, and losartan are all drugs widely used for the treatment of hypertension. As both of hypertension and GERD are common diseases, the effects of anti-hypertensive drugs on the esophageal motor activity are important.
This is a well-designed study with limited new information about drug effects on esophageal motility. The references are well selected and the discussion is good.
Peer reviewer: Andrew Ukleja, MD, Assistant Professor, Clinical Assistant Professor of Medicine, Director of Nutrition Support Team, Director of Esophageal Motility Laboratory, Cleveland Clinic Florida, Department of Gastroenterology, 2950 Cleveland Clinic Blvd., Weston, FL 33331, United States
S- Editor Wang YR L- Editor Stewart GJ E- Editor Ma WH
| 1. | Achem AC, Achem SR, Stark ME, DeVault KR. Failure of esophageal peristalsis in older patients: association with esophageal acid exposure. Am J Gastroenterol. 2003;98:35-39. |
| 2. | Boeckxstaens GE. Review article: the pathophysiology of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2007;26:149-160. |
| 3. | Bruley des Varannes S, Scarpignato C. Current trends in the management of achalasia. Dig Liver Dis. 2001;33:266-277. |
| 4. | Konrad-Dalhoff I, Baunack AR, Rämsch KD, Ahr G, Kraft H, Schmitz H, Weihrauch TR, Kuhlmann J. Effect of the calcium antagonists nifedipine, nitrendipine, nimodipine and nisoldipine on oesophageal motility in man. Eur J Clin Pharmacol. 1991;41:313-316. |
| 5. | Ishikawa H, Iwakiri K, Sugiura T, Kobayashi M. Effect of nifedipine administration (10 mg) on esophageal acid exposure time. J Gastroenterol. 2000;35:43-46. |
| 6. | Hamada A, Ishii J, Doi K, Hamada N, Miyazaki C, Hamada T, Ohwaki Y, Wada M, Nakashima K. Increased risk of exacerbating gastrointestinal disease among elderly patients following treatment with calcium channel blockers. J Clin Pharm Ther. 2008;33:619-624. |
| 7. | Casselbrant A, Edebo A, Wennerblom J, Lönroth H, Helander HF, Vieth M, Lundell L, Fändriks L. Actions by angiotensin II on esophageal contractility in humans. Gastroenterology. 2007;132:249-260. |
| 8. | Tanaka Y, Horinouchi T, Koike K. New insights into beta-adrenoceptors in smooth muscle: distribution of receptor subtypes and molecular mechanisms triggering muscle relaxation. Clin Exp Pharmacol Physiol. 2005;32:503-514. |
| 9. | Clouse RE, Staiano A. Topography of the esophageal peristaltic pressure wave. Am J Physiol. 1991;261:G677-G684. |
| 10. | Clouse RE, Alrakawi A, Staiano A. Intersubject and interswallow variability in topography of esophageal motility. Dig Dis Sci. 1998;43:1978-1985. |
| 11. | Ghosh SK, Janiak P, Schwizer W, Hebbard GS, Brasseur JG. Physiology of the esophageal pressure transition zone: separate contraction waves above and below. Am J Physiol Gastrointest Liver Physiol. 2006;290:G568-G576. |
| 12. | Clouse RE, Prakash C. Topographic esophageal manometry: an emerging clinical and investigative approach. Dig Dis. 2000;18:64-74. |
| 13. | Clouse RE, Staiano A, Alrakawi A, Haroian L. Application of topographical methods to clinical esophageal manometry. Am J Gastroenterol. 2000;95:2720-2730. |
| 14. | Wilson JA, Vela MF. New esophageal function testing (impedance, Bravo pH monitoring, and high-resolution manometry): clinical relevance. Curr Gastroenterol Rep. 2008;10:222-230. |
| 15. | Crist J, Gidda JS, Goyal RK. Intramural mechanism of esophageal peristalsis: roles of cholinergic and noncholinergic nerves. Proc Natl Acad Sci USA. 1984;81:3595-3599. |
| 16. | Park H, Conklin JL. Neuromuscular control of esophageal peristalsis. Curr Gastroenterol Rep. 1999;1:186-197. |
| 17. | Goyal RK, Chaudhury A. Physiology of normal esophageal motility. J Clin Gastroenterol. 2008;42:610-619. |
| 18. | Pandolfino JE, Ghosh SK, Zhang Q, Jarosz A, Shah N, Kahrilas PJ. Quantifying EGJ morphology and relaxation with high-resolution manometry: a study of 75 asymptomatic volunteers. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1033-G1040. |
| 19. | Spak E, Casselbrant A, Olbers T, Lönroth H, Fändriks L. Angiotensin II-induced contractions in human jejunal wall musculature in vitro. Acta Physiol (Oxf). 2008;193:181-190. |
| 20. | Murray JA, Clouse RE, Conklin JL. Components of the standard oesophageal manometry. Neurogastroenterol Motil. 2003;15:591-606. |
| 21. | Iwakiri K, Sugiura T, Kotoyori M, Yamada H, Hayashi Y, Nakagawa Y, Kawakami A, Kobayashi M. Effect of body position on lower esophageal sphincter pressure. J Gastroenterol. 1999;34:305-309. |
| 22. | Tutuian R, Elton JP, Castell DO, Gideon RM, Castell JA, Katz PO. Effects of position on oesophageal function: studies using combined manometry and multichannel intraluminal impedance. Neurogastroenterol Motil. 2003;15:63-67. |