Published online Feb 28, 2010. doi: 10.3748/wjg.v16.i8.1025
Revised: December 31, 2009
Accepted: January 7, 2010
Published online: February 28, 2010
AIM: To investigate differential points of solid-pseudopapillary neoplasm (SPN) of the pancreas and pancreatic endocrine tumor (PET).
METHODS: Ten cases of SPN and fourteen cases of PET were studied in this retrospective study. Clinical and pathologic features, immunostaining reactions and β-catenin gene mutations were analyzed.
RESULTS: The mean age of SPN patients was 25.6 years and these patients had no specific symptoms. The mean diameter of the tumors was 11.0 cm, 9/10 cases were cystic or a mixture of solid and cystic structures, and there was hemorrhage and necrosis on the cut surface in 8/10 (80%) cases. Characteristic pseudopapillary structure and discohesive appearance of the neoplastic cells were observed in all 10 (100%) cases. The results of immunostaining showed that nuclear expression of β-catenin and loss of E-cadherin in all the cases, was only seen in SPN. Molecular studies discovered that 9/10 (90%) cases harbored a point mutation of exon 3 in β-catenin gene. On the other hand, the mean age of PET patients was 43.1 years. Eight of 14 cases presented with symptoms caused by hypoglycemia, and the other 6 cases presented with symptoms similar to those of SPN. The mean size of the tumors was 2.9 cm, most of the tumors were solid, only 3/14 (21%) were a mixture of solid and cystic structures, and macroscopic hemorrhage and necrosis were much less common (3/14, 21%). Histologically, tumor cells were arranged in trabecular, acinar or solid patterns and demonstrated no pseudopapillary structure and discohesive appearance in all 14 (100%) cases. The results of immunostaining and mutation detection were completely different with SPN that membrane and cytoplastic expression of β-catenin without loss of E-cadherin, as well as no mutation in β-catenin gene in all the cases.
CONCLUSION: Both macroscopic and microscopic features of SPN are quite characteristic. It is not difficult to distinguish it from PET. If necessary, immunostaining of β-catenin and E-cadherin is quite helpful to make the differential diagnosis.
- Citation: Liu BA, Li ZM, Su ZS, She XL. Pathological differential diagnosis of solid-pseudopapillary neoplasm and endocrine tumors of the pancreas. World J Gastroenterol 2010; 16(8): 1025-1030
- URL: https://www.wjgnet.com/1007-9327/full/v16/i8/1025.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i8.1025
Solid-pseudopapillary neoplasm (SPN) of the pancreas is a relatively rare and its histogenesis is still controversial. There are some similarities between SPN and pancreatic endocrine tumor (PET), especially the non-functioning ones, in clinical and pathological manifestations[1-3]. Both have few specific clinical symptoms and signs and lack exclusive features on ultrasonography, imaging examination and laboratory tests. Histopathologically, both may be very similar and the results of immunohistochemistry reported in the literature showed that expression profiles of the two tumors overlapped[4,5], which sometimes results in difficulty in distinguishing the two entities. In recent years, studies have shown that the vast majority of SPN harbored a point mutation on exon 3 of β-catenin gene, which has not yet been discovered in other pancreas tumors. In this study we took the mutation of β-catenin gene as major diagnostic evidence and explored the major points of pathological differential diagnosis of SPN and non-functioning PET.
A total of 24 cases pathologically diagnosed as SPN or PET were retrieved from the files of Department of Pathology, Xiangya Hospital, Central South University, China, during the period from 1999 to 2008.
The clinical data, description of gross morphology, H&E sections and immunohistochemical staining of all the cases were reviewed and the pathological diagnoses were re-evaluated.
Immunohistochemical stains were performed on formalin-fixed, paraffin-embedded 5 μm sections from all patients. Eleven consecutive sections were prepared from each tissue block and stained for the following markers: pan cytokeratin (pan CK, DAKO), anti-trypsin (ACT, DAKO), anti-chymotrypsin (AACT, DAKO), vimentin (Vim, DAKO), synaptophysin (Syn, DAKO), chromogranin (CgA, Santa cruz), neuron-specific enolase (NSE, DAKO), insulin (Ins, Santa cruz), somatostatin (Som, Santa cruz), glucagon (Glu, Santa cruz), pancreatic polypeptide (PP, Santa cruz), E-cadherin (E-cad, Santa cruz), β-catenin (Santa cruz) and Ki-67 (DAKO). The sections were deparaffinized in xylene and rehydrated through graded alcohols. Antigen retrieval was performed in 1 mmol/L of EDTA (pH 8.0) in a microwave oven at 98°C. Endogenous peroxidases were inactivated by immersing the sections in 0.3% hydrogen peroxide for 20 min. Staining was performed with the DAKO En Vision Kit (DAKO) and the sections were developed with 3,3’-diaminobenzidine tetrahydrochloride and counterstained with hematoxylin.
Tumor tissue was microdissected from formalin-fixed, paraffin-embedded blocks using a scalpel and placed into microcentrifuge tubes for DNA extraction. DNA extraction was performed with a FFPE DNA Isolation Kit from Omega Biotek following the manufacturers’ protocol. Exon 3 of β-catenin was amplified by PCR. The primer sequences were as follows: sense: 5'-ATGGAACCAGACAGAAAAGC-3'; anti-sense: 5'-TTCCCACTCATACAGGACTT-3'. PCR cycling conditions were: 2 mmol/L MgCl2 and 1 U Platinum-Taq polymerase (Takara), initial denaturation 5 min 94°C, 40 denaturation cycles 30 s 94°C, 30 s annealing 52-60°C, 30 s elongation 72°C, and final elongation 7 min 72°C. Using a PCR Purification Kit (Takara), purification of the PCR product was done essentially as recommended by the manufacturer.
DHPLC analysis was carried out on a WAVE DNA fragment analysis system (Transgenomic Inc.) equipped with a DNASep® Column. PCR products were denatured for 5 min at 95°C before being gradually reannealed by decreasing the sample temperature from 95 to 45°C over a period of 50 min to enable the formation of heteroduplexes. DHPLC analysis was carried out at a flow-rate of 0.9 mL/min and buffer B (0.1 mol/L TEAA, 25% acetonitrile), with a gradient increase of 2% per min for 4 min. Sequencing reactions were set up with 30 ng purified PCR fragment template and 10 pmol sequencing primer in 10 μL total reaction volume following a dye terminator protocol. Sequencing primers were identical to amplification primers. Sequence alterations were verified by sequencing both DNA strands and by analyzing an independently generated PCR amplicon.
Twenty-four cases, including 6 SPN and 18 PET, were re-evaluated according to clinical characteristics, gross morphology and microscopic features, results of immunohistochemistry and molecular findings. Six cases of SPN continued to have the original diagnosis, 14 cases of 18 PET were thought to be PET, the other 4 cases of PET were revised as SPN, and no other kind of tumor was found. Therefore there were 10 cases in the SPN group and 14 cases in the PET group.
There were 7 females and 3 males in the SPN group and 9 females and 5 males in the PET group. The mean age was 25.6 years (range: 14-43 years) in the SPN group and 43.1 years (range: 16-52 years) in the PET group. Eight PET cases presented with symptoms of endocrine disorders, such as confusion, psychiatric disturbances, and even coma caused by hypoglycemia. All patients in the SPN group and 6 cases in the PET group had nonspecific and similar clinical symptoms, including a dull aching pain in the abdomen (6 cases in the SPN group and 2 in the PET group); a painless abdominal mass in the epigastric region (3 in the SPN and 1 in the PET group); and non-specific abdominal symptoms for which they had been examined (1 case in the SPN group and 3 in the PET group).
Macroscopically all the SPN and PET cases were well-circumscribed single masses except for 1 PET case, which showed multiple masses. The mean diameters were 11.0 cm (range: 5-20 cm) in the SPN group and 2.9 cm (range: 1.5-5.7 cm) in the PET group. Cystic or cystic-solid areas with zones of hemorrhage and necrosis, or cystic spaces filled with necrotic debris were seen in 8/10 (80%) cases of SPN, and only 3/14 (21%) cases of PET. For both tumors there was no preferential localization within the pancreas.
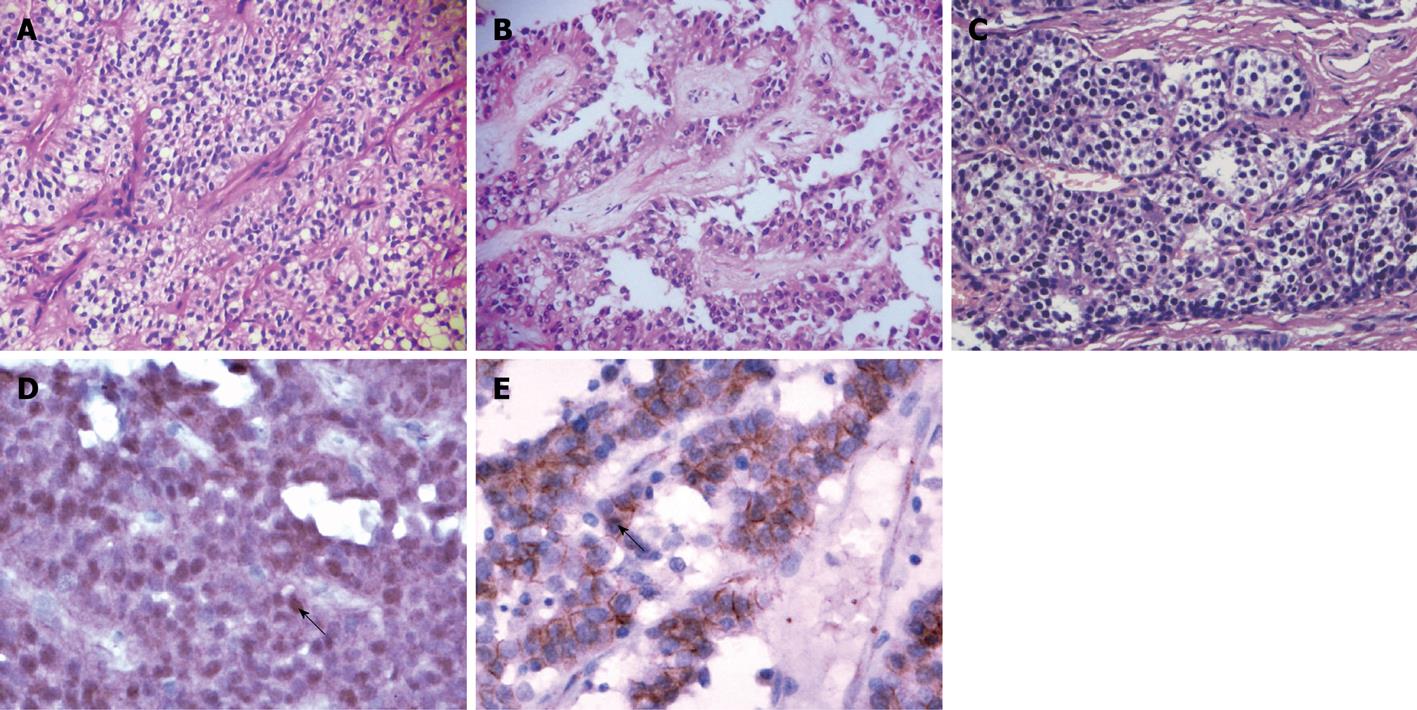
On microscopy, SPN was composed of cells arranged in the form of solid sheets (Figure 1A), microcysts and pseudopapillary areas which showed characteristic pseudopapillae with the fibrovascular axis of the branch-shaped area surrounded by several layers of polygonal epithelioid cells (Figure 1B). The cells had moderate amounts of eosinophilic to vacuolated cytoplasm. The nuclei were ovoid and folded with indistinct nucleoli and few mitoses. Regional cystic degeneration, hemorrhage, necrosis, aggregates of foamy histiocytes, cholesterol clefts were common. PET were usually arranged in three patterns: (1) trabecular or gyrus-like; (2) acinar- or duct-like (Figure 1C); and (3) solid or diffuse structure. The tumor cells were usually small and round with a granular eosinophilic or clear cytoplasm. Hemorrhage and necrosis were seen in only 3 cases, and pseudopapillary structures and cellular dyscohesive degeneration were not observed in all cases. The clinical and pathological features of SPN and PET are summarized in Table 1.
| Case No. | Sex/age (yr) | Tumor location | Size (cm) | Cystic or solid | H/N | PP areas |
| SPN group | ||||||
| 1 | F/17 | Body | 9.5 | Cystic | Present | Present |
| 2 | F/19 | Tail | 6.0 | Cystic | Present | Present |
| 3 | F/14 | Head | 8.0 | Cystic | Present | Present |
| 4 | M/20 | Body & tail | 10.0 | Cystic & solid | Present | Present |
| 5 | F/24 | Head | 10.0 | Cystic | Present | Present |
| 6 | F/33 | Body & tail | 5.0 | Solid | Absent | Present |
| 7 | F/21 | Body & tail | 20.0 | Cystic & solid | Present | Present |
| 8 | M/43 | Body | 10.5 | Cystic & solid | Present | Present |
| 9 | F/27 | Tail | 12.0 | Cystic& solid | Present | Present |
| 10 | F/38 | Unclear | 19.5 | Cystic & solid | Present | Present |
| PET group | ||||||
| 11 | F/16 | Head | 1.5 | Solid | Absent | Absent |
| 12 | F/53 | Body | 2.0 | Solid | Absent | Absent |
| 13 | M/44 | Tail | 1.5 | Solid | Absent | Absent |
| 14 | F/40 | Head | 4.5 | Solid | Absent | Absent |
| 15 | M/43 | Head | 3.0 | Solid | Absent | Absent |
| 16 | F/49 | Head | 1.5 | Solid | Absent | Absent |
| 17 | F/34 | Head | 2.0 | Solid | Absent | Absent |
| 18 | F/46 | Head | 1.5 | Solid | Absent | Absent |
| 19 | F/49 | Tail | 5.5 | Solid | Absent | Absent |
| 20 | M/52 | Head | 3.0 | Cystic & solid | Present | Absent |
| 21 | F/50 | Tail | 1.5 | Solid | Absent | Absent |
| 22 | M/37 | Tail | 4.2 | Cystic & solid | Present | Absent |
| 23 | F/45 | Body | 3.4 | Solid | Absent | Absent |
| 24 | M/46 | Tail | 5.7 | Cystic & solid | Present | Absent |
The immunohistochemical results are summarized in Table 2. Nuclear translocation and accumulation of β-catenin protein was seen in neoplastic cells in all SPN cases (Figure 1D). The tumor cells of PET cases showed only normal membranous and cytoplastic β-catenin labeling, but were negative for nuclear accumulation (Figure 1E). The other immunostains showed an overlap, even including synaptophysin and chromogranin A, between these two tumors. Ki-67 proliferative index was less than 1% in most of the tumors in both groups.
| Case No. | AACT | AAT | Vim | NSE | Syn | CgA | CK | EMA | Ins | Som | Glu | PP | E-cad | Beta | Ki-67 (%) |
| SPN group | |||||||||||||||
| 1 | + | + | + | - | - | - | - | + | - | - | - | - | - | N/C | > 1 |
| 2 | + | + | + | + | + | - | + | + | - | - | - | - | - | N/C | > 1 |
| 3 | + | + | - | + | - | + | - | - | - | - | - | - | - | N/C | > 1 |
| 4 | + | + | - | + | + | - | - | - | - | - | - | - | - | N/C | > 1 |
| 5 | + | + | + | + | + | - | - | + | - | - | - | - | - | N/C | > 1 |
| 6 | - | + | + | + | + | - | + | - | - | - | - | - | - | N/C | > 1 |
| 7 | + | + | + | + | - | + | - | - | - | - | - | - | - | N/C | > 1 |
| 8 | + | - | + | - | + | - | - | - | - | - | - | - | - | N/C | > 1 |
| 9 | - | + | - | - | - | - | + | - | - | - | - | - | - | N/C | > 1 |
| 10 | + | - | - | - | + | + | + | - | - | - | - | - | - | N/C | 2 |
| PET group | |||||||||||||||
| 11 | - | - | - | + | + | - | + | + | + | - | - | - | + | C/M | > 1 |
| 12 | - | - | - | + | + | + | - | + | + | + | - | + | C/M | > 1 | |
| 13 | - | - | - | + | + | + | - | - | + | - | - | - | + | C/M | > 1 |
| 14 | - | - | + | + | + | + | + | + | - | - | - | - | + | C/M | > 1 |
| 15 | - | - | - | + | + | + | + | + | - | - | +- | + | + | C/M | > 1 |
| 16 | - | - | - | + | + | + | + | - | - | - | - | - | + | C/M | > 1 |
| 17 | - | - | + | + | + | + | + | + | + | + | - | - | + | C/M | > 1 |
| 18 | - | - | - | + | + | + | + | + | + | - | - | - | + | C/M | > 1 |
| 19 | + | - | - | + | + | + | - | - | - | - | - | - | + | C/M | 2 |
| 20 | - | - | - | - | + | + | - | - | - | - | - | - | + | C/M | > 1 |
| 21 | + | - | - | + | + | + | + | + | + | - | - | - | + | C/M | > 1 |
| 22 | - | + | - | - | - | + | + | - | - | - | - | - | + | C/M | 3 |
| 23 | - | + | + | - | + | - | + | + | - | - | - | - | + | C/M | > 1 |
| 24 | + | - | + | - | - | - | - | + | - | - | - | - | + | C/M | 4 |
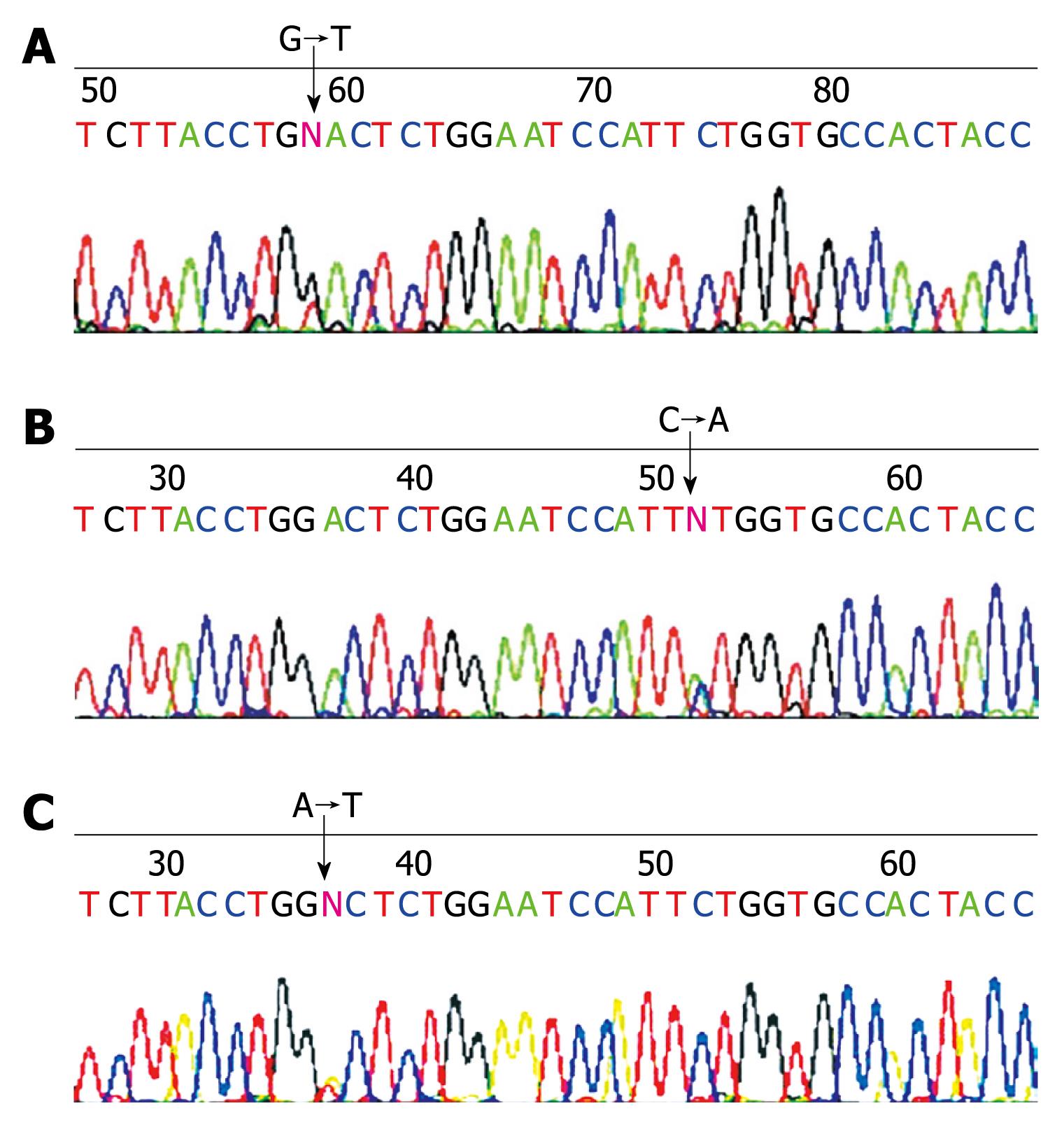
Nine of 10 SPN cases presented DHPLC heteroduplex bimodal or shoulder-type peaks on DHPLC mutation detection, which indicated mutations on exon 3 of β-catenin gene.
All 14 samples of PET showed a single peak on DHPLC mutation detection indicating no mutations of β-catenin gene exon 3 in PCR amplified fragments.
Sequencing confirmed results of DHPLC mutation detection that 9 cases of SPN had a point mutation of β-catenin gene exon 3. Mutations involving codons were as follows: 4 cases at codon 32, 2 at codon 33, 2 at codon 34 and 1 case at codon 37 (Table 3, Figure 2).
| Case No. | Codon | Base substitution | Amino acid conversion |
| 1 | 33 | TCT→TGT | Ser→Cys |
| 2 | 37 | TCT→TAT | Ser→Tyr |
| 3 | 34 | GGA→GTA | Gly→Val |
| 4 | Wild type | ||
| 5 | 32 | GAC→TAC | Asp→Tyr |
| 6 | 34 | GGA→AGA | Gly→Arg |
| 7 | 33 | TCT→CCT | Ser→Pro |
| 8 | 32 | GAC→TAC | Asp→Tyr |
| 9 | 32 | GAC→GTC | Asp→Val |
| 10 | 32 | GAC→TAC | Asp→Tyr |
SPN of the pancreas is an uncommon tumor, constituting only about 1%-2% of exocrine pancreatic neoplasms[6]. SPN is clinically and histologically quite similar to the more common PETs, especially the non-functioning ones.
SPN predominantly occurs in adolescent girls and young women with a reported frequency of 87% to 90% (mean age of 22 to 25 years)[7]. Such a striking gender and age predilection of SPN is different from PET.
Functioning PET demonstrates specific clinical symptoms and signs which provides a suggestion for consideration of endocrine tumors. However, the clinical manifestations of SPN and non-functioning PET in our cases as mentioned in literature are not specific[8]. The patients in both groups present with unclear clinical features including abdominal discomfort, mild abdominal pain, poor appetite and nausea which are related to tumor compression on the adjacent organs, and a palpable mass in the abdomen[9,10]. Occasionally a mass may be found in the abdomen during complementary imaging studies such as ultrasound or CT scan[11,12].
Both SPN and non-functioning PET have not been associated with specific clinical laboratory test findings including serum tumor markers[12,13]. The imaging features of both tumors present similarities in most cases, including ultrasonography, CT scanning, MRI and other imaging examinations. For both SPN and PET the best treatment is surgical resection. The routine imaging examinations are satisfactory for preoperative tumor location and understanding of the relationship between tumors and surrounding tissue. However, it is difficult for imaging studies to determine the nature of the lesions.
Both SPN and PET can occur in any part of the pancreas, though PET occurring in the pancreatic tail is more common[8]. SPN usually has much larger dimensions than PET. In contrast to the smaller mass (mean diameter 2.7 cm) of non-functioning PET, SPN is often much larger in size (mean of 10 cm at presentation in our study). Most of the cases in both groups are characterized with a tumor that is separated from the normal pancreas by a complete or incomplete fibrous capsule. On the cut surface, hemorrhagic and cystic areas are much more common in SPN than in PET.
Histologically, both SPN and PET could be arranged as solid areas and the size and shape of the tumor cells were relatively uniform with round or oval nuclei and vacuolated or eosinophilic large cytoplasms. It was difficult to distinguish these two kinds of tumor if only such histological pattern was used as the diagnostic basis. However, the unique morphological characteristics of SPN, large areas composing of pseudopapillary structures indicating evidence of cellular dyscohesive degeneration and cholesterol clefts, aggregation of foamy histiocytes, and characteristic microscopic features of SPN allow easy differential diagnosis from PET.
Immunohistochemical findings reported in early literature were of variation and absence of a specific profile. There was overlap of positive expression on immunostaining using such markers as α1-antitrypsin, α1-antichymotrypsin, NSE, Syn, progesterone receptor, carcinoembryonic antigen, pan CK, vimentin, CD10, CD56, and cyclin D1, so that immunohistochemistry was incapable of giving much helpful additional information for the differential diagnosis of SPN. However, more recently, the situation was changed by applications of E-cadherin and β-catenin which possess highly sensitivity and specificity. Literature reports[14-17] and our results showed that nuclear expression of β-catenin and loss of E-cadherin were seen in nearly all cases of SPN. On the contrary, there was cytoplasmic and membrane expression of β-catenin and strong expression of E-cadherin in PET cases. The exclusive expression of β-catenin and E-cadherin in SPN can be applied to make definite differentiation from PET.
Most SPNs harbor mutations in the β-catenin gene and, as a result, most SPNs have an abnormal nuclear expression of β-catenin protein. We found 1 (case 4) in our cases without mutation in exon 3, however, it showed cystic and necrosis on macroscopy, pseudopapillary structure and discohesive appearance of the neoplastic cells on microscopy, cytoplasmic and nuclear staining for β-catenin, and loss of E-cadherin, which indicated alteration of β-catenin gene and suggested that there might exist a mutation on another exon of β-catenin gene.
In conclusion, SPN of the pancreas, compared with PET, is a cystic-solid or cystic tumor with a larger size mostly seen in young women, and has the morphological features of hemorrhage and necrosis on the cut surface and exclusive pseudopapillary structures on light microscopy caused by cellular dyscohesive degeneration. If one is aware of its clinical and histopathologic features, with sufficient sampling of the tumor, one usually does not confuse SPN with PET in most cases. If necessary, immunostaining of β-catenin and E-cadherin is quite helpful to make the differential diagnosis.
Solid-pseudopapillary neoplasm (SPN) is an uncommon pancreatic tumor with low malignant potential and unknown cell origin, constituting only about 1% of pancreatic neoplasms. Since it is rare and maybe morphologically similar to pancreatic endocrine tumor (PET), sometimes there is overlapped expression of convensional immunohistochemical markers in both tumors and a pathological misdiagnosis may take place.
In recent years, mutations of β-catenin, which are sufficient to induce pancreas tumorigenesis, have been found in SPN and the aberrant activation of the Wnt-β-catenin pathway appears to be a constant feature in SPN. The mutations lead to the displacement of β-catenin and E-cadherin from their normal membrane location, and, as a result, SPNs have an abnormal pattern of labeling with antibodies to the β-catenin and E-cadherin protein which helps in differential diagnosis.
In this study, mutations of the β-catenin gene were analyzed and immunostaining of β-catenin and E-cadherin protein were done in both SPN (10 cases) and PET (14 cases). Mutations of β-catenin and delocalization of β-catenin and E-cadherin were exclusively found in SPN.
Characteristic morphological features and specific expressive patterns of β-catenin and E-cadherin make it to be easy to differentiate SPN from other pancreatic tumors
β-catenin (or Beta-catenin) gene is also known as CTNNB1 gene which encodes the protein β-catenin. β-catenin is a subunit of the cadherin complex and has been implicated as an integral component in the Wnt signaling pathway.
This is a nice retrospective analysis of the histological features of SPN of the pancreas compared with endocrine tumors and highlights some important differences.
Peer reviewer: Aaron Vinik, MD, PhD, FCP, MACI, Professor of Medicine/Pathology/Neurobiology, Director of Research and Neuroendocrine Unit, Eastern Virginia Medical School, Strelitz Diabetes Center, 855 W Brambleton Avenue, Norfolk, VA 23510, United States
S- Editor Wang YR L- Editor O’Neill M E- Editor Zheng XM
| 1. | Wick MR, Graeme-Cook FM. Pancreatic neuroendocrine neoplasms: a current summary of diagnostic, prognostic, and differential diagnostic information. Am J Clin Pathol. 2001;115 Suppl:S28-S45. |
| 2. | Comper F, Antonello D, Beghelli S, Gobbo S, Montagna L, Pederzoli P, Chilosi M, Scarpa A. Expression pattern of claudins 5 and 7 distinguishes solid-pseudopapillary from pancreatoblastoma, acinar cell and endocrine tumors of the pancreas. Am J Surg Pathol. 2009;33:768-774. |
| 3. | Serra S, Chetty R. Revision 2: an immunohistochemical approach and evaluation of solid pseudopapillary tumour of the pancreas. J Clin Pathol. 2008;61:1153-1159. |
| 4. | Notohara K, Hamazaki S, Tsukayama C, Nakamoto S, Kawabata K, Mizobuchi K, Sakamoto K, Okada S. Solid-pseudopapillary tumor of the pancreas: immunohistochemical localization of neuroendocrine markers and CD10. Am J Surg Pathol. 2000;24:1361-1371. |
| 5. | Kosmahl M, Seada LS, Jänig U, Harms D, Klöppel G. Solid-pseudopapillary tumor of the pancreas: its origin revisited. Virchows Arch. 2000;436:473-480. |
| 6. | Canzonieri V, Berretta M, Buonadonna A, Libra M, Vasquez E, Barbagallo E, Bearz A, Berretta S. Solid pseudopapillary tumour of the pancreas. Lancet Oncol. 2003;4:255-256. |
| 7. | Kosmahl M, Pauser U, Peters K, Sipos B, Lüttges J, Kremer B, Klöppel G. Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch. 2004;445:168-178. |
| 8. | Liang H, Wang P, Wang XN, Wang JC, Hao XS. Management of nonfunctioning islet cell tumors. World J Gastroenterol. 2004;10:1806-1809. |
| 9. | Martin RC, Klimstra DS, Brennan MF, Conlon KC. Solid-pseudopapillary tumor of the pancreas: a surgical enigma? Ann Surg Oncol. 2002;9:35-40. |
| 10. | Mulkeen AL, Yoo PS, Cha C. Less common neoplasms of the pancreas. World J Gastroenterol. 2006;12:3180-3185. |
| 11. | Salvia R, Bassi C, Festa L, Falconi M, Crippa S, Butturini G, Brighenti A, Capelli P, Pederzoli P. Clinical and biological behavior of pancreatic solid pseudopapillary tumors: report on 31 consecutive patients. J Surg Oncol. 2007;95:304-310. |
| 12. | Frago R, Fabregat J, Jorba R, García-Borobia F, Altet J, Serrano MT, Valls C. Solid pseudopapillary tumors of the pancreas: diagnosis and curative treatment. Rev Esp Enferm Dig. 2006;98:809-816. |
| 13. | Yoon DY, Hines OJ, Bilchik AJ, Lewin K, Cortina G, Reber HA. Solid and papillary epithelial neoplasms of the pancreas: aggressive resection for cure. Am Surg. 2001;67:1195-1199. |
| 14. | Tanaka Y, Notohara K, Kato K, Ijiri R, Nishimata S, Miyake T, Fukunaga M, Horisawa M, Nakatani Y. Usefulness of beta-catenin immunostaining for the differential diagnosis of solid-pseudopapillary neoplasm of the pancreas. Am J Surg Pathol. 2002;26:818-820. |
| 15. | Tanaka Y, Kato K, Notohara K, Hojo H, Ijiri R, Miyake T, Nagahara N, Sasaki F, Kitagawa N, Nakatani Y. Frequent beta-catenin mutation and cytoplasmic/nuclear accumulation in pancreatic solid-pseudopapillary neoplasm. Cancer Res. 2001;61:8401-8404. |
| 16. | Abraham SC, Klimstra DS, Wilentz RE, Yeo CJ, Conlon K, Brennan M, Cameron JL, Wu TT, Hruban RH. Solid-pseudopapillary tumors of the pancreas are genetically distinct from pancreatic ductal adenocarcinomas and almost always harbor beta-catenin mutations. Am J Pathol. 2002;160:1361-1369. |
| 17. | Kim MJ, Jang SJ, Yu E. Loss of E-cadherin and cytoplasmic-nuclear expression of beta-catenin are the most useful immunoprofiles in the diagnosis of solid-pseudopapillary neoplasm of the pancreas. Hum Pathol. 2008;39:251-258. |