Published online Dec 14, 2010. doi: 10.3748/wjg.v16.i46.5845
Revised: July 27, 2010
Accepted: August 3, 2010
Published online: December 14, 2010
AIM: To study whether immune-activation stage in serum of adult Crohn’s disease (CD) patients correlates with disease activity and with treatment response to anti-tumor necrosis factor-α (TNF-α) therapy.
METHODS: Serum samples were obtained from 15 adult CD patients introduced to anti-TNF-α therapy. The individual stage of immune activation was studied applying our new in vitro assay, in which target cells (donor derived peripheral blood mononuclear cells) were cultured with patient serum and the T-cell activation induced by the patient serum was studied using a panel of markers for effector [interferon γ (IFNγ), interleukin (IL)-5] and regulatory T-cells [forkhead transcription factor 3 (FOXP3) and glucocorticoid-induced tumour necrosis factor receptor (GITR)]. The endoscopic disease activity was assessed with the Crohn’s disease endoscopic index of severity (CDEIS) before and 3 mo after therapy with an anti-TNF-α agent.
RESULTS: Low induction of FOXP3 and GITR in target cells cultured in the presence of patient serum was associated with high disease activity i.e. CDEIS assessed before therapy (r = -0.621, P = 0.013 and r = -0.625, P = 0.013, respectively). FOXP3 expression correlated inversely with pre-treatment erythrocyte sedimentation rate (r = -0.548, P = 0.034). Low serum induced FOXP3 (r = -0.600, P = 0.018) and GITR (r = -0.589, P = 0.021) expression and low IFNγ secretion from target cells (r = -0.538, P = 0.039) associated with treatment response detected as a decrease in CDEIS.
CONCLUSION: The immune-activation potency in the patient serum prior to anti-TNF-α therapy reflected intestinal inflammation and the therapeutic response.
- Citation: Rintamäki H, Sipponen T, Salo HM, Vaarala O, Kolho KL. Serum immune-activation potency and response to anti-TNF-α therapy in Crohn's disease. World J Gastroenterol 2010; 16(46): 5845-5851
- URL: https://www.wjgnet.com/1007-9327/full/v16/i46/5845.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i46.5845
Tumor necrosis factor-α (TNF-α), a pro-inflammatory protein secreted mainly by monocytes, macrophages and T-cells, has a central role in the disease pathogenesis of Crohn’s disease (CD)[1-3]. TNF-α is elevated in stools and mucosa of CD patients[4-6].
The chimeric TNF-α-antibody infliximab and human IgG1 TNF-α-antibody adalimumab are indicated for the treatment of moderate to severe CD[7,8]. During treatment with an anti-TNF-α agent, clinical improvement is accompanied by significant healing of endoscopic lesions and the disappearance of mucosal inflammatory infiltrate[9]. The therapeutic response, however, is not uniform and a significant proportion of patients are non-responders. In the ACCENT I study, 58% of patients with moderate CD responded to the first infusion of infliximab and with adalimumab 24%-36% of TNF-α-antibody naïve patients responded to the induction of therapy[7,8].
There are a few studies exploring immunological markers that reflect treatment response to anti-TNF-α therapy in CD. Elevated concentration of mucosal nuclear NFκBp65 and high secretion of TNF-α by cells of peripheral blood cultivation precede clinical relapse[10]. In the study by Mäkitalo et al[11], the expression profile of the macrophage tissue inhibitor of metalloproteinase (TIMP)-1 and stromal TIMP-3 in the intestine correlated positively with the Crohn’s disease endoscopic index of severity (CDEIS) and the down regulation of matrix metalloproteinase-9 (MMP9) production of macrophages correlated with histological improvement during anti-TNF-α therapy. Further, in another study serum MMP9 levels seemed to decrease particularly in those CD patients who responded to infliximab therapy[12].
Recently, we described a novel approach to study individual treatment responses at an early phase of glucocorticoid therapy. In that study we investigated pediatric patients with inflammatory bowel disease (IBD) introduced to systemic glucocorticoids and showed that patient serum modified the expression of T-cell signalling markers on target cells (peripheral blood mononuclear cells from a healthy donor)[13].
Encouraged by this finding we applied the same method to study the individual immune-activation potency in adult CD patients starting anti-TNF therapy. We measured the expression of the regulatory T-cell markers forkhead transcription factor 3 (FOXP3) and glucocorticoid-induced tumour necrosis factor receptor (GITR), and cytokines interferon γ (IFNγ), interleukin-5 (IL-5) and IL-17 induced in the target cells by patient serum in 15 adult CD patients at the beginning of anti-TNF-α therapy. We found that prior to anti-TNF-α therapy the ability of patient serum to modulate the FOXP3 and GITR activation of the target cells mirrored the disease activity and the individual therapeutic response in the gut assessed with the CDEIS.
Fifteen adult patients (6 females) with established CD were introduced to an anti-TNF-α agent due to an acute flare (n = 6), chronic active disease (6), or rapid postoperative reoccurrence of the disease (3; Table 1). Fourteen patients received infliximab infusion 5 mg/kg at week 0 and 8. One patient received an adalimumab induction dose 80 mg subcutaneously (s.c.) at week 0, followed by 40 mg s.c. every other week until week 8. After the beginning of the anti-TNF-α treatment, corticosteroids were tapered off.
| Age (yr): median (range) | 25 (19-44) | |
| Disease duration, years median (range) | 5.1 (0.4-27) | |
| No. of patients | % | |
| Disease location | ||
| Ileum | 2 | 13 |
| Colon | 4 | 27 |
| Ileocolon | 9 | 60 |
| Disease type | ||
| Inflammatory | 7 | 47 |
| Stricturing | 5 | 33 |
| Inflammatory + perianal | 3 | 20 |
| Prior anti-TNF-therapy | 4 | 27 |
| Prior bowel operation | 4 | 27 |
| Smokers | 7 | 47 |
| Baseline concomitant medication | ||
| Azathioprine/6-mercaptopurine | 10 | 67 |
| Methotrexate | 2 | 13 |
| Corticosteroids | 10 | 67 |
| Mesalamine or sulphasalazine | 13 | 87 |
| Week 12 concomitant medication | ||
| Azathioprine/6-mercaptopurine | 13 | 87 |
| Methotrexate | 2 | 13 |
| Corticosteroids | 1 | 6.7 |
| Mesalamine or sulphasalazine | 11 | 73 |
| CDAI at baseline, median (range) | 158 (49-605) | |
| CDAI at week 12, median (range) | 66 (24-202) |
All patients underwent an ileocolonoscopy before the introduction of anti-TNF-α therapy (median 7 d, range 1-38 d) and the endoscopic assessment of treatment response was performed at week 12 (week 10 for the adalimumab-treated patient). The endoscopic activity was graded according to the CDEIS[14,15]. This score is based on the presence of superficial or deep ulcerations, proportion of affected and ulcerated surface, and presence of either ulcerated or non-ulcerated stenosis in the terminal ileum and four segments of the colon (right, transverse, left colon and sigmoid, and rectum)[14]. Clinical disease activity was assessed with the Crohn’s disease activity index (CDAI)[16].
Exclusion criteria were contraindication to anti-TNF-α treatment, pregnancy, history of extensive bowel resection, ostomy, long-term use of nonsteroidal anti-inflammatory drugs, or perianal fistulating disease without luminal inflammation.
A serum sample for the target cell assay was provided at the time of the first ileocolonoscopy. The routine blood samples for serum C-reactive protein (CRP, normal value < 10 mg/L), erythrocyte sedimentation rate (ESR) and fecal samples for measurement of calprotectin (PhiCal Test, Calpro AS, Oslo, Norway[17,18]) were obtained by the time of the endoscopies and 3 mo after the first anti-TNF-α dose[15].
The assay for the assessment of individual stages of immunoactivation by applying patient serum in an in vitro culture of donor derived peripheral blood mononuclear cells (PBMC, target cells) is described recently in detail[13]. In brief, healthy donor (male 34 years) derived PBMC were separated by Ficoll-Paque (Amersham Biosciences) centrifugation (800 × G, 25 min) and cultured in the presence of the patients inactivated (35 min in 56°C) serum at an end concentration of 8%, either at resting state or activated with mitogen phytohemagglutin (PHA, 5 μg/mL). Serum of a healthy donor (male 27 years) was used as the methodological control between cell culture plates. After 72 h incubation at 37°C in humified atmosphere with 5% CO2/air the supernatants were collected and stored at -70°C. This assay was performed with serum samples drawn prior to first anti-TNF-α infusion.
IFNγ, IL-5 and IL-17 were measured with ELISA in duplicate from the supernatants collected from the target cell cultures (see above) incubated with patient serum. IFNγ and IL-5 was detected as described before[19,20]. IL-17 was measured according to the manufacturer’s protocol (Catalogue no: DY317; R&D Systems, United Kingdom). We subtracted the non-stimulated value from the stimulated value to obtain the ∆-value for statistical analyses.
Quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) process was recently described in detail[13]. Briefly, total RNA was isolated from cell samples with the GenElute Mammalian total RNA miniprep kit (Sigma-Aldrich), and the RNA concentration was measured by a spectrophotometer (ND-1000, NanoDrop Technologies Inc, Wilmington, DE, USA). Reverse transcription was performed by using TaqMan Reverse Transcription reagents (Applied Biosystems, Foster City, CA, USA) with additional treatment of total RNA at 10 ng/μL with DNAse I (0.01 U/μL) (Roche Diagnostics, Mannheim, Germany) to eliminate genomic DNA. Quantitative RT-PCR was performed using predesigned FAM-labelled TaqMan Gene Expression Assay reagents (Applied Biosystems) and the ABI Prism 7700 Sequence Detection System (Applied Biosystems) in triplicate wells. Assay reagents for FOXP3 (Hs00203958_m1), GITR (Hs00188346_m1), IFNγ (Hs00174143_m1) and 18s RNA (Hs99999901_s1) were used. The difference value (ΔCt) is the normalised quantitative value of the expression level of the target gene achieved by subtracting the Ct value of the reference gene (18s) from the Ct value of the target gene. An exogenous cDNA pool calibrator was collected from PHA stimulated PBMC and considered as an interassay standard to which normalized samples were compared. ΔΔCt is the difference between the ΔCt of the analyzed sample and ΔCt of the calibrator. Calculation of 2-ΔΔCt gives a relative amount of the target gene in analyzed sample compared with the calibrator, both normalized to an endogenous control (18S). For presentations the relative amount of target genes was multiplied by 1000 and expressed as relative units.
Results are reported as median. Comparison between two dependent samples was calculated with Wilcoxon rank test and two independent samples was calculated with Mann-Whitney t-test. The two-tailed Spearman’s rho was used for calculation of the correlations and Kruskall-Wallis test served in exploring associations between groups (SPSS 16.0 program). P < 0.05 was set for statistical significance.
All patients gave their informed written consent for participation in this study approved by the ethics committee of the Helsinki University Central Hospital.
The expression levels of IFNγ, FOXP3 and GITR specific mRNA in both resting and activated target cells cultured in the presence of CD patient serum obtained before anti-TNF-α therapy is shown in Table 2. Also, the secretion of IFNγ, IL-5 and IL-17 from activated target cells is shown in Table 2. The secretion of IFNγ, IL-5 and IL-17 from resting target cells was below detection limits.
| RT-qPCR | ELISA | ||||||||
| Patient No. | FOXP3 | FOXP3 PHA | GITR | GITR PHA | IFNγ | IFNγ PHA | IFNγ PHA | IL-5 PHA | IL-17 PHA |
| 1 | 4.9 | 53.8 | 10.8 | 65.0 | 1.8 | 11.7 | 1580.0 | 8.4 | 0.0 |
| 2 | 8.2 | 65.4 | 18.7 | 175.9 | 3.8 | 30.5 | 37 400.0 | 80.5 | 19.4 |
| 3 | 12.9 | 127.7 | 30.8 | 452.9 | 11.6 | 128.9 | 172 000.0 | 187.0 | 239.0 |
| 4 | 9.2 | 153.7 | 32.5 | 399.2 | 5.0 | 52.0 | 97 600.0 | 154.0 | 265.0 |
| 5 | 10.0 | 68.4 | 22.8 | 183.2 | 8.2 | 22.6 | 38 100.0 | 81.5 | 118.0 |
| 6 | 4.0 | 4.6 | 8.9 | 55.2 | 1.3 | 10.4 | 0.0 | 0.5 | 42.7 |
| 7 | 11.5 | 36.7 | 22.4 | 107.0 | 6.1 | 19.3 | 1460.0 | 8.5 | 20.9 |
| 8 | 7.9 | 97.0 | 17.93 | 171.0 | 5.8 | 26.2 | 0.0 | 6.5 | 82.0 |
| 9 | 4.6 | 40.7 | 9.3 | 66.6 | 3.0 | 4.5 | 0.0 | 1.0 | 0.0 |
| 10 | 6.4 | 68.3 | 15.8 | 145.8 | 4.2 | 15.8 | 28.8 | 4.1 | 0.0 |
| 11 | 7.4 | 96.9 | 17.0 | 251.4 | 3.4 | 36.1 | 173.0 | 7.4 | 0.0 |
| 12 | 6.9 | 86.4 | 15.1 | 217.4 | 4.3 | 36.2 | 223 000.0 | 144.0 | 638.0 |
| 13 | 5.2 | 32.7 | 14.1 | 83.0 | 3.8 | 5.3 | 11 600.0 | 3.3 | 341.0 |
| 14 | 3.8 | 81.6 | 12.7 | 180.7 | 2.9 | 13.5 | 1660.0 | 0.5 | 181.0 |
| 15 | 5.4 | 31.2 | 9.9 | 54.4 | 3.6 | 3.3 | 16 100.0 | 5.5 | 310.0 |
The type of CD or localization was not associated with the level of IFNγ, FOXP3 and GITR specific mRNA expression or IFNγ, IL-5 and IL-17 secretion from target cells (all P = NS).
During anti-TNF-α therapy the CDEIS decreased from a median of 13 points (range 1.8-25) to 4.8 points (range 0-11, P = 0.002). 12/15 patients responded to therapy, while 3 patients had no decrease in the CDEIS.
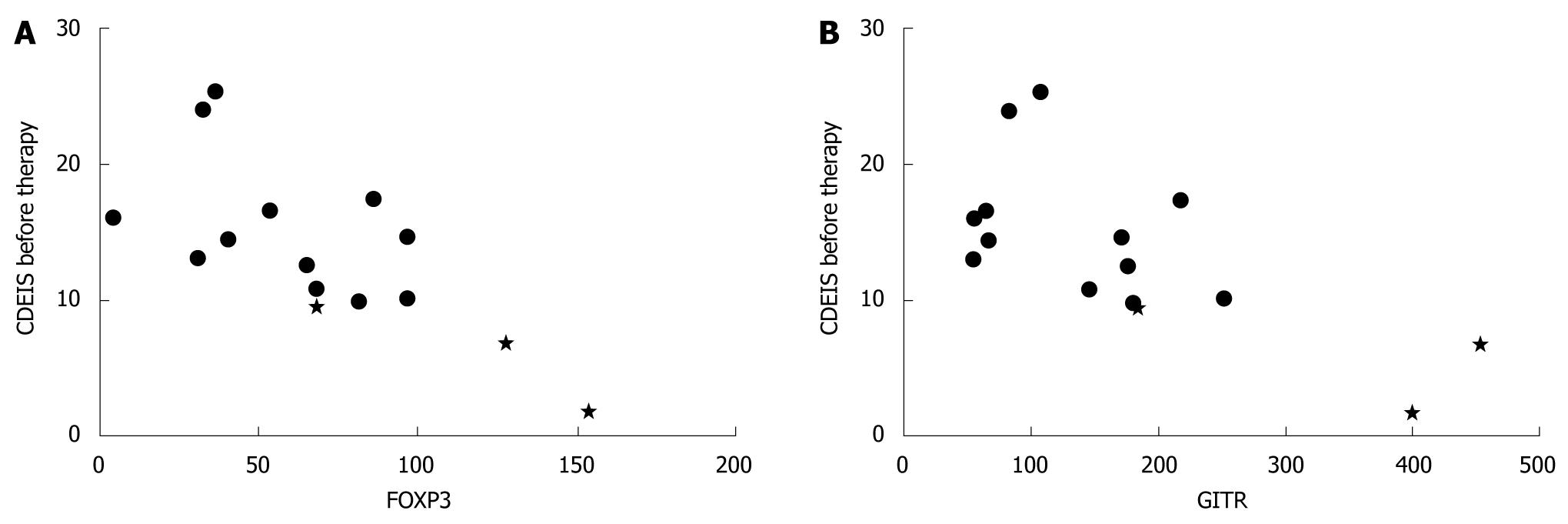
The expression of regulatory T-cell markers FOXP3 and GITR specific mRNA in activated target cells cultured with patient serum correlated inversely with the pre-treatment CDEIS (FOXP3 r = -0.621, P = 0.013 and GITR r = -0.625, P = 0.013; Figure 1). A trend towards an inverse correlation between IFNγ mRNA expression and the pre-treatment CDEIS was observed (r = -0.446, P = 0.095). There was no correlation between IFNγ, IL-5 or IL-17 secretion from target cells and the pre-treatment CDEIS (P = 0.241 for IFNγ, P = 0.286 for IL-5 and P = 0.980 for IL-17).
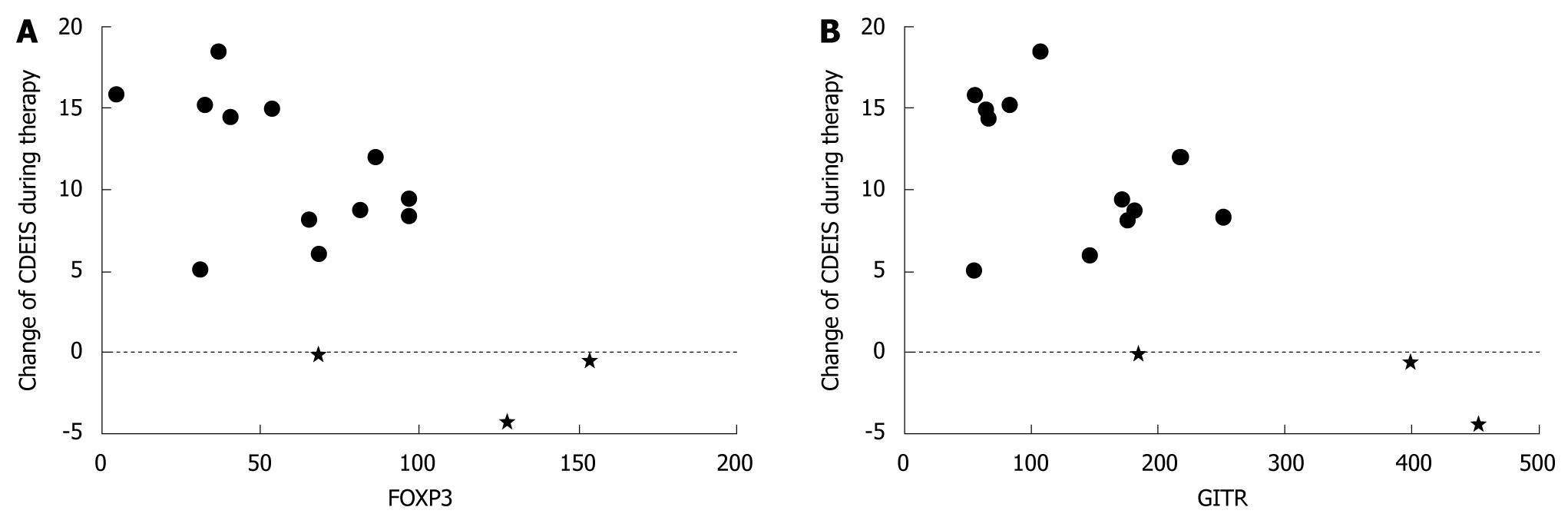
Low patient serum induced FOXP3, GITR and IFNγ specific mRNA expression in target cells was associated with a remarkable change of CDEIS observed during 3 mo therapy (FOXP3 r = -0.600, P = 0.018; GITR r = -0.589, P = 0.021; IFNγr = -0.486, P = 0.066; Figure 2). Accordingly, in resting target cells GITR specific mRNA expression correlated with the change of CDEIS (r = -0.550, P = 0.034).
Also low serum induced IFNγ and IL-5 secretion from activated target cells was associated with a high change of CDEIS (r = -0.538, P = 0.039; r = -0.504, P = 0.055). IL-17 secretion from activated target cells did not correlate with the change of CDEIS (P = 0.467).
Fecal calprotectin decreased from a median of 1170 μg/g (range 88-15300) to a median of 130 μg/g (range 13-1400) within the 3 mo anti-TNF-α treatment (P = 0.001). No correlation was observed between target cell responses and calprotectin levels before or after treatment.
ESR decreased from a median of 18 mm/h (range 6-58) to a median of 10.6 mm/h (range 1-40; P = 0.001) and CRP decreased from a median of 10 mg/L (range 0-54) to a median of < 5 mg/L (range < 5-11, P = 0.005) within 3 mo after introduction of anti-TNF-α therapy.
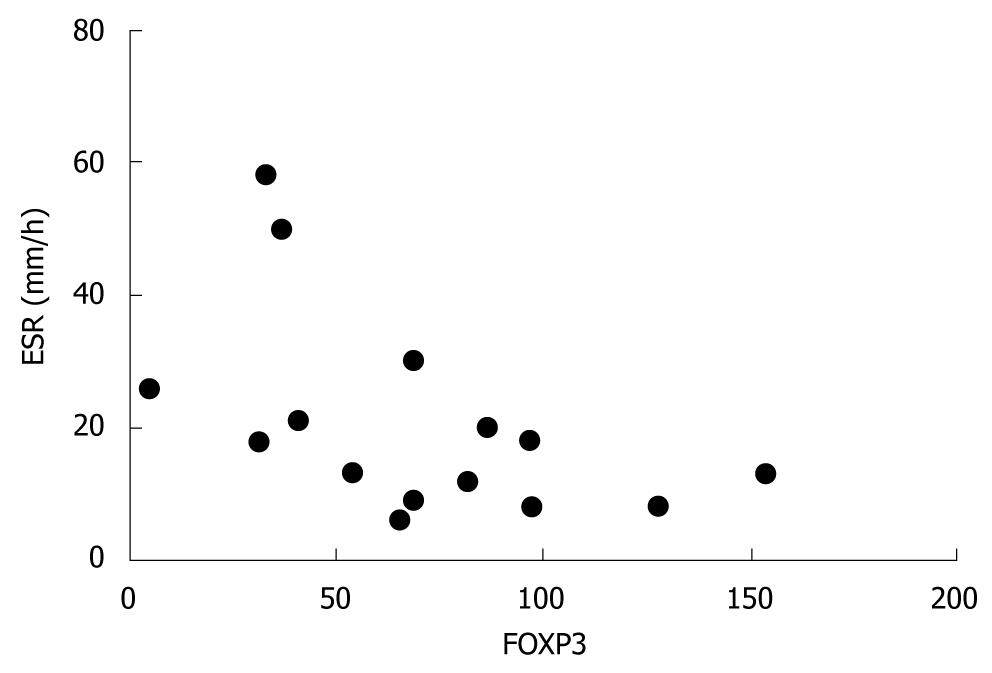
FOXP3 specific mRNA expression in activated target cells correlated negatively with pre-treatment ESR (r = -0.548, P = 0.034, Figure 3). There was no correlation between pre-treatment ESR and GITR (P≥ 0.210) or IFNγ (P≥ 0.109) specific mRNA expression or cytokine secretion from target cells (IFNγP = 0.755, IL-5 P = 0.434, IL-17 P = 0.511).
FOXP3, GITR or IFNγ specific mRNA expression in target cells or IFNγ, IL-5 or IL-17 secretion from target cells did not correlate with the change of ESR or CRP during 3 mo anti-TNF-α therapy (all P = NS, data not shown).
We found that serum samples obtained from patients with CD before the introduction of anti-TNF-α therapy modulated the expression of regulatory T-cell (T-reg) markers FOXP3 and GITR and secretion of inflammatory cytokines IFNγ, IL-5 and IL-17 from target cells (peripheral blood mononuclear cells from healthy donors). The serum induced FOXP3, GITR and IFNγ responses of target cells correlated with the pre-treatment endoscopic status and also with therapeutic responses, i.e. mucosal improvement assessed with CDEIS within 3 mo.
In our previous study of children with IBD we found that the attenuation of systemic inflammation after the start of oral glucocorticoids was mirrored in the target cell responses induced by the patient serum[13]. We underline that we measured cytokine secretion and transcription markers of the target cells (donor derived PBMCs) modulated by the patient serum and not the activation stage of the PBMC from the patient. To our knowledge the ability of patient serum to activate signalling of normal T-cells has not previously been studied in adult CD patients at induction of anti-TNF-α therapy. It is remarked that post-treatment samples are not comparable here since the serum taken after the treatment contains an anti-TNF-α agent.
We found an inverse correlation between the expression of T-reg markers FOXP3 and GITR in target cells and the endoscopic disease activity before therapy. A similar inverse correlation existed also between FOXP3 and pre-treatment ESR. Since FOXP3 inhibits T-cell activation by its suppressive effect on transcription of cytokine genes[21] it is reasonable to assume that those patients whose serum environment seemed to mediate enhanced FOXP3 up-regulation as a response to T-cell stimulation had endoscopically milder disease. However, the serum induced expression of FOXP3 in target cells was not directly reflected in the cytokine activation of the target cells and cytokine response did not correlate with the pre-treatment CDEIS. Impaired up-regulation of GITR in the patient serum environment may be related to poor suppression of T-cell activation[22,23].
Interestingly, we also found that low expression of FOXP3 and GITR specific mRNA induced by patient serum obtained prior to therapy was associated with a good therapeutic response within 3 mo. These parameters were associated with high clinical activity and thus enhanced inflammation in vivo. High inflammatory activity at the early phase of anti-TNF-α therapy has been connected to the lack of therapeutic response in rheumatoid arthritis (RA). Previously non-responders to anti-TNF-α agents had a higher number of blood T-cells expressing chemokine receptors (CCR 3 and CCR 5) before the introduction of therapy[24]. In another study, high levels of serum IL-2 were associated with poor therapeutic response[25]. In our study, the group of patients that had high potency for FOXP3 induction in target cells showed poor clinical response to anti-TNF treatment. It was also evident that their disease activity before treatment was milder. TNF blocking has been shown to induce FOXP3 expression in patients with RA. Recovery of regulatory mechanisms has been proposed to be one of the mechanisms of action for TNF blocking[26]. It is possible that non-responders whose serum induced high FOXP3 up-regulation in target cells do not benefit from further activation of FOXP3 but their disease activity should be down-regulated by other mechanisms.
The majority of CD patients driven to anti-TNF-α therapy are on immunosuppressive medication such as azathioprine or methotrexate as here also. However, there was no correlation between patient serum induced expression or secretion of inflammatory cytokines from target cells and pre-treatment disease activity. We suggest that individual differences in target cell responses mediated by patient serum represents the net effect of maintenance medication, disease activity and patients immunological heterogeneity that together reflect the patients further capability to respond to biological therapy.
Fecal calprotectin correlates with the CDEIS and CRP[15,27]. In this study we failed to find statistically significant correlations between calprotectin or CRP and target cell responses. This finding was similar to the finding in our previous study of IBD children. Fecal calprotectin excretion reflects increased neutrophils and mononuclear cell migration into the gut lumen through the inflamed mucosa[28] and CRP is an acute phase protein produced predominantly in the liver in response to stimulation by IL-6, TNF-α and IL-1β[29]. It seems that the ability of patient serum to activate target cells reflects the immunological net effects in circulation in CD patients rather than inflammatory cell accumulation in the intestine reflected in fecal calprotectin.
To conclude, there are few studies of the mechanisms of treatment failure during anti-TNF-α therapy. An impaired response to anti-TNF-α therapy in CD has been suggested to be a result of early reactivation of the inflammatory cascade caused by individual intrinsic immunological mechanisms[10]. Also the inflammatory activity of the disease itself may play a role in the therapeutic response. We found that the immune activation potency of the patient serum that is monitored by gene expression profile of human PBMC is individual and correlates to later mucosal healing during anti-TNF-α therapy. Characterization of the key factors in serum that mediated the effects observed with this method, such as up-regulation of FOXP3 and GITR, could be one step toward better understanding of in vivo actions of anti-TNF-α therapies.
Since the FDA approval of infliximab in 1999 for the treatment of severe Crohn’s disease (CD), the use of tumor necrosis factor-α (TNF-α)-antagonist agents has emerged in treatment of severe inflammatory bowel disease. A significant proportion of patients, however, do not respond to the treatment. To date there are no means to foresee the therapeutic response or to monitor the response at an early phase of therapy with an TNF-α-antagonist agent.
Recently, the authors applied a novel immunological assay for assessment of therapeutic response to glucocorticoids in pediatric patients with inflammatory bowel disease. In that study they showed that the therapeutic effect of corticosteroid therapy can be measured from patient serum at an early phase of the therapy. In the in vitro assay, a sample of patient serum is used to stimulate human white blood cells and the effect on specific white blood cell (T cell) markers is assessed. This kind of testing seems a promising means to predict individual responses to immunological therapies.
There are few studies exploring the immunological markers that reflect treatment response to anti-TNF-α therapy in CD. Here the authors used a recently described assay to measure therapeutic response to TNF-α-antagonist therapy from a patient serum sample. Disease activity and response to therapy is reflected in the patient serum and can be measured before the introduction of therapy. Serum induced changes in the specific white blood cell markers [forkhead transcription factor 3 (FOXP3), glucocorticoid-induced tumour necrosis factor receptor (GITR)] seemed to reflect individual response to anti-TNF-α therapy.
The results suggest that the effect of therapy with an anti-TNF-α agent can be measured from patient serum at an early phase. The study group was small and the results are preliminary, thus more studies are warranted to establish whether this kind of serum testing is suitable for predicting the individual response to anti-TNF-α therapy in clinical practice.
Forkhead box P3, FOXP3, is a gene regulating the development and function of specific white cells, regulatory T cells. GITR is glucocorticoid-induced tumour necrosis factor receptor. FOXP3 and GITR are commonly used markers for regulatory T-cell activity.
This study raises some interesting points never raised before about the T regulatory cell responses with biological agents.
Peer reviewer: Paul J Ciclitira, Professor, The Rayne Institute (GKT), St Thomas’ hospital, London NW32QG, United Kingdom
S- Editor Wang YR L- Editor O’Neill M E- Editor Ma WH
| 1. | MacDonald TT, Hutchings P, Choy MY, Murch S, Cooke A. Tumour necrosis factor-alpha and interferon-gamma production measured at the single cell level in normal and inflamed human intestine. Clin Exp Immunol. 1990;81:301-305. |
| 3. | Schreiber S, Nikolaus S, Hampe J, Hämling J, Koop I, Groessner B, Lochs H, Raedler A. Tumour necrosis factor alpha and interleukin 1beta in relapse of Crohn's disease. Lancet. 1999;353:459-461. |
| 4. | Braegger CP, Nicholls S, Murch SH, Stephens S, MacDonald TT. Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet. 1992;339:89-91. |
| 5. | Murch SH, Lamkin VA, Savage MO, Walker-Smith JA, MacDonald TT. Serum concentrations of tumour necrosis factor alpha in childhood chronic inflammatory bowel disease. Gut. 1991;32:913-917. |
| 6. | Murch SH, Braegger CP, Walker-Smith JA, MacDonald TT. Location of tumour necrosis factor alpha by immunohistochemistry in chronic inflammatory bowel disease. Gut. 1993;34:1705-1709. |
| 7. | Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541-1549. |
| 8. | Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh D, Panaccione R, Wolf D, Pollack P. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323-333. |
| 9. | D'haens G, Van Deventer S, Van Hogezand R, Chalmers D, Kothe C, Baert F, Braakman T, Schaible T, Geboes K, Rutgeerts P. Endoscopic and histological healing with infliximab anti-tumor necrosis factor antibodies in Crohn's disease: A European multicenter trial. Gastroenterology. 1999;116:1029-1034. |
| 10. | Nikolaus S, Raedler A, Kühbacker T, Sfikas N, Fölsch UR, Schreiber S. Mechanisms in failure of infliximab for Crohn's disease. Lancet. 2000;356:1475-1479. |
| 11. | Mäkitalo L, Sipponen T, Kärkkäinen P, Kolho KL, Saarialho-Kere U. Changes in matrix metalloproteinase (MMP) and tissue inhibitors of metalloproteinases (TIMP) expression profile in Crohn's disease after immunosuppressive treatment correlate with histological score and calprotectin values. Int J Colorectal Dis. 2009;24:1157-1167. |
| 12. | Gao Q, Meijer MJ, Schlüter UG, van Hogezand RA, van der Zon JM, van den Berg M, van Duijn W, Lamers CB, Verspaget HW. Infliximab treatment influences the serological expression of matrix metalloproteinase (MMP)-2 and -9 in Crohn's disease. Inflamm Bowel Dis. 2007;13:693-702. |
| 13. | Rintamäki H, Salo HM, Vaarala O, Kolho KL. New means to monitor the effect of glucocorticoid therapy in children. World J Gastroenterol. 2010;16:1104-1109. |
| 14. | Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn's disease: a prospective multicentre study. Groupe d'Etudes Thérapeutiques des Affections Inflammatoires du Tube Digestif (GETAID). Gut. 1989;30:983-989. |
| 15. | Sipponen T, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Färkkilä M. Crohn's disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn's disease activity index and endoscopic findings. Inflamm Bowel Dis. 2008;14:40-46. |
| 16. | Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70:439-444. |
| 17. | von Roon AC, Karamountzos L, Purkayastha S, Reese GE, Darzi AW, Teare JP, Paraskeva P, Tekkis PP. Diagnostic precision of fecal calprotectin for inflammatory bowel disease and colorectal malignancy. Am J Gastroenterol. 2007;102:803-813. |
| 18. | Kolho KL, Raivio T, Lindahl H, Savilahti E. Fecal calprotectin remains high during glucocorticoid therapy in children with inflammatory bowel disease. Scand J Gastroenterol. 2006;41:720-725. |
| 19. | Halminen M, Klemetti P, Vaarala O, Hurme M, Ilonen J. Interferon-gamma production in antigen specific T cell response: quantitation of specific mRNA and secreted protein. Scand J Immunol. 1997;46:388-392. |
| 20. | Honkanen J, Skarsvik S, Knip M, Vaarala O. Poor in vitro induction of FOXP3 and ICOS in type 1 cytokine environment activated T-cells from children with type 1 diabetes. Diabetes Metab Res Rev. 2008;24:635-641. |
| 21. | Corthay A. How do regulatory T cells work? Scand J Immunol. 2009;70:326-336. |
| 22. | Nocentini G, Giunchi L, Ronchetti S, Krausz LT, Bartoli A, Moraca R, Migliorati G, Riccardi C. A new member of the tumor necrosis factor/nerve growth factor receptor family inhibits T cell receptor-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:6216-6221. |
| 23. | McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311-323. |
| 24. | Nissinen R, Leirisalo-Repo M, Tiittanen M, Julkunen H, Hirvonen H, Palosuo T, Vaarala O. CCR3, CCR5, interleukin 4, and interferon-gamma expression on synovial and peripheral T cells and monocytes in patients with rheumatoid arthritis. J Rheumatol. 2003;30:1928-1934. |
| 25. | Kuuliala A, Nissinen R, Kautiainen H, Repo H, Leirisalo-Repo M. Low circulating soluble interleukin 2 receptor level predicts rapid response in patients with refractory rheumatoid arthritis treated with infliximab. Ann Rheum Dis. 2006;65:26-29. |
| 26. | Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277-285. |
| 27. | Sipponen T, Savilahti E, Kärkkäinen P, Kolho KL, Nuutinen H, Turunen U, Färkkilä M. Fecal calprotectin, lactoferrin, and endoscopic disease activity in monitoring anti-TNF-alpha therapy for Crohn's disease. Inflamm Bowel Dis. 2008;14:1392-1398. |
| 28. | Røseth AG, Schmidt PN, Fagerhol MK. Correlation between faecal excretion of indium-111-labelled granulocytes and calprotectin, a granulocyte marker protein, in patients with inflammatory bowel disease. Scand J Gastroenterol. 1999;34:50-54. |
| 29. | Florin TH, Paterson EW, Fowler EV, Radford-Smith GL. Clinically active Crohn's disease in the presence of a low C-reactive protein. Scand J Gastroenterol. 2006;41:306-311. |