Published online Dec 14, 2010. doi: 10.3748/wjg.v16.i46.5790
Revised: October 26, 2010
Accepted: November 2, 2010
Published online: December 14, 2010
The role of chronic inflammation, acting as an independent factor, on the onset of gastrointestinal carcinogenesis is now well accepted. However, even if there is an increase in the number of elements directly involving polymorphonuclear leukocytes (PMNL), as a major actor in digestive carcinogenesis, the different cellular and molecular events occurring in this process are still not completely understood. The transepithelial migration of PMNL, which is the ultimate step of the afflux of PMNL into the digestive mucosa, is a complex phenomenon involving sequential interaction of molecules expressed both on PMNL and on digestive epithelial cells. Chronic inflammatory areas rich in PMNL [so-called (chronic active inflammation)] and iterative transepithelial migration of PMNL certainly evoke intracellular signals, which lead toward progressive transformation of epithelia. Among these different signals, the mutagenic effect of reactive oxygen species and nitrates, the activation of the nuclear factor-κB pathway, and the modulation of expression of certain microRNA are key actors. Following the initiation of carcinogenesis, PMNL are involved in the progression and invasion of digestive carcinomas, with which they interact. It is noteworthy that different subpopulations of PMNL, which can have some opposite effects on tumor growth, in association with different levels of transforming growth factor-β and with the number of CD8 positive T lymphocytes, could be present during the development of digestive carcinoma. Other factors that involve PMNL, such as massive elastase release, and the production of angiogenic factors, can participate in the progression of neoplastic cells through tissues. PMNL may play a major role in the onset of metastases, since they allow the tumor cells to cross the endothelial barrier and to migrate into the blood stream. Finally, PMNL play a role, alone or in association with other cell parameters, in the initiation, promotion, progression and dissemination of digestive carcinomas. This review focuses on the main currently accepted cellular and molecular mechanisms that involve PMNL as key actors in digestive carcinogenesis.
- Citation: Hofman PM. Pathobiology of the neutrophil-intestinal epithelial cell interaction: Role in carcinogenesis. World J Gastroenterol 2010; 16(46): 5790-5800
- URL: https://www.wjgnet.com/1007-9327/full/v16/i46/5790.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i46.5790
The link between a chronic active inflammatory process (i.e. chronic inflammation rich in neutrophils) and the onset of carcinoma, in association or not with another factor such as a pathogen, is now convincingly demonstrated with epidemiological, experimental, and molecular data obtained for different tissues[1-10]. In particular, this relationship is well-established at the gastric and intestinal mucosal level[11-18]. Different factors are involved in digestive carcinogenesis, but the association of these factors and their importance in cancer onset are certainly variable from one disease to another and among individuals. Thus, predisposing genetic factors, infectious factors and inflammatory factors can be involved in digestive carcinogenesis[19]. Inappropriate innate immunity induces cellular infiltration of the digestive mucosa composed of polymorphonuclear leukocytes (PMNL), dendritic cells, natural killer cells, and then secondarily, an afflux of adaptive immune cells such as T lymphocytes. The intensity of this polymorphous cellular infiltrate varies according to the period of the active phases of the digestive disease[20]. In this regard, inflammatory infiltration can be present at variable time periods and at a variable frequency. Among the different populations of cells which migrate into the digestive mucosa, the PMNL play a central role in the pathophysiology of inflammatory digestive diseases[21]. Thus, previous epidemiological and histological studies have convincingly demonstrated a direct link between the clinical symptoms (pain and diarrhea) and the presence of PMNL in the digestive mucosa. More particularly, the periods of acute diarrhea certainly correlate with transepithelial migration of PMNL into the digestive lumen. It is noteworthy that during interaction between the intestinal epithelial cells (IEC) and PMNL different intracellular events are triggered, leading to neoplastic transformation of the digestive epithelia. The molecular phases involved in PMNL transepithelial migration are complex, but it is crucial to understand these phases to better comprehend the initial steps in digestive carcinogenesis. The progression from an in situ carcinoma to a microinvasive and invasive digestive carcinoma is associated with several molecular events, in particular, cytoskeleton modification, modulation of adherence molecules and metalloprotease production. Among these different events, some directly implicate PMNL. Currently, the pros and cons of the role of PMNL in tumor progression are debatable[22,23]. PMNL produce elastases[24], which favor tumor cell extracellular matrix invasion and release of pro-angiogenic factors, which creates a favorable microenvironment for tumor progression[25-30], but also produce defensins, which have an anti-tumor effect. Recently, a dual function of PMNL, in regard to their action on carcinoma cells, has been proposed[31,32]. Thus, two different populations of PMNL can be present in tumors, a population that favors tumour progression, the tumor-associated neutrophils 1 (TAN1) and a population that decreases tumor progression, the TAN2[31,32]. Accordingly to the proportion of TAN1 and TAN2 in a carcinoma the level of tumor progression can vary. This phenomenon can be present in colonic adenocarcinomas. Finally, previous studies implicate PMNL in the pathophysiology of metastases. This phenomenon can occur in colonic adenocarcinoma dissemination. In particular, PMNL allow transendothelial migration of tumor cells and then their migration into the blood stream.
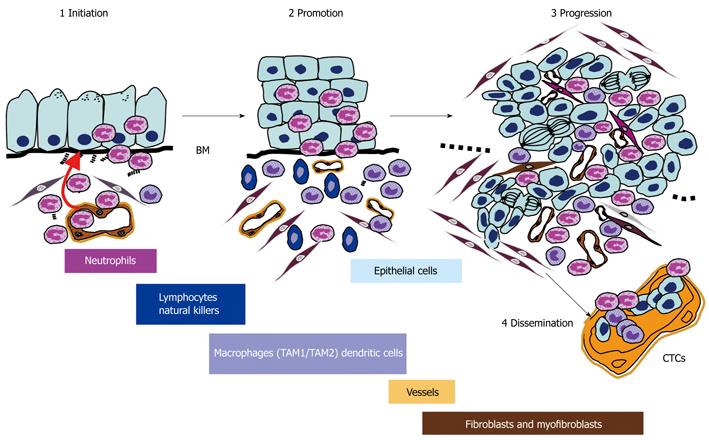
Previous studies and reviews have focused on the role of the immune system during cancer development[33] but the impact of PMNL in the different phases of the natural history of cancer (Figure 1) has been poorly described to date. In this review, I describe the role of PMNL and the direct events induced by PMNL in the mechanisms of the different steps in digestive carcinogenesis (cancer initiation, progression and dissemination).
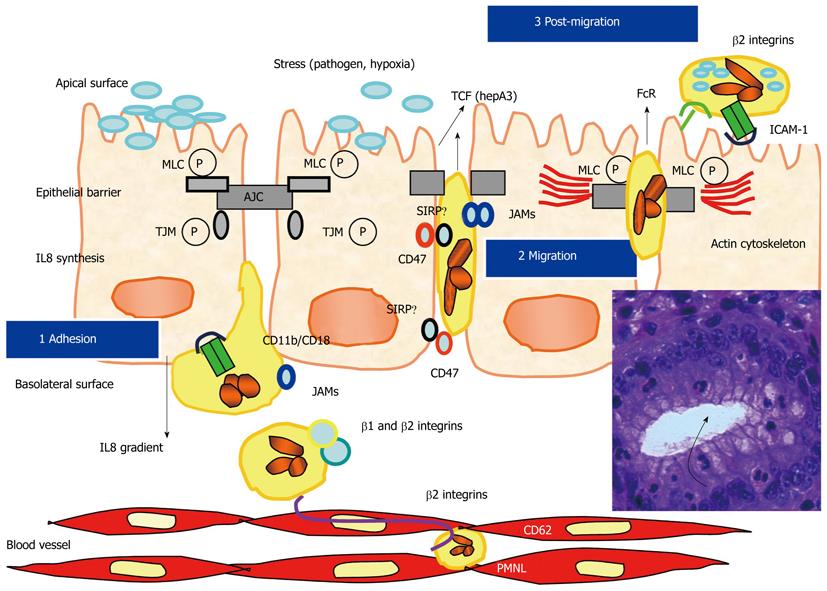
After transendothelial migration, following the crossing of the matrix of the lamina propria, which is mainly induced by a gradient of interleukin (IL) 8[34], PMNL adhere to the basal side of the glandular and crypt cell epithelium, and then transmigrate to the digestive lumen. This transepithelial migration is associated with sequential steps and with dynamic and transitory interactions between some surface molecules that are present on cytoplasmic membranes of PMNL and IEC[35,36] (Figure 2). Studies using in vitro models, such as the T84 model, have greatly improved our knowledge concerning these different cellular interactions. Thus, PMNL transepithelial migration can be induced by different stresses on epithelial cells, such as bacteria, bacterial products, toxins, or hypoxia[37,38]. Using this T84 model, the different steps of PMNL transepithelial migration and the different mechanisms involved in cell-cell interactions have been described[39-41]. Briefly, PMNL adhere to the basal side of the digestive epithelia through their CD11b/CD18 molecules (for which the ligand on epithelia is still unknown), then they migrate using a paracellular pathway through an homophilic CD47 interaction, which is expressed both on PMNL and IEC[42,43]. A more recent study showed that CD47 regulates neutrophil transmigration through close cross-talk with one toll-like receptor, TLR-2[44]. Other interactions occur at the desmosome and tight junction levels, which involve JAM and SIRPα[45-47]. After crossing the epithelial barrier PMNL interact with ICAM1 at the apical membrane through CD11b/CD18. During this transepithelial migration, the actin cytoskeleton of epithelial cells is reorganized[48]. Activated PMNL release 5’-adenosine monophosphate, which is secondarily cleaved by an epithelial membrane ectonucleotidase into adenosine, and finally produce chloride secretion on the epithelial apical side[49,50]. More recently, other molecular mechanisms have been described to occur during interaction between PMNL and the IEC[44,51]. Serine protease-mediated activation of epithelial protease-activated receptors has been shown to increase permeability. It has been demonstrated that transmigrating PMNL can regulate barrier function through epithelial protease-activated receptor activation[51]. Thus, transepithelial resistance decreased significantly after contact of PMNL with basolateral surfaces of T84 monolayers or after incubation with PMNL elastase and proteinase-3[51].
Beside these different events, which are associated with rapid paracellular migration of PMNL, different studies using the T84 model demonstrated the modulation of different molecules expressed on epithelial cells, which may be potentially involved in the initiation of carcinogenesis in direct or indirect pathways, by inducing an amplified inflammatory response rich in PMNL[52,53]. Moreover, paracellular migration of PMNL induced the onset of apoptosis, and, then potentially increases turnover of epithelium regeneration[54]. Thus, there is certainly a tight association between this chronic active inflammation and the onset of digestive carcinoma. An increased level in oxidative stress is present in the mucosa of inflammatory bowel diseases[55-57]. In this regard, an inflammatory microenvironment rich in PMNL can increase the rate of mutation, in addition to enhancing the proliferation of mutated cells[58]. Activated PMNL serve as sources of reactive oxygen species (ROS) and reactive nitrogen intermediates that are capable of inducing DNA damage and genomic instability[59]. Interestingly, release of ROS can occur during epithelium adhesion, but also during transepithelial migration and during post transepithelial migration of PMNL[60]. Alternatively, activated PMNL may use cytokines such as tumor necrosis factor (TNF)-α, which is implicated in carcinogenesis, to stimulate ROS and nitric oxide accumulation in neighboring epithelial cells[61,62]. Moreover, nitric oxide synthase can activate cyclooxygenase-2 in epithelial cells[63]. Different studies focus primarily on the effect of early mediators of inflammation, such as TNF-α, in stimulating tumor cell growth by activating nuclear factor (NF)-κB[64]. Conversely, decreased production of TNF-α in mice can reduce digestive carcinogenesis associated with chronic colitis[65]. However, chronic inflammation involves many other cytokines in the host microenvironment, which may also affect tumor growth in an NF-κB-dependent manner. While most inflammatory cytokines are released from activated macrophages following stimulus-induced transcription, others are secreted from intracellular pools and display later kinetics during the inflammatory response. Furthermore, the fact that NF-κB inhibition does not completely prevent tumor formation in these studies suggests that cytokines could also promote tumorigenesis via alternative pathways[66]. Mutations in p53, caused by oxidative damage, were found in both cancer cells and in a nondysplastic epithelium in cancer associated colitis, suggesting that chronic inflammation causes genomic changes[67]. Finally, ROS can also cause direct oxidative inactivation of mismatch repair enzymes[5].
Other mechanisms have been described, which involve PMNL in the early steps of initiation of carcinogenesis. Using animal models that reproduce digestive carcinogenesis linked to colitis, the molecule vanin 1 has been recently implicated in the onset of carcinoma[68]. Interestingly, it has been described that protein expression of cyclooxygenase-2 and the hypoxia-inducible factor-1 is up-regulated and associated with inflammation in early steps of digestive carcinoma[69]. The role of ROS and nitrates, largely suggested by previous studies, has been highlighted by different recent studies[70-76]. Interestingly, the myeloperoxidase (MPO) released by activated PMNL can inhibit nucleotide excision repair in certain epithelial cell lines[77]. In this regard, mutagenic products of MPO such as 5-chlorouracil and 5-bromouracil are released into inflammatory tissues. Moreover, the role of PMNL in initiation of carcinogenesis is probably more complex[78-80].
MicroRNA have been mainly investigated in oncology. However, microRNA are also implicated in inflammatory mechanisms, and their deregulation during some inflammatory diseases, in particular at the digestive level, could be associated with the molecular events that link chronic inflammation to cancer development[81-87]. The action of PMNL in this process is currently difficult to define, but through ROS release, and/or by the production of different enzymes, PMNL probably participate in deregulation of the RNA network in digestive epithelial cells.
Recent studies have demonstrated that the presence of intratumoral PMNL can be associated with shorter disease specific survival in certain cancer patients[88]. Following the initiation of digestive carcinoma, processes allow the tumor to grow from a single initiated cell into a developed primary adenocarcinoma. In this context, tumor growth depends on increased cell proliferation and reduced cell death, both of which can be stimulated by PMNL-driven mechanisms. This inflammation-induced tumor promotion may occur early or late in tumor development and leads to activation of premalignant lesions that have been dormant for many years. As for tumor-associated macrophages[89-91], PMNL probably promote tumor growth but the putative mechanisms have not yet been determined. However, it has been shown that accelerated intestinal epithelial cell turnover caused by chronic active inflammation and epithelial damage might predispose the mucosa to DNA damage, resulting in an elevated risk of mutation, which is in line with dysplasia and carcinoma development in patients with ulcerative colitis[92]. In parallel, the repeated inflammatory process could act on COX-2 expression which is down-regulated by the adenomatous polyposis coli (APC) gene and up-regulated by nuclear beta-catenin accumulation, and additionally implicate the Wnt signaling transduction pathway in colon carcinogenesis[93].
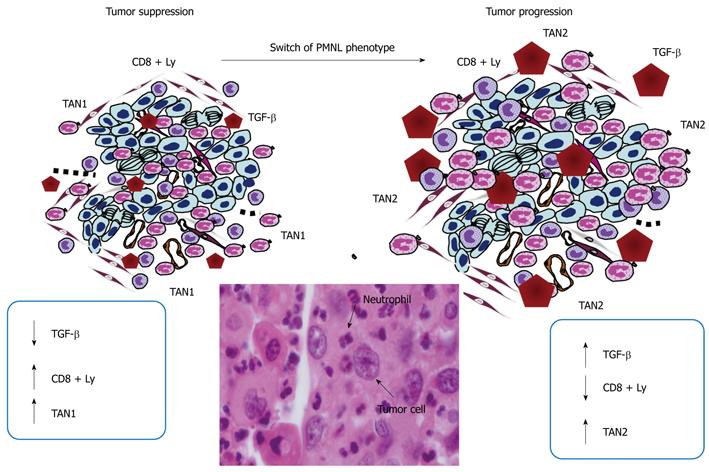
Secreted PMNL factors, such as human neutrophil peptides 1-3 (HNP1-3), have been found to be elevated in patients with digestive carcinoma, both in tissues and plasma, and to correlate with Dukes’ stages[94]. Other molecules such as neutrophil gelatinase-associated lipocalin or neutrophil elastase are able to suppress or to increase the invasion of carcinoma cells[95-97]. Among the cytokines involved in carcinoma progression, Transforming growth factor (TGF)-β is certainly one of the most studied, to date. It has been reported recently in a mouse model of carcinoma that TGF-β controls maturation of a sub-type of PMNL, the so-called TAN-2. TANs could function in parallel with tumor-associated macrophages (TAMs)[98,99]. Conversely, inhibition of the TGF-β activity leads to the differentiation of PMNL in anti-tumor TAN-1 cells (Figure 3). While TAN-2 inhibit the cytotoxic response of CD8+-T lymphocytes, which infiltrate the intestinal mucosa and thereby allow tumor cells to circumvent immune surveillance, TAN-1 enhance the anti-tumor action of CD8+ T-lymphocytes. TGF-β blockade not only activates CD8+T cells, but also increases the recruitment of hypersegmented neutrophils, their NI polarization (high expression of TNF-α, ICAM-1 and FAS) and their anti-tumor activity. Moreover, N1 neutrophils produce T cell-attracting chemokines including CCL3, CXCL9 and CXCL10. By contrast, TGF-β stimulation polarizes PMNL to the so-called N2 state with increased expression of arginase and chemokines such as CCL2 and CCL5. N1 are cytotoxic for tumors, whereas N2 display pro-tumor properties.
We may speculate that this mechanism is universally found in carcinomas arising in different organs. Finally, it is noteworthy that the prognostic value of a high number of PMNL in different carcinomas correlates with poor outcome in previous studies[100].
In addition to TGF-β, other cytokines produced by PMNL may be involved in carcinoma progression. Thus, TNF-β secreted by PMNL can stimulate a positive loop of inflammation by inducing production of chemokines such as IL8 and Groα by epithelial tumor cells and probably inducing renewed recruitment of PMNL[101]. Moreover, other mechanisms may exist such as carcinoma cell stimulation of PMNL to produce oncostatin M[102].
Although it is not yet established, we can speculate that some miRNA expressed by PMNL, in particular mir-223, may also play a crucial role in modulating progression of digestive tumors. Mir-223 was found to possess a crucial role in regulating neutrophil proliferation and activation[103]. Moreover, the expression of mir-223 may be modulated by some cytokines released by tumor cells and may influence the phenotype of TAN-1 or TAN-2. In this regard, different molecules have recently been reported as markers and/or promoters of inflammation-associated cancers[104]. Thus, we can speculate that the level of expression of mir-223 in carcinoma might be a marker of tumor progression.
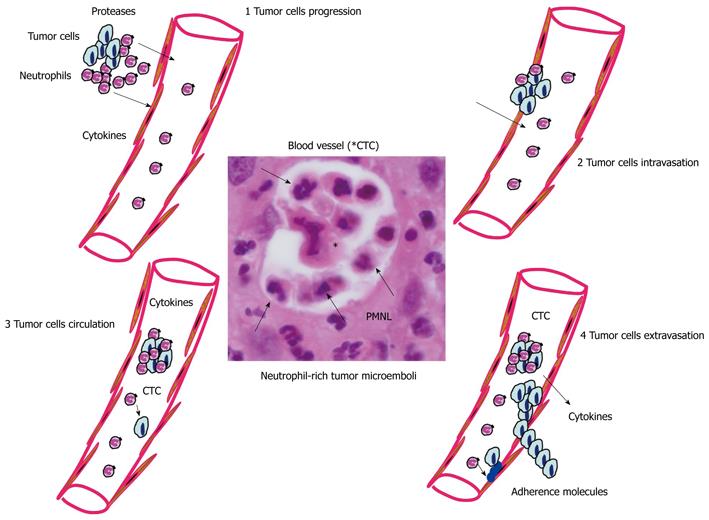
Inflammation is a key actor of metastasis onset[105]. In this regard, different studies have demonstrated the role of PMNL in tumor metastasis through different steps[106,107]. PMNL can participate in the transendothelial migration of adenocarcinoma cells, as well as their dissemination into the blood (Figure 4)[108,109]. Cytokines produced by PMNL can increase vascular permeability and upregulation of certain adhesion molecules located on endothelial cells[110]. In addition, PMNL are important sources of proteases that degrade the extracellular matrix and may alter the vascular barrier allowing entry of tumor cells into the blood stream. Interestingly, in a model of invasive colon cancer, CCR1+ myeloid cells, the recruitment of which is driven by the chemokine CCL9 produced by cancer cells, promote invasiveness through secretion of the matrix metalloproteinases MMP2 and MMP9[111]. It has been demonstrated that extracellular ATP can be released by activated PMNL[112]. This release of ATP occurs through a conformational opening of membrane Cx43 hemichannels in response to PMNL activation[113]. Moreover, the extracellular ATP released by activated PMNL may act both on epithelial cells, through activation of some purinergic receptors expressed by epithelial cells[53], and on endothelial cells[112]. More specifically, ATP released by activated PMNL is auto-hydrolyzed to AMP through CD39 on the surface of PMNL. CD39 may function as an immunomodulatory control point, requiring a close and special relationship with CD73-positive cells, such as endothelial cells. In addition to regulating the endothelial barrier function, a role for PMNL-dependent ATP release in directed movement of PMNL has been reported[114]. ROS released by activated PMNL can generate mitochondrial DNA mutations that regulate tumor cell metastasis[115].
Once metastatic cells enter the circulation, they need to survive in suspension and resist detachment-induced cell death or anoikis. The survival of circulating cancer cells is affected by inflammatory mediators released by immune cells in response to cancer-derived stimuli[116]. In the same way, the presence of a variety of cytokines released by activated PMNL present in the tumor microenvironment, including TNF-α, can promote the survival of circulating metastatic seeds[117]. PMNL can also favor the circulation in the blood of tumor cells, in a similar way to that of platelets or blood macrophages which can be physically linked to cancer cells, allowing them to travel together through the circulation[118]. Thus, single circulating tumor cells (CTC), which are no longer present in an immunosuppressive environment, may be targeted again by immunosurveillance. In this regard, the interaction of circulating cancer cells with PMNL may protect them from cell death, thereby overcoming immunosurveillance[119]. The journey of CTC ends upon integrin-dependent arrest on the endothelium, followed by extravasation. In this regard, systemic inflammation enhances attachment of CTC to endothelial cells, and this process is governed by neutrophil-dependent upregulation of adhesion molecules[120]. Thus, the production of high levels of proinflammatory cytokines by the PMNL can upregulate expression of certain adhesion molecules on endothelial cells and thereby increase the probability of metastatic cell attachment and potentialize the passage of tumor cells from the circulation into the extracellular space and then to develop micrometastases[90,105].
Different proinflammatory molecules and inflammatory cells have been suggested to be potential candidate targets for therapeutic strategies for cancer[99,121,122]. One study has shown that different drugs that prevent inflammation can inhibit carcinogenesis[123].
The role of PMNL in the onset and progression of digestive carcinoma, in particular those occurring in inflammatory bowel diseases, is complex. However, recent studies highlight new aspects of the pathophysiology of the PMNL-epithelial cells interaction, in particular, the effect of ROS release by activated PMNL on digestive epithelial cells at the molecular level or the effect of different TAN on tumor progression. Interestingly, these novel findings on the role of PMNL in the initiation and progression of carcinogenesis open up therapeutic avenues for the treatment of digestive cancers[124]. It is noteworthy that immunotherapy against cancer has been explored as a coadjuvant and has been based mostly on the properties of the adaptative immune system (i.e. B and T lymphocytes, dendritic cells) and of some components of the innate system (macrophages, NK cells, or complement proteins)[125,126]. PMNL have been rarely considered as a weapon against cancer. However, studies highlighting the anti-tumor efficacy of PMNL have been published. For example, suppression of the secreted protein acidic and rich in cystein, which is associated with the capacity of tumor cells to migrate and invade tissues, in malignant cells, led to the promotion of PMNL recruitment and induced tumor rejection[127]. However, the mode of action of PMNL that leads to the killing of tumor cells is not fully understood. It probably depends on the maturation of PMNL since in an animal model of lung tumors, only a subpopulation of PMNL i.e. TAN2 had an anti-tumor effect[31]. PMNL produce cytotoxic agents such as proteases, ROS, and defensins, all of which can directly damage the target cells. However, the cytotoxic effect of PMNL on tumors is greatly enhanced in the presence of target-specific antibodies. Finally, another strong argument for the anti-cancer effect of PMNL comes from studies using animal models in which tumor cells were genetically engineered to release immunoregulatory molecules (cytokines and chemokines). These molecules did not affect the proliferation of the tumors directly, but activated a host immune reaction that was strong enough to overcome their oncogenic capacity. For instance, G-CSF-releasing colon adenocarcinoma cells were found to lose their tumorigenic activity through the massive attraction of PMNL to the tumor injection site[128]. These PMNL distinguished between G-CSF-producing and nonproducing cancer cells. Moreover, tumor inhibition in vivo was accompanied by intimate physical contact between PMNL and G-CSF-producing tumor cells[129]. However, future research should be done in order to better target the different subpopulations of TAN, since only one population of PMNL would have an anti-tumor effect and should be considered.
Peer reviewer: Julio Mayol, MD, PhD, Department of Digestive surgery, Hospital Clinico San Carlos, Martin-Lagos S/n, Madrid, 28040, Spain
S- Editor Sun H L- Editor O’Neill M E- Editor Ma WH
| 1. | Allavena P, Garlanda C, Borrello MG, Sica A, Mantovani A. Pathways connecting inflammation and cancer. Curr Opin Genet Dev. 2008;18:3-10. |
| 3. | Borrello MG, Degl'Innocenti D, Pierotti MA. Inflammation and cancer: the oncogene-driven connection. Cancer Lett. 2008;267:262-270. |
| 4. | Cataisson C, Ohman R, Patel G, Pearson A, Tsien M, Jay S, Wright L, Hennings H, Yuspa SH. Inducible cutaneous inflammation reveals a protumorigenic role for keratinocyte CXCR2 in skin carcinogenesis. Cancer Res. 2009;69:319-328. |
| 5. | Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073-1081. |
| 6. | Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344-1367. |
| 7. | Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373-2380. |
| 8. | Meira LB, Bugni JM, Green SL, Lee CW, Pang B, Borenshtein D, Rickman BH, Rogers AB, Moroski-Erkul CA, McFaline JL. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J Clin Invest. 2008;118:2516-2525. |
| 9. | Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436-444. |
| 10. | Walser T, Cui X, Yanagawa J, Lee JM, Heinrich E, Lee G, Sharma S, Dubinett SM. Smoking and lung cancer: the role of inflammation. Proc Am Thorac Soc. 2008;5:811-815. |
| 11. | Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854-862. |
| 12. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. |
| 13. | Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228-1233. |
| 14. | Ekbom A, Helmick C, Zack M, Adami HO. Increased risk of large-bowel cancer in Crohn's disease with colonic involvement. Lancet. 1990;336:357-359. |
| 15. | Erdman SE, Rao VP, Poutahidis T, Rogers AB, Taylor CL, Jackson EA, Ge Z, Lee CW, Schauer DB, Wogan GN. Nitric oxide and TNF-alpha trigger colonic inflammation and carcinogenesis in Helicobacter hepaticus-infected, Rag2-deficient mice. Proc Natl Acad Sci USA. 2009;106:1027-1032. |
| 16. | Gillen CD, Walmsley RS, Prior P, Andrews HA, Allan RN. Ulcerative colitis and Crohn's disease: a comparison of the colorectal cancer risk in extensive colitis. Gut. 1994;35:1590-1592. |
| 17. | Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285-296. |
| 18. | Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127-1131. |
| 19. | Hofman VJ, Moreilhon C, Brest PD, Lassalle S, Le Brigand K, Sicard D, Raymond J, Lamarque D, Hébuterne XA, Mari B. Gene expression profiling in human gastric mucosa infected with Helicobacter pylori. Mod Pathol. 2007;20:974-989. |
| 20. | Sandborn WJ, Feagan BG, Hanauer SB, Lochs H, Löfberg R, Modigliani R, Present DH, Rutgeerts P, Schölmerich J, Stange EF. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn's disease. Gastroenterology. 2002;122:512-530. |
| 21. | Grisham MB, Granger DN. Neutrophil-mediated mucosal injury. Role of reactive oxygen metabolites. Dig Dis Sci. 1988;33:6S-15S. |
| 22. | Di Carlo E, Forni G, Lollini P, Colombo MP, Modesti A, Musiani P. The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood. 2001;97:339-345. |
| 23. | Jaganjac M, Poljak-Blazi M, Zarkovic K, Schaur RJ, Zarkovic N. The involvement of granulocytes in spontaneous regression of Walker 256 carcinoma. Cancer Lett. 2008;260:180-186. |
| 24. | Sato T, Takahashi S, Mizumoto T, Harao M, Akizuki M, Takasugi M, Fukutomi T, Yamashita J. Neutrophil elastase and cancer. Surg Oncol. 2006;15:217-222. |
| 25. | Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci USA. 2007;104:20262-20267. |
| 26. | Benelli R, Morini M, Carrozzino F, Ferrari N, Minghelli S, Santi L, Cassatella M, Noonan DM, Albini A. Neutrophils as a key cellular target for angiostatin: implications for regulation of angiogenesis and inflammation. FASEB J. 2002;16:267-269. |
| 27. | Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618-631. |
| 28. | Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci USA. 2006;103:12493-12498. |
| 29. | Yasuda M, Shimizu S, Ohhinata K, Naito S, Tokuyama S, Mori Y, Kiuchi Y, Yamamoto T. Differential roles of ICAM-1 and E-selectin in polymorphonuclear leukocyte-induced angiogenesis. Am J Physiol Cell Physiol. 2002;282:C917-C925. |
| 30. | Zijlstra A, Seandel M, Kupriyanova TA, Partridge JJ, Madsen MA, Hahn-Dantona EA, Quigley JP, Deryugina EI. Proangiogenic role of neutrophil-like inflammatory heterophils during neovascularization induced by growth factors and human tumor cells. Blood. 2006;107:317-327. |
| 31. | Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell. 2009;16:183-194. |
| 32. | Mantovani A. The yin-yang of tumor-associated neutrophils. Cancer Cell. 2009;16:173-174. |
| 33. | de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24-37. |
| 34. | McCormick BA, Hofman PM, Kim J, Carnes DK, Miller SI, Madara JL. Surface attachment of Salmonella typhimurium to intestinal epithelia imprints the subepithelial matrix with gradients chemotactic for neutrophils. J Cell Biol. 1995;131:1599-1608. |
| 35. | Edens HA, Levi BP, Jaye DL, Walsh S, Reaves TA, Turner JR, Nusrat A, Parkos CA. Neutrophil transepithelial migration: evidence for sequential, contact-dependent signaling events and enhanced paracellular permeability independent of transjunctional migration. J Immunol. 2002;169:476-486. |
| 36. | McCormick BA, Nusrat A, Parkos CA, D'Andrea L, Hofman PM, Carnes D, Liang TW, Madara JL. Unmasking of intestinal epithelial lateral membrane beta1 integrin consequent to transepithelial neutrophil migration in vitro facilitates inv-mediated invasion by Yersinia pseudotuberculosis. Infect Immun. 1997;65:1414-1421. |
| 37. | Bétis F, Brest P, Hofman V, Guignot J, Bernet-Camard MF, Rossi B, Servin A, Hofman P. The Afa/Dr adhesins of diffusely adhering Escherichia coli stimulate interleukin-8 secretion, activate mitogen-activated protein kinases, and promote polymorphonuclear transepithelial migration in T84 polarized epithelial cells. Infect Immun. 2003;71:1068-1074. |
| 38. | Hofman P, Le Negrate G, Mograbi B, Hofman V, Brest P, Alliana-Schmid A, Flatau G, Boquet P, Rossi B. Escherichia coli cytotoxic necrotizing factor-1 (CNF-1) increases the adherence to epithelia and the oxidative burst of human polymorphonuclear leukocytes but decreases bacteria phagocytosis. J Leukoc Biol. 2000;68:522-528. |
| 39. | Chin AC, Parkos CA. Neutrophil transepithelial migration and epithelial barrier function in IBD: potential targets for inhibiting neutrophil trafficking. Ann N Y Acad Sci. 2006;1072:276-287. |
| 40. | Hofman P. Pathological interactions of bacteria and toxins with the gastrointestinal epithelial tight junctions and/or the zonula adherens: an update. Cell Mol Biol (Noisy-le-grand). 2003;49:65-75. |
| 41. | Hofman P. Regulation of polymorphonuclear leukocyte-intestinal epithelial cell interactions: signalling events and potential drug targets. Curr Sign Trans Ther. 2007;2:11-19. |
| 42. | Parkos CA, Delp C, Arnaout MA, Madara JL. Neutrophil migration across a cultured intestinal epithelium. Dependence on a CD11b/CD18-mediated event and enhanced efficiency in physiological direction. J Clin Invest. 1991;88:1605-1612. |
| 43. | Parkos CA, Colgan SP, Liang TW, Nusrat A, Bacarra AE, Carnes DK, Madara JL. CD47 mediates post-adhesive events required for neutrophil migration across polarized intestinal epithelia. J Cell Biol. 1996;132:437-450. |
| 44. | Chin AC, Fournier B, Peatman EJ, Reaves TA, Lee WY, Parkos CA. CD47 and TLR-2 cross-talk regulates neutrophil transmigration. J Immunol. 2009;183:5957-5963. |
| 45. | Kucharzik T, Walsh SV, Chen J, Parkos CA, Nusrat A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am J Pathol. 2001;159:2001-2009. |
| 46. | Liu Y, Bühring HJ, Zen K, Burst SL, Schnell FJ, Williams IR, Parkos CA. Signal regulatory protein (SIRPalpha), a cellular ligand for CD47, regulates neutrophil transmigration. J Biol Chem. 2002;277:10028-10036. |
| 47. | Zen K, Babbin BA, Liu Y, Whelan JB, Nusrat A, Parkos CA. JAM-C is a component of desmosomes and a ligand for CD11b/CD18-mediated neutrophil transepithelial migration. Mol Biol Cell. 2004;15:3926-3937. |
| 48. | Hofman P, D'Andrea L, Carnes D, Colgan SP, Madara JL. Intestinal epithelial cytoskeleton selectively constrains lumen-to-tissue migration of neutrophils. Am J Physiol. 1996;271:C312-C320. |
| 49. | Madara JL, Patapoff TW, Gillece-Castro B, Colgan SP, Parkos CA, Delp C, Mrsny RJ. 5'-adenosine monophosphate is the neutrophil-derived paracrine factor that elicits chloride secretion from T84 intestinal epithelial cell monolayers. J Clin Invest. 1993;91:2320-2325. |
| 50. | Strohmeier GR, Reppert SM, Lencer WI, Madara JL. The A2b adenosine receptor mediates cAMP responses to adenosine receptor agonists in human intestinal epithelia. J Biol Chem. 1995;270:2387-2394. |
| 51. | Chin AC, Lee WY, Nusrat A, Vergnolle N, Parkos CA. Neutrophil-mediated activation of epithelial protease-activated receptors-1 and -2 regulates barrier function and transepithelial migration. J Immunol. 2008;181:5702-5710. |
| 52. | Cesaro A, Abakar-Mahamat A, Brest P, Lassalle S, Selva E, Filippi J, Hébuterne X, Hugot JP, Doglio A, Galland F. Differential expression and regulation of ADAM17 and TIMP3 in acute inflamed intestinal epithelia. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1332-G1343. |
| 53. | Cesaro A, Brest P, Hofman V, Hébuterne X, Wildman S, Ferrua B, Marchetti S, Doglio A, Vouret-Craviari V, Galland F. Amplification loop of the inflammatory process is induced by P2X7R activation in intestinal epithelial cells in response to neutrophil transepithelial migration. Am J Physiol Gastrointest Liver Physiol. 2010;299:G32-G42. |
| 54. | Le'Negrate G, Selva E, Auberger P, Rossi B, Hofman P. Sustained polymorphonuclear leukocyte transmigration induces apoptosis in T84 intestinal epithelial cells. J Cell Biol. 2000;150:1479-1488. |
| 55. | Lih-Brody L, Powell SR, Collier KP, Reddy GM, Cerchia R, Kahn E, Weissman GS, Katz S, Floyd RA, McKinley MJ. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig Dis Sci. 1996;41:2078-2086. |
| 56. | Roessner A, Kuester D, Malfertheiner P, Schneider-Stock R. Oxidative stress in ulcerative colitis-associated carcinogenesis. Pathol Res Pract. 2008;204:511-524. |
| 57. | Seril DN, Liao J, Yang GY, Yang CS. Oxidative stress and ulcerative colitis-associated carcinogenesis: studies in humans and animal models. Carcinogenesis. 2003;24:353-362. |
| 58. | Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis. 2000;21:361-370. |
| 59. | Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79-82. |
| 60. | Le'Negrate G, Rostagno P, Auberger P, Rossi B, Hofman P. Downregulation of caspases and Fas ligand expression, and increased lifespan of neutrophils after transmigration across intestinal epithelium. Cell Death Differ. 2003;10:153-162. |
| 61. | Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361-371. |
| 62. | Ohshima H, Bartsch H. Chronic infections and inflammatory processes as cancer risk factors: possible role of nitric oxide in carcinogenesis. Mutat Res. 1994;305:253-264. |
| 63. | Kim SF, Huri DA, Snyder SH. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science. 2005;310:1966-1970. |
| 64. | Ditsworth D, Zong WX. NF-kappaB: key mediator of inflammation-associated cancer. Cancer Biol Ther. 2004;3:1214-1216. |
| 65. | Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560-570. |
| 66. | Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461-466. |
| 67. | Kraus S, Arber N. Inflammation and colorectal cancer. Curr Opin Pharmacol. 2009;9:405-410. |
| 68. | Pouyet L, Roisin-Bouffay C, Clément A, Millet V, Garcia S, Chasson L, Issaly N, Rostan A, Hofman P, Naquet P. Epithelial vanin-1 controls inflammation-driven carcinogenesis in the colitis-associated colon cancer model. Inflamm Bowel Dis. 2010;16:96-104. |
| 69. | Mariani F, Sena P, Marzona L, Riccio M, Fano R, Manni P, Gregorio CD, Pezzi A, Leon MP, Monni S. Cyclooxygenase-2 and Hypoxia-Inducible Factor-1alpha protein expression is related to inflammation, and up-regulated since the early steps of colorectal carcinogenesis. Cancer Lett. 2009;279:221-229. |
| 70. | Babbs CF. Oxygen radicals in ulcerative colitis. Free Radic Biol Med. 1992;13:169-181. |
| 71. | Cerda S, Weitzman SA. Influence of oxygen radical injury on DNA methylation. Mutat Res. 1997;386:141-152. |
| 72. | D'Incà R, Cardin R, Benazzato L, Angriman I, Martines D, Sturniolo GC. Oxidative DNA damage in the mucosa of ulcerative colitis increases with disease duration and dysplasia. Inflamm Bowel Dis. 2004;10:23-27. |
| 73. | Gasche C, Chang CL, Rhees J, Goel A, Boland CR. Oxidative stress increases frameshift mutations in human colorectal cancer cells. Cancer Res. 2001;61:7444-7448. |
| 75. | Hofseth LJ, Saito S, Hussain SP, Espey MG, Miranda KM, Araki Y, Jhappan C, Higashimoto Y, He P, Linke SP. Nitric oxide-induced cellular stress and p53 activation in chronic inflammation. Proc Natl Acad Sci USA. 2003;100:143-148. |
| 76. | McKenzie SJ, Baker MS, Buffinton GD, Doe WF. Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J Clin Invest. 1996;98:136-141. |
| 77. | Güngör N, Godschalk RW, Pachen DM, Van Schooten FJ, Knaapen AM. Activated neutrophils inhibit nucleotide excision repair in human pulmonary epithelial cells: role of myeloperoxidase. FASEB J. 2007;21:2359-2367. |
| 78. | Knaapen AM, Schins RP, Borm PJ, van Schooten FJ. Nitrite enhances neutrophil-induced DNA strand breakage in pulmonary epithelial cells by inhibition of myeloperoxidase. Carcinogenesis. 2005;26:1642-1648. |
| 79. | Knaapen AM, Güngör N, Schins RP, Borm PJ, Van Schooten FJ. Neutrophils and respiratory tract DNA damage and mutagenesis: a review. Mutagenesis. 2006;21:225-236. |
| 80. | Schabath MB, Spitz MR, Hong WK, Delclos GL, Reynolds WF, Gunn GB, Whitehead LW, Wu X. A myeloperoxidase polymorphism associated with reduced risk of lung cancer. Lung Cancer. 2002;37:35-40. |
| 81. | Bhaumik D, Patil CK, Campisi J. MicroRNAs: an important player in maintaining a balance between inflammation and tumor suppression. Cell Cycle. 2009;8:1822. |
| 82. | Bi Y, Liu G, Yang R. MicroRNAs: novel regulators during the immune response. J Cell Physiol. 2009;218:467-472. |
| 83. | Lu LF, Liston A. MicroRNA in the immune system, microRNA as an immune system. Immunology. 2009;127:291-298. |
| 84. | Schetter AJ, Nguyen GH, Bowman ED, Mathé EA, Yuen ST, Hawkes JE, Croce CM, Leung SY, Harris CC. Association of inflammation-related and microRNA gene expression with cancer-specific mortality of colon adenocarcinoma. Clin Cancer Res. 2009;15:5878-5887. |
| 85. | Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103:12481-12486. |
| 86. | Wu F, Zikusoka M, Trindade A, Dassopoulos T, Harris ML, Bayless TM, Brant SR, Chakravarti S, Kwon JH. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology. 2008;135:1624-1635.e24. |
| 87. | Xiao B, Liu Z, Li BS, Tang B, Li W, Guo G, Shi Y, Wang F, Wu Y, Tong WD. Induction of microRNA-155 during Helicobacter pylori infection and its negative regulatory role in the inflammatory response. J Infect Dis. 2009;200:916-925. |
| 88. | Jensen HK, Donskov F, Marcussen N, Nordsmark M, Lundbeck F, von der Maase H. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol. 2009;27:4709-4717. |
| 89. | Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006;25:315-322. |
| 90. | Mantovani A, Romero P, Palucka AK, Marincola FM. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet. 2008;371:771-783. |
| 91. | Sica A, Allavena P, Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett. 2008;267:204-215. |
| 92. | Arai N, Mitomi H, Ohtani Y, Igarashi M, Kakita A, Okayasu I. Enhanced epithelial cell turnover associated with p53 accumulation and high p21WAF1/CIP1 expression in ulcerative colitis. Mod Pathol. 1999;12:604-611. |
| 93. | Araki Y, Okamura S, Hussain SP, Nagashima M, He P, Shiseki M, Miura K, Harris CC. Regulation of cyclooxygenase-2 expression by the Wnt and ras pathways. Cancer Res. 2003;63:728-734. |
| 94. | Albrethsen J, Møller CH, Olsen J, Raskov H, Gammeltoft S. Human neutrophil peptides 1, 2 and 3 are biochemical markers for metastatic colorectal cancer. Eur J Cancer. 2006;42:3057-3064. |
| 95. | Lee HJ, Lee EK, Lee KJ, Hong SW, Yoon Y, Kim JS. Ectopic expression of neutrophil gelatinase-associated lipocalin suppresses the invasion and liver metastasis of colon cancer cells. Int J Cancer. 2006;118:2490-2497. |
| 96. | Sun Z, Yang P. Role of imbalance between neutrophil elastase and alpha 1-antitrypsin in cancer development and progression. Lancet Oncol. 2004;5:182-190. |
| 97. | Yang P, Bamlet WR, Sun Z, Ebbert JO, Aubry MC, Krowka MJ, Taylor WR, Marks RS, Deschamps C, Swensen SJ. Alpha1-antitrypsin and neutrophil elastase imbalance and lung cancer risk. Chest. 2005;128:445-452. |
| 98. | Allavena P, Sica A, Garlanda C, Mantovani A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev. 2008;222:155-161. |
| 99. | Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717-727. |
| 100. | Caruso RA, Bellocco R, Pagano M, Bertoli G, Rigoli L, Inferrera C. Prognostic value of intratumoral neutrophils in advanced gastric carcinoma in a high-risk area in northern Italy. Mod Pathol. 2002;15:831-837. |
| 101. | Cuenca RE, Azizkhan RG, Haskill S. Characterization of GRO alpha, beta and gamma expression in human colonic tumours: potential significance of cytokine involvement. Surg Oncol. 1992;1:323-329. |
| 102. | Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, Jorcyk CL. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res. 2005;65:8896-8904. |
| 103. | Johnnidis JB, Harris MH, Wheeler RT, Stehling-Sun S, Lam MH, Kirak O, Brummelkamp TR, Fleming MD, Camargo FD. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature. 2008;451:1125-1129. |
| 104. | Darash-Yahana M, Gillespie JW, Hewitt SM, Chen YY, Maeda S, Stein I, Singh SP, Bedolla RB, Peled A, Troyer DA. The chemokine CXCL16 and its receptor, CXCR6, as markers and promoters of inflammation-associated cancers. PLoS One. 2009;4:e6695. |
| 106. | Remedi MM, Donadio AC, Chiabrando GA. Polymorphonuclear cells stimulate the migration and metastatic potential of rat sarcoma cells. Int J Exp Pathol. 2009;90:44-51. |
| 107. | Tazawa H, Okada F, Kobayashi T, Tada M, Mori Y, Une Y, Sendo F, Kobayashi M, Hosokawa M. Infiltration of neutrophils is required for acquisition of metastatic phenotype of benign murine fibrosarcoma cells: implication of inflammation-associated carcinogenesis and tumor progression. Am J Pathol. 2003;163:2221-2232. |
| 108. | Slattery MJ, Liang S, Dong C. Distinct role of hydrodynamic shear in leukocyte-facilitated tumor cell extravasation. Am J Physiol Cell Physiol. 2005;288:C831-C839. |
| 109. | Wu QD, Wang JH, Condron C, Bouchier-Hayes D, Redmond HP. Human neutrophils facilitate tumor cell transendothelial migration. Am J Physiol Cell Physiol. 2001;280:C814-C822. |
| 110. | De Larco JE, Wuertz BR, Furcht LT. The potential role of neutrophils in promoting the metastatic phenotype of tumors releasing interleukin-8. Clin Cancer Res. 2004;10:4895-4900. |
| 111. | Kitamura T, Kometani K, Hashida H, Matsunaga A, Miyoshi H, Hosogi H, Aoki M, Oshima M, Hattori M, Takabayashi A. SMAD4-deficient intestinal tumors recruit CCR1+ myeloid cells that promote invasion. Nat Genet. 2007;39:467-475. |
| 112. | Eltzschig HK, Macmanus CF, Colgan SP. Neutrophils as sources of extracellular nucleotides: functional consequences at the vascular interface. Trends Cardiovasc Med. 2008;18:103-107. |
| 113. | Eltzschig HK, Eckle T, Mager A, Küper N, Karcher C, Weissmüller T, Boengler K, Schulz R, Robson SC, Colgan SP. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99:1100-1108. |
| 114. | Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792-1795. |
| 115. | Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, Nakada K, Honma Y, Hayashi J. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320:661-664. |
| 116. | Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102-106. |
| 117. | Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274-284. |
| 118. | Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263-266. |
| 119. | Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Hu Z, Barney KA, Degen JL. Tumor cell-associated tissue factor and circulating hemostatic factors cooperate to increase metastatic potential through natural killer cell-dependent and-independent mechanisms. Blood. 2007;110:133-141. |
| 120. | McDonald B, Spicer J, Giannais B, Fallavollita L, Brodt P, Ferri LE. Systemic inflammation increases cancer cell adhesion to hepatic sinusoids by neutrophil mediated mechanisms. Int J Cancer. 2009;125:1298-1305. |
| 121. | Shen HM, Tergaonkar V. NFkappaB signaling in carcinogenesis and as a potential molecular target for cancer therapy. Apoptosis. 2009;14:348-363. |
| 122. | Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735-6741. |
| 123. | Saini RK, Sanyal SN. Chemopreventive effect of nonsteroidal anti-inflammatory drugs on 9,10-dimethylbenz[a]anthracene-induced lung carcinogenesis in mice. Oncol Res. 2009;17:505-518. |
| 124. | Hofman P. Molecular regulation of neutrophil apoptosis and potential targets for therapeutic strategy against the inflammatory process. Curr Drug Targets Inflamm Allergy. 2004;3:1-9. |
| 125. | Bhardwaj N. Harnessing the immune system to treat cancer. J Clin Invest. 2007;117:1130-1136. |
| 126. | Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704-2715. |
| 127. | Alvarez MJ, Prada F, Salvatierra E, Bravo AI, Lutzky VP, Carbone C, Pitossi FJ, Chuluyan HE, Podhajcer OL. Secreted protein acidic and rich in cysteine produced by human melanoma cells modulates polymorphonuclear leukocyte recruitment and antitumor cytotoxic capacity. Cancer Res. 2005;65:5123-5132. |
| 128. | Colombo MP, Ferrari G, Stoppacciaro A, Parenza M, Rodolfo M, Mavilio F, Parmiani G. Granulocyte colony-stimulating factor gene transfer suppresses tumorigenicity of a murine adenocarcinoma in vivo. J Exp Med. 1991;173:889-897. |
| 129. | Colombo MP, Lombardi L, Stoppacciaro A, Melani C, Parenza M, Bottazzi B, Parmiani G. Granulocyte colony-stimulating factor (G-CSF) gene transduction in murine adenocarcinoma drives neutrophil-mediated tumor inhibition in vivo. Neutrophils discriminate between G-CSF-producing and G-CSF-nonproducing tumor cells. J Immunol. 1992;149:113-119. |