Published online Dec 7, 2010. doi: 10.3748/wjg.v16.i45.5752
Revised: August 6, 2010
Accepted: August 13, 2010
Published online: December 7, 2010
AIM: To identify a method for efficient large-scale purification of functional hepatitis B virus polymerase (HBV-Pol) without addition of cellular factors.
METHODS: Full-length HBV-Pol (843 amino acids) tagged with 5’ end Polyhistidine was expressed at a high level in an Escherichia coli (E. coli) system. Sodium dodecyl sulfate lysis buffer was utilized to dissolve insoluble HBV-Pol, and Ni-NTA resin affinity chromatography was utilized for HBV-Pol purification. Most recombinant HBV-Pol was eluted with 100 mmol/L imidazole in the presence of NP-40, a weak detergent that keeps HBV-Pol in solution. A reducing agent was utilized throughout the purification steps to keep soluble HBV-Pol from redundant disulfide bond formation.
RESULTS: The large-scale production of functional intact human HBV-Pol was achieved in an E. coli expression system. Purified HBV-Pol showed stable reverse transcriptase activity and DNA polymerase activity. The purified protein was of high purity and had stable reverse transcriptase activity.
CONCLUSION: Large-scale production of HBV-Pol in pure form should facilitate crystallization and detailed analysis of the structure and mechanism of HBV-Pol. Ability of this purification approach to obtain human HBV-Pol in an enzymatically active form should be helpful for development of drugs for treatment of chronic hepatitis B.
- Citation: Yu Y, Pandeya DR, Liu ML, Liu MJ, Hong ST. Expression and purification of a functional human hepatitis B virus polymerase. World J Gastroenterol 2010; 16(45): 5752-5758
- URL: https://www.wjgnet.com/1007-9327/full/v16/i45/5752.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i45.5752
Hepatitis B virus (HBV) infection is a global public health problem. It is estimated that between 350 and 400 million people worldwide are chronically infected, and a significant proportion of chronic infection patients ultimately develop life-threatening liver disease such as cirrhosis, hepatocellular carcinoma (HCC) and other complications[1]. HBV replicates via a reverse transcription step, using the polymerase (HBV-Pol) that is encoded by its own genome. HBV-Pol is a multifunctional protein, with protein-priming activity[2-4], DNA polymerase, reverse transcriptase[2,5] and RNase H activity, but it is short of proofreading activity[6]. Most approved medications for chronic hepatitis B (CHB) infection are nucleotide reverse transcriptase inhibitors (NRTIs) that target HBV-Pol[7]. Although NRTIs have been used in CHB infection for several decades, their therapeutic efficacy has been limited by high frequency appearance of mutants during treatment[8]. Therefore, a quick and easy way to obtain a large quantity of functionally intact human HBV-Pol is required for selection of sensitive CHB medications, mutated HBV strains research, and mass high-throughput screening.
It is known that expression of an enzymatically active HBV-Pol in heterologous systems, or purification of useful quantities of human HBV-Pol from virions is difficult to achieve. Due to these problems, drug development for HBV infection has not progressed satisfactorily, and biological studies of hepadnaviral polymerase have been conducted by using duck HBV[9]. Several groups have succeeded in achieving heterologous in vitro expression of full-length polymerase proteins of duck HBV that exhibit DNA-dependent DNA polymerase (DDDP) activity and RNA-dependent DNA polymerase (RDDP) activity. However human HBV-Pol is expressed by in vitro translation with a rabbit reticulocyte lysate system[10], and in-vitro-translated human HBV-Pol shows only DDDP activity and fails to show RDDP activity. RDDP activity of human HBV-Pol has been observed in Escherichia coli (E. coli) as a fusion protein in frame with maltose-binding protein[11]. The enzymatically active HBV-Pol has also been obtained in E. coli by co-expression of the polymerase with the chaperone GRP94[12]. However, the stable and large-scale heterologous expression of intact human HBV-Pol without co-expression of molecular chaperon in common hosts such as E. coli or yeast has not been reported.
In this study, a full-length HBV-Pol with a 6 × His tag was expressed in E. coli. HBV-Pol is a large molecule with approximately 2.5% cysteine residues[2], therefore, the protein is expected to be present as inclusion bodies. For this reason, the total lysate was dissolved by applying high concentration of sodium dodecyl sulfate (SDS) and reducing agents to dissolve inclusion bodies, and then SDS was replaced by weak detergent for renaturation during washing. Finally, the target protein was purified with nickel-based chromatography. Purified HBV-Pol showed RDDP and DDDP activity. This is believed to be the first time that functional intact human HBV-Pol has been expressed in E. coli without co-expression molecules or in the presence of certain helper chaperons. The functional HBV-Pol might be helpful for development of potential pharmaceutical agents for CHB treatment.
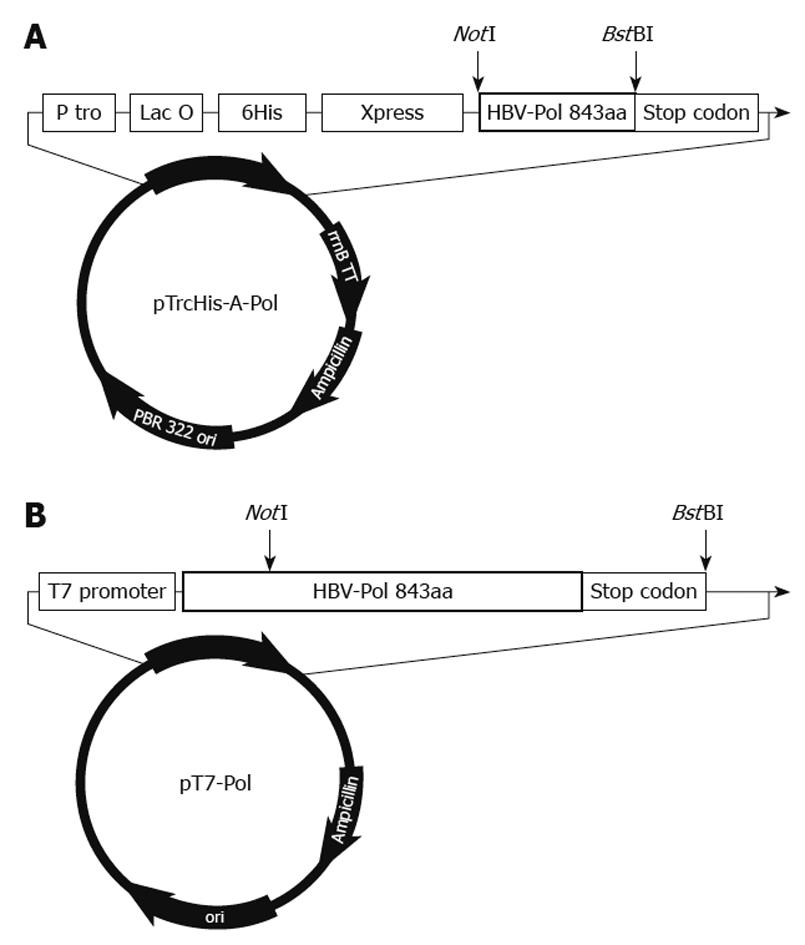
Liver tissues were obtained from a chronic hepatitis B surface antigen carrier who developed HCC and underwent surgical resection. The tumor tissues were dissected and immediately cut into small pieces and stored into liquid nitrogen until use. The cellular DNA was isolated from tissues by SDS-protease K digestion and phenol-chloroform extraction as described previously[13]. HBV-Pol sequence [spanning 2307 to 1623 bp, 843 amino acids] were amplified with PrimeSTAR™ high fidelity polymerase using CPNotIF01: GTTGCGGCCGCATAATGGCCCTATCTTATC and CPBstBIR01: ATTTTCGAATTCTCACGGTGGTTTCCA for complete P gene. The vectors were designed as follows (Figure 1).
The pTrcHis-A-Pol and pT7-Pol were constructed by the following procedure. HBV-Pol full-length sequence was cloned into NotI and BstBI sites of the two expression vector pTrcHis-A (Invitrogen, Carlsbad, CA, USA) and pT7 vector (Promega, Madison, WI, USA). For expression of HBV-Pol in E. coli, the plasmid pTrcHis-A-Pol was constructed as showed in Figure 1A. The HBV-Pol frame was under the control of the Trc promoter and Lac operator. His-tag and Xpress antigen were fused at the 5’ end of the HBV-Pol sequence. For expression of HBV-Pol in the rabbit reticulocyte lysate system, the plasmid pT7-Pol was constructed as showed in Figure 1B. The HBV-Pol frame was driven by T7-promoter.
Plasmid pTrcHis-A-Pol was chemically transformed in competent DH5α (F-,φ80dlacZΔM15,Δ(lacZYA-argF)U169,deoR,recA1,endA1,hsdR17(rk-,mk+),phoA,supE44,λ-,thi-1,gyrA96,relA1) cells following the manual supplied by Real Biotech Corporation (Taipei, Taiwan, China). Isopropylthiogalactopyranoside (IPTG, Sigma, St Louis, MO, USA) was added into 5 mL overnight-cultured E. coli, to a final concentration of 1 mmol/L, and incubated for 4, 8, 12 and 16 h, respectively, at 18°C, 24°C, 30°C, 37°C and 42°C, as indicated. Cells were harvested by centrifugation and disrupted with lysis-buffer (50 mmol/L phosphate buffer, pH 8.0; 0.5 mmol/L NaCl; 1% SDS; 10 mmol/L imidazole; 0.1 mg/mL lysozyme; 20 mmol/L 2-mercaptoethanol; 1 × Roche protein inhibitor cocktail). Protein expression level was determined with dot blot using anti-Xpress antibody (Invitrogen).
Transformed cells were grown in 100 mL LB broth until the culture reached OD600 0.6-0.8. IPTG was added to a final concentration of 1 mmol/L and incubated for 8-10 h at 18°C with shaking. Induced cells were harvested by centrifugation at 2000 g for 20 min at 4°C. The ratio of lysis-buffer volume and wet weight of cell pellet was about 4 to 1. The lysate was incubated at room temperature for 30 min and sonicated for 10 × 10 s. The samples were cooled on ice for 5-10 s between each sonication. The suspension was centrifuged at 20 000 g for 30 min at room temperature. The supernatant was transferred to an Ni-NTA resin (Invitrogen) column (bed volume: 2 mL), which had been equilibrated with 16 mL equilibration buffer (same components of previous lysis buffer without lysozyme). Resin and lysate supernatant were mixed thoroughly but gently for 45-60 min at room temperature. The resin was washed with 8 ml washing buffer (50 mmol/L phosphate buffer, pH 8.0, 0.5 mmol/L NaCl, 1% NP-40, 10 mmol/L imidazole, 20 mmol/L 2-mercaptoethanol, and 1 × Roche protein inhibitor cocktail) for 6-8 times. The HBV-Pol fractions were eluted with 6 mL E-50, E-75, and E-100 Elution buffer (50 mmol/L phosphate buffer, pH 8.0, 0.5 mmol/L NaCl, 1% NP-40, 1 × Roche protein inhibitor cocktail), and dithiothreitol (DTT) was added to the harvest tube to achieve a final concentration of 5 mmol/L. The concentration of imidazole in E-50, E-75, and E-100 buffer was 50 mmol/L, 75 mmol/L and100 mmol/L, respectively. HBV-Pol elution fractions were harvested and stored at -70°C. The concentration of purified protein samples was determined using a BCA assay with bovine serum albumin as a standard.
Anti-reverse transcriptase (RT) polyclonal antibody was prepared before full-length polymerase purification (data not shown). A recombinant RT domain (spanning 304-693 amino acids) was purified from E. coli. Fifty micrograms purified RT and Freund’s complete adjuvant were injected subcutaneously into C57BL/6 mice (Macrogen, Seoul, South Korea). Another 50 μg purified RT and Freund’s incomplete adjuvant were injected 2 wk after the first injection. The mice were sacrificed 2 wk after the second injection for serum extraction of anti-RT polyclonal antibody. The antibody titers against the RT peptides, monitored with ELISA (Sigma), were > 1:32 000.
Total lysate and other washing/elution fractions were heated at 95°C for 5 min for denaturation. Proteins were separated by SDS-PAGE. Gels were stained with Coomassie Blue. For dot blot analysis, 1 μL of each sample was dropped on nitrocellulose membranes (Amersham, Bucks, UK), and the membranes were treated first with a 1/4000 dilution of anti-Xpress antibody and then goat-anti-mouse conjugated alkaline phosphatase (AP; Invitrogen). For Western blotting analysis, proteins were electrophoretically transferred to a polyvinylidene difluoride blotting membrane (Amersham, Piscataway, NY, USA), and membranes were treated first with a 1/4000 dilution of anti-RT antibody and then goat-anti-mouse conjugated AP.
The recombinant plasmid pT7-Pol was purified with Qiagen Midiprep DNA purification kit. In vitro transcription and translation reactions were performed using the TNT T7 coupled reticulocyte lysate system (Promega). Two micrograms of the plasmid DNA template was transcribed and the protein was translated in each 50-mL reaction in the presence or absence of 40 mCi of [35-S]-methionine (1000 Ci/mmol) (Amersham) at 30°C for 75 min[10]. The in vitro translation reaction was stopped by the addition of 0.1 mg/mL cycloheximide for the polymerase activity assay, or SDS sample buffer for checking the efficiency of translation. The in vitro translated proteins were separated by 4%-12% SDS-PAGE and dried prior to autoradiography.
DDDP and RT/RDDP were monitored by synthesis of DNA using poly (dA) • oligo (dT)12-18 and poly (rA) • oligo (dT)12-18 as template primer (Amersham Biosciences), respectively. The standard enzyme reaction (50 mL) contained 50 mmol/L Tris-HCl pH 7.4, 50 mmol/L KCl, 10 mmol/L MgCl2, 1 mmol/L DTT, 0.01% Nonidet P-40, 50 ng homopolymer template [poly(dA) • oligo(dT)12-18 for DDDP activity assay and poly(rA) • oligo(dT)12-18 for RDDP activity assay], and 2 mCi of [α-32P]dTTP (3000 Ci/mmol), (PerkinElmer, USA). For RDDP activity assay, RNase inhibitor and RNase-free water were employed in the reaction[10,11]. Reactions were started by the addition of 0.5 mg of the purified HBV-Pol or 5 μL products from the TNT-T7 coupled reticulocyte lysate system into the reaction buffer. The endogenous DNA polymerase activity from the reticulocyte lysate was suppressed by the addition of 60 mmol/L aphidicolin and 1 mmol/L NEM. After incubation at 37°C for 75 min, reactions were stopped by the addition of 0.2 mg/mL protease K in the presence of 0.5% SDS. Incubation was continued for another 20 min, followed by spotting on Whatman DE81 filter paper. Filters were washed three times with 0.5 mol/L Na2HPO4, and once with distilled H2O[11]. Incorporation of radioactivity was determined by liquid scintillation counting in a Packard Tri-Carb Series 2300 liquid scintillation counter.
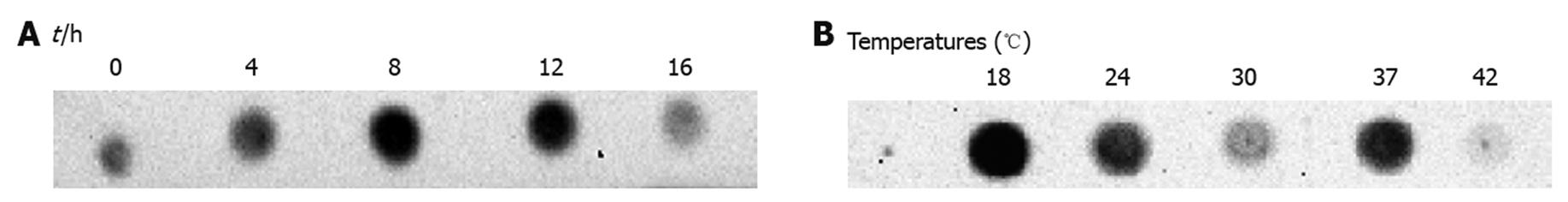
A high expression level of target protein is crucial to obtain an ideal elution result in affinity chromatography. To achieve the highest expression level, expression level was examined at various conditions. Recombinant HBV-Pol is a large molecule of 95 kDa full-length HBV-Pol plus a 5-kDa fused tag, and a low induction temperature and short or long induction time were considered for expression. It is known that heterologous protein was stabilized at low temperature[14] and formation of inclusion bodies was restrained[15]. Decreasing the temperature of induction medium should lower the rate of association of folding intermediates, which allows unfolded proteins and partially folded proteins to have more time to refold into native, soluble tertiary structures. Appropriate induction time is another important factor to obtain sufficient recombinant polymerase. The quantity of expressed protein varies with different induction times.
HBV-Pol level was monitored in the total lysate of E. coli transformed with HBV-Pol recombinant plasmid, pTrcHis-A-Pol (Figure 2). HBV-Pol expression level was the highest at 8 h induction compared to shorter or longer incubation (Figure 2A). In terms of temperature, the lowest temperature tested, 18°C, showed the highest level of expression compared to other samples expressed at higher temperatures (Figure 2B). Based on these data, incubation of HBV-Pol expression was carried out under these conditions throughout the experiment. For HBV-Pol purification, a denaturing-renaturing purification protocol was applied. According to previous lysis data under native conditions, inclusion bodies do not dissolve in the presence of weak detergent at 4°C, which decreased the quantity of target protein in the binding step. A strong detergent (SDS) was employed in the lysis step to dissolve overexpressed HBV-Pol in inclusion bodies, and to denature some protease present in the total lysate.
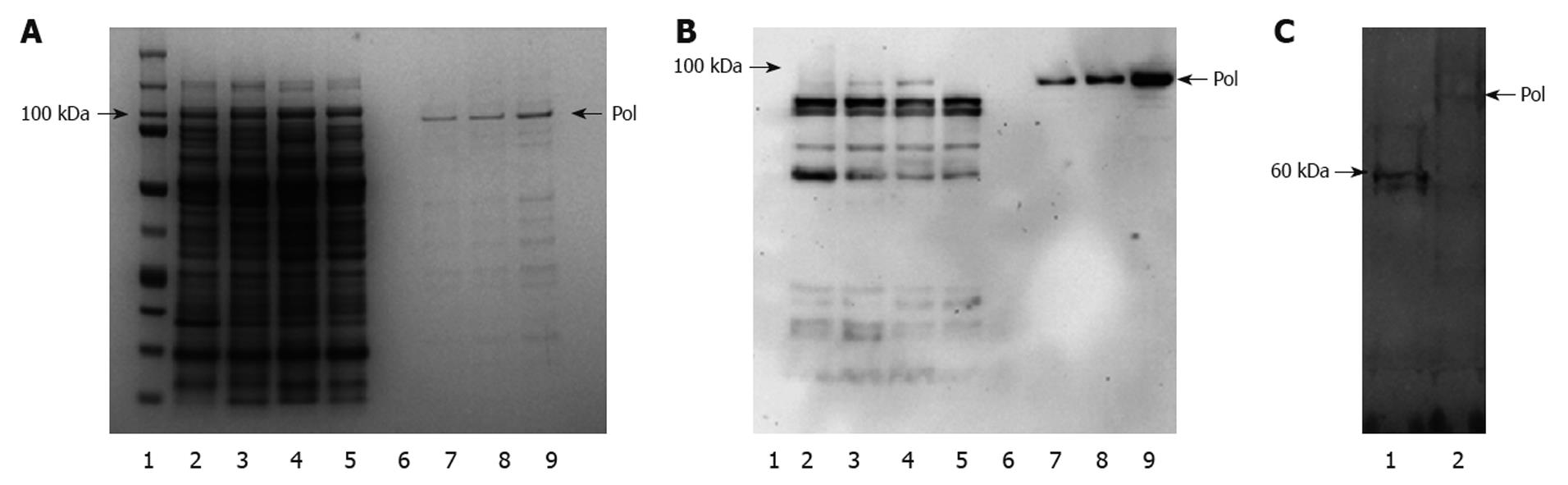
HBV-Pol fractions were eluted with elution buffers (50, 75, 100 mmol/mL imidazole). Figure 3A shows that a prominent HBV-Pol band was detected with SDS-PAGE, and analysis by Western blotting confirmed the purification results (Figure 3B). DTT, a stronger reducing agent than 2-mercaptoethanol, is suitable for long-term protein storage and is used in many experiments for HBV-Pol purification at a high concentration[2,5,6,16]. Although nickel resin is easily reduced by strong detergent, in the present study, 2-mercaptoethanol was employed in all steps except elution. DTT was finally added into HBV-Pol fractions for long-term storage. SDS-PAGE and Western blotting clearly showed high efficiency of purification. Involvement of the denaturing-renaturing purification process also suggested that purified recombinant HBV-Pol is a stable, single-component product. HBV-Pol was purified up to 5 mg/mL.
There have been many reports about purified or partially purified active HBV-Pol in eukaryotic systems. In the present study, recombinant HBV-Pol was also expressed in a reticulocyte lysate system as a positive control in the activity assay. The protein products from in vitro expression labeled with [35S]-methionine are shown in Figure 3C. Approximately 95-kDa HBV-Pol protein was detected as predicted from the nucleotide sequence of the HBV-Pol-ORF.
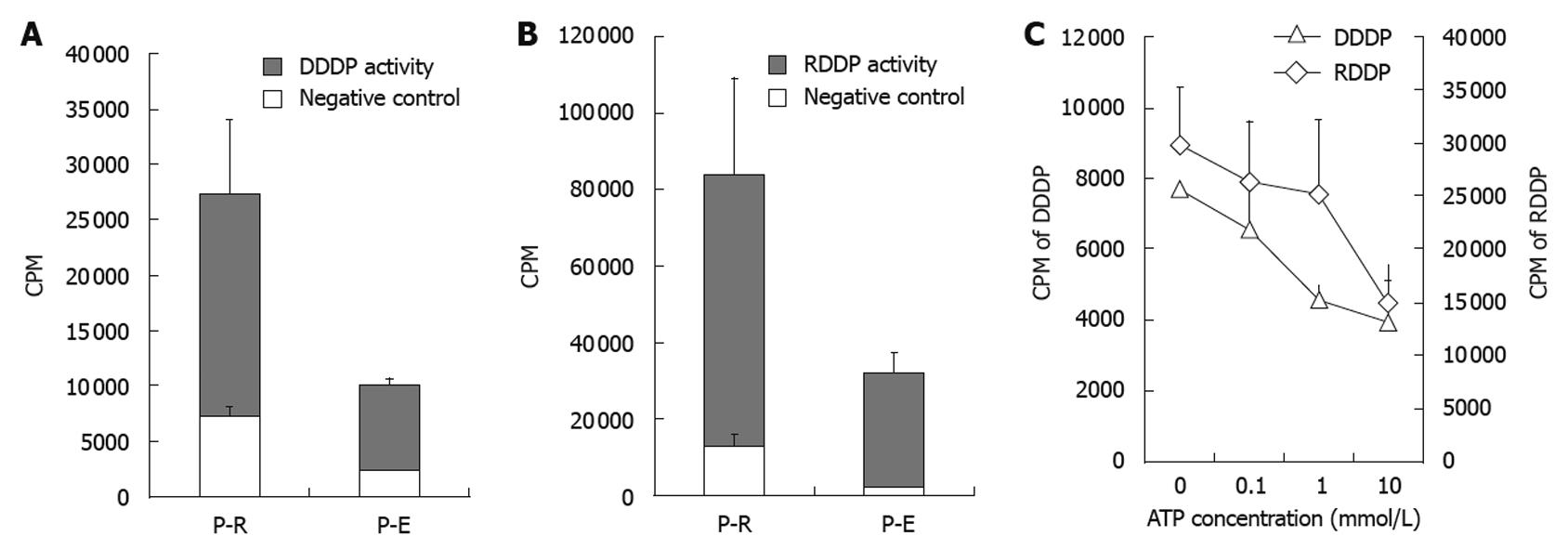
To measure the enzymatic activity of purified HBV-Pol, the enzymatic activity assays for DDDP and RDDP were performed by using purified HBV-Pol (expressed in recombinant E. coli, named P-E) and HBV-Pol (expressed in reticulocyte system, named P-R). pT7 plasmid was expressed in the reticulocyte lysate system as a negative control for P-R enzyme activity assays. The solvent of P-E was regarded as a negative control for P-E enzyme activity assays. Enzyme activities of P-R and P-E were compared (Figure 4A-C). P-R showed medium DDDP activity and much higher RDDP activity under these reaction conditions. Homopolymer primer-template has been used for many groups[10,11] in HBV-Pol activity assays. Activity assay results showed that P-E and P-R have high RDDP activity and relatively weak DDDP activity compared to the negative controls. This pattern had been observed previously with partially purified HBV-Pol[9].
It has been shown that adenosine triphosphate (ATP) participates in HBV-Pol activation during duck HBV replication[17]. To study the effect of ATP on purified HBV-Pol, different concentrations of ATP were added to the enzyme activity assays. As shown in Figure 4B, RDDP and DDDP activities of P-R were affected by ATP concentration. In the presence of ATP (0.1, 1 and 10 mmol/L), RDDP and DDDP activities of P-R showed a declining trend in a dose-dependent manner.
Human HBV-Pol is crucial for HBV genome DNA replication. It shows RDDP activity in minus strand DNA synthesis and DDDP activity in plus strand synthesis. Among six medications approved by the United States Food and Drug Administration for the treatment of CHB, lamivudine, adefovir dipivoxil, entecavir and telbivudine are HBV-Pol inhibitors[7]. Blocking HBV DNA replication is still the main target for CHB therapy. However, stable and large-scale heterologous expression of intact human HBV-Pol in common hosts such as E. coli or yeast has not been successfully achieved[9]. Although recombinant HBV-Pol has been expressed in different systems and purified in various ways, obtaining high-purity functional HBV-Pol without coexpression of a chaperon is still problematic[2,5,9,12]. Therefore, we developed a reliable purification method for further investigation of HBV-Pol activities.
The major obstacle for a large multi-functional protein such as HBV-Pol is its instability in heterologous expression systems and during purification[9]. It is known that inclusion bodies are formed during expression of human HBV-Pol, not only in E. coli expression systems, but also in eukaryotic expression systems[2]. In accordance with this, the target protein was present in total lysate and pellet rather than supernatant in detergent-free lysis buffer. Our preliminary study showed that expressed human HBV-Pol was degraded easily under native purification conditions, even with the presence of protease inhibitor. Choi et al[9] also have observed this phenomenon in a yeast expression system. Therefore, the key points of successful large-scale purification of human HBV-Pol are the solubilization and stabilization of the target protein. So far, nobody has reported a high level of expression and purification of HBV-Pol by using an E. coli expression system, in which we purified the protein up to 5 mg/mL. This is far higher than the earlier studies done by Choi et al[9], who used a Pichia methanolica expression system and Qadri et al[5] who used Saccharomyces cerevisiae.
In this study, we tried to achieve a high level of recombinant HBV-Pol with application of the following strategies. First, an amino-terminal 6 × His tag was used to facilitate rapid purification. Also, a sequence that encoded the Xpress epitope was fused to the N terminus of HBV-Pol so that it could be recognized rapidly by anti-Xpress primary antibody. Second, induction temperatures were lowered to maximize target protein expression. Third, for cell lysate purification, strong detergent lysis was followed by weak detergent washing and elution. Fourth, a high-concentration reducing agent was present during all the purification steps to keep HBV-Pol in soluble form by inhibiting disulfide bond formation, because approximately 2.5% of amino acid residues of HBV-Pol are cysteine.
It has been reported that structural and biochemical investigations of HBV-Pol have been complicated by the requirement of cellular factors such as HSP90[18], HSP60[3] and other cofactors[19] during purification. However, we developed a new method for large-scale purification of fully active HBV-Pol without addition of cellular factors by adjustment of salts during the purification steps. A binding step under denaturation conditions was used to isolate target protein from total lysate. With the presence of 1% SDS in the lysate, insoluble human HBV-Pol was dissolved rapidly. Proteases released from E. coli were also inactive in this SDS denaturing step. Soluble HBV-Pol was washed and eluted from nickel resin using the buffer in the presence of weak detergent and reducing agent. SDS was replaced by NP-40 during washing and elution. To prevent target protein from oxidization and to keep cysteine residues in a reduced state, a high concentration of reducing agents was employed throughout the purification steps.
The large-scale production of functionally intact human HBV-Pol was achieved in the E. coli expression system in this study. The availability of this recombinant protein in pure form should facilitate the crystallization and detailed analysis of the structure and mechanism of HBV-Pol. The availability of a large quantity of functional human HBV-Pol will help in high-throughput screening assays for development of potential pharmaceutical agents for CHB treatment.
Hepatitis B virus polymerase (HBV-Pol) is a multifunctional protein, which has intrinsic RNA-dependent reverse transcriptase (RT), DNA-dependent DNA polymerase, and RNase H activity. HBV-Pol is limited by difficulties in expressing and purifying the proteins in a heterologous system. This is believed to be the first time that functionally intact human HBV-Pol was expressed in Escherichia coli (E. coli) without co-expression of other molecules or in the presence of certain helper chaperons. Purified HBV-Pol showed stable RT activity and DNA polymerase activity. Functional HBV-Pol might be helpful for development of potential pharmaceutical agents for chronic hepatitis B (CHB) treatment.
HBV is a small DNA virus that replicates by reverse transcription of pre-genomic RNA, and is a major threat to human health. Most approved medications for CHB infection are nucleotide reverse transcriptase inhibitors that target HBV-Pol. Analysis of HBV-Pol has been hampered by the inability to express the functional enzyme in a recombinant system. For this reason, we applied various strategies to the production of functionally intact human HBV-Pol on a large scale by using an E. coli expression system.
This is believed to be the first time that functionally intact human HBV-Pol has been expressed in E. coli without co-expression of other molecules or in the presence of certain helper chaperons. To achieve high levels of recombinant HBV-Pol, an N-terminal 6×His tag and Xpress epitope were fused to the N terminus of HBV-Pol. Second, the induction temperature was lowered to maximize target protein expression, and a high concentration of reducing agent was present during all the purification steps to keep HBV-Pol in a soluble form. The availability of this recombinant protein in pure form should facilitate the crystallization and detailed analysis of the structure and mechanism of HBV-Pol.
The availability of a large quantity of functional human HBV-Pol can be helpful in high-throughput screening assay for potential pharmaceutical agents for CHB treatment.
This is a well-written paper and documents the expression and purification of full-length HBV-Pol in an E. coli system with promising results that could be the basis of forthcoming research on potential pharmaceutical agents for CHB treatment.
Peer reviewer: Stephan Menne, Assistant Professor of Virology, Department of Clinical Sciences/GI Unit, College of Veterinary Medicine, Cornell University, C2-005 Veterinary Medical Center, Ithaca, NY 14853, United States
S- Editor Sun H L- Editor Kerr C E- Editor Lin YP
| 1. | Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev. 2006;28:112-125. |
| 2. | Lanford RE, Notvall L, Beames B. Nucleotide priming and reverse transcriptase activity of hepatitis B virus polymerase expressed in insect cells. J Virol. 1995;69:4431-4439. |
| 3. | Park SG, Jung G. Human hepatitis B virus polymerase interacts with the molecular chaperonin Hsp60. J Virol. 2001;75:6962-6968. |
| 4. | Wang GH, Seeger C. The reverse transcriptase of hepatitis B virus acts as a protein primer for viral DNA synthesis. Cell. 1992;71:663-670. |
| 5. | Qadri I, Siddiqui A. Expression of hepatitis B virus polymerase in Ty1-his3AI retroelement of Saccharomyces cerevisiae. J Biol Chem. 1999;274:31359-31365. |
| 6. | Wei X, Peterson DL. Expression, purification, and characterization of an active RNase H domain of the hepatitis B viral polymerase. J Biol Chem. 1996;271:32617-32622. |
| 7. | Rivkin A. Entecavir: a new nucleoside analogue for the treatment of chronic hepatitis B. Drugs Today (Barc). 2007;43:201-220. |
| 8. | Ling R, Mutimer D, Ahmed M, Boxall EH, Elias E, Dusheiko GM, Harrison TJ. Selection of mutations in the hepatitis B virus polymerase during therapy of transplant recipients with lamivudine. Hepatology. 1996;24:711-713. |
| 9. | Choi J, Kim EE, Park YI, Han YS. Expression of the active human and duck hepatitis B virus polymerases in heterologous system of Pichia methanolica. Antiviral Res. 2002;55:279-290. |
| 10. | Kim Y, Jung G. Active human hepatitis B viral polymerase expressed in rabbit reticulocyte lysate system. Virus Genes. 1999;19:123-130. |
| 11. | Jeong JH, Kwak DS, Rho HM, Jung G. The catalytic properties of human hepatitis B virus polymerase. Biochem Biophys Res Commun. 1996;223:264-271. |
| 12. | Kim SS, Shin HJ, Cho YH, Rho HM. Expression of stable hepatitis B viral polymerase associated with GRP94 in E. coli. Arch Virol. 2000;145:1305-1320. |
| 13. | Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. 3rd Edition. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press 2001; 6.28-6.29. |
| 14. | Chesshyre JA, Hipkiss AR. Low temperatures stabilize interferon α-2 against proteolysis in Methylophilus methylotrophus and Escherichia coli. Appl Microbio and Biotech J. 1989;31:158-162. |
| 15. | Bollag DM, Rozycki MD, Edelstein SJ. Protein Methods. 2nd Edition. New York: Wiley-Liss 1996; . |
| 16. | Weber M, Bronsema V, Bartos H, Bosserhoff A, Bartenschlager R, Schaller H. Hepadnavirus P protein utilizes a tyrosine residue in the TP domain to prime reverse transcription. J Virol. 1994;68:2994-2999. |
| 17. | Beck J, Nassal M. Efficient Hsp90-independent in vitro activation by Hsc70 and Hsp40 of duck hepatitis B virus reverse transcriptase, an assumed Hsp90 client protein. J Biol Chem. 2003;278:36128-36138. |
| 18. | Cho G, Park SG, Jung G. Localization of HSP90 binding sites in the human hepatitis B virus polymerase. Biochem Biophys Res Commun. 2000;269:191-196. |