Copyright
©2010 Baishideng Publishing Group Co.
World J Gastroenterol. Dec 7, 2010; 16(45): 5752-5758
Published online Dec 7, 2010. doi: 10.3748/wjg.v16.i45.5752
Published online Dec 7, 2010. doi: 10.3748/wjg.v16.i45.5752
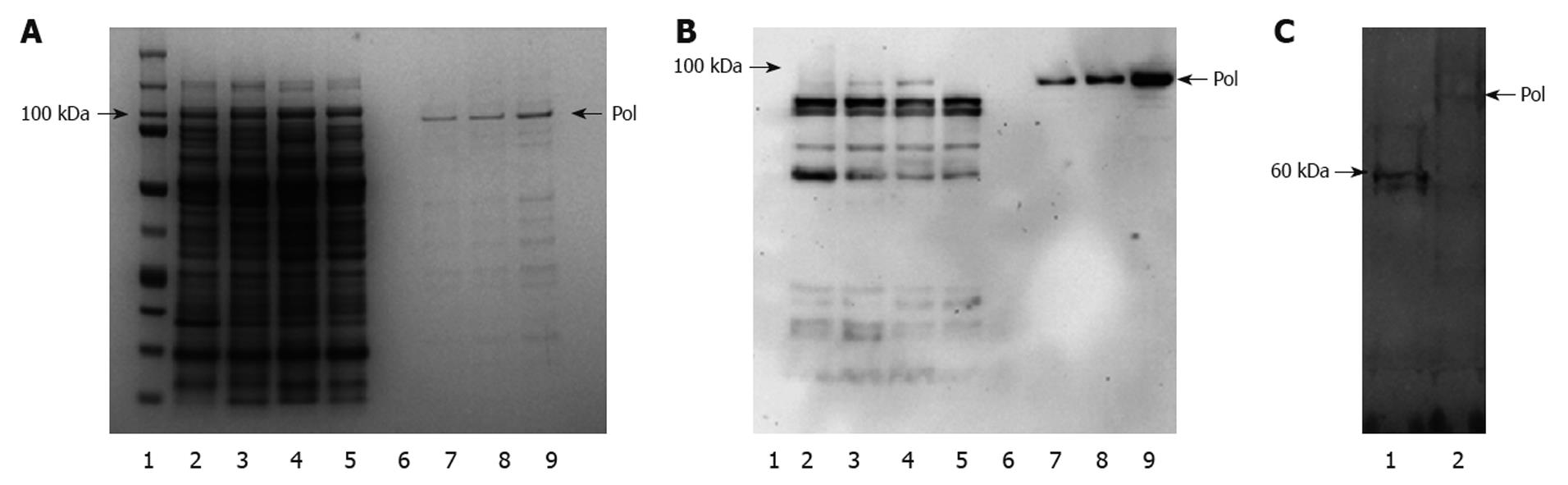
Figure 3 Expression and purification of hepatitis B virus polymerase.
Recombinant human hepatitis B virus polymerase (HBV-Pol) was produced in Escherichia coli (E. coli) cells transformed by pTrcHis-A-Pol and purified with nickel-based resin. Protein samples were analyzed by 4%-12% sodium dodecyl sulfate (SDS)-PAGE, and gels were stained with Coomassie Blue (A). HBV-Pol bands were located at the expected molecular mass, approximate 98 kDa (lane 1). Although HBV-Pol bands were also detected in 50 mmol/L (lane 7) and 75 mmol/L (lane 8) imidazole washing fractions, most HBV-Pol was eluted by 100 mmol/L imidazole (lane 9). Purified recombinant human HBV-Pol was also analyzed by immunoblotting with an anti-reverse transcriptase (RT) antibody (B). HBV-Pol bands were detected in total lysate of uninduced transformant (lane 3) and total lysate of induced transformant (lane 4), but not in untransformed E. coli (lane 2), unbound fraction (lane 5) and fifth washing fraction with 10 mmol/L imidazole (lane 6). Some positive small bands were also detected in lanes 2-5, which was mainly because some components from total lysate of E. coli were recognized by anti-RT polyclonal antibody. Expression of human HBV-Pol in TNT T7 transcription-translation-coupled rabbit reticulocyte lysate expression system is shown in C. PT7-pol and pT7-luciferase (1650 bp, ORF size approximate 61 kDa) were added to in vitro transcription-translation reactions for 75 min at 30°C in the presence of [35S]-methionine. Five microliters of each sample was incubated in SDS sample buffer at 95°C for 3 min, and electrophoresed through 4%-12% SDS-PAGE. A band of pT7-luciferase (lane 1) and pT7-pol (lane 2) were detected by autoradiography.
- Citation: Yu Y, Pandeya DR, Liu ML, Liu MJ, Hong ST. Expression and purification of a functional human hepatitis B virus polymerase. World J Gastroenterol 2010; 16(45): 5752-5758
- URL: https://www.wjgnet.com/1007-9327/full/v16/i45/5752.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i45.5752