Published online Dec 7, 2010. doi: 10.3748/wjg.v16.i45.5701
Revised: July 13, 2010
Accepted: July 20, 2010
Published online: December 7, 2010
AIM: To investigate the effects of dextrans of various molecular weights (Mw) during a 12 h cold storage time-course on energetics, histology and mucosal infiltration of fluorescein isothiocyanate (FITC)-dextran.
METHODS: Rodent intestines were isolated and received a standard University of Wisconsin vascular flush followed by intraluminal administration of a nutrient-rich preservation solution containing dextrans of varying Mw: Group D1, 73 kdal; Group D2, 276 kdal; Group D3, 534 kdal; Group D4, 1185 kdal; Group D5, 2400 kdal.
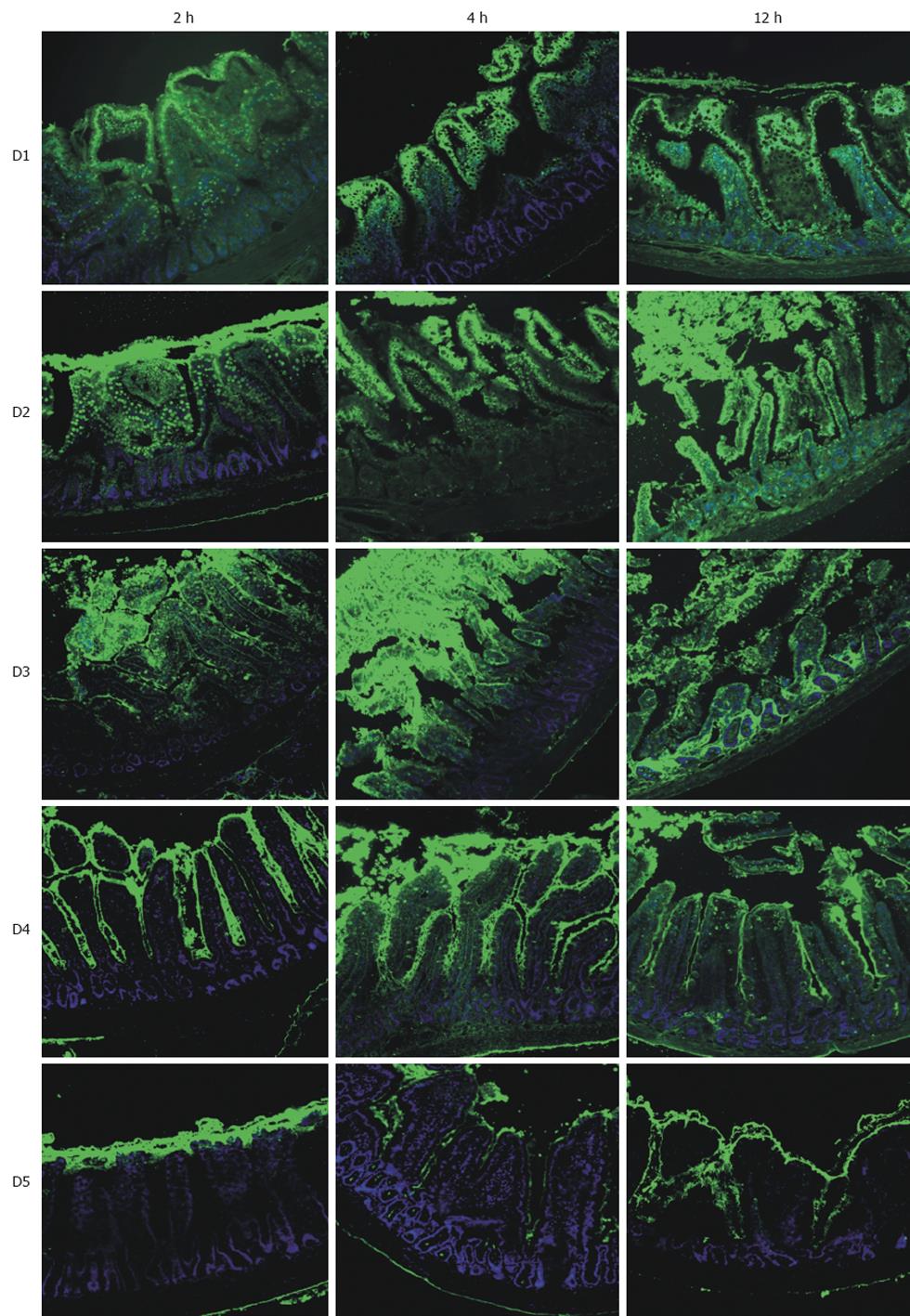
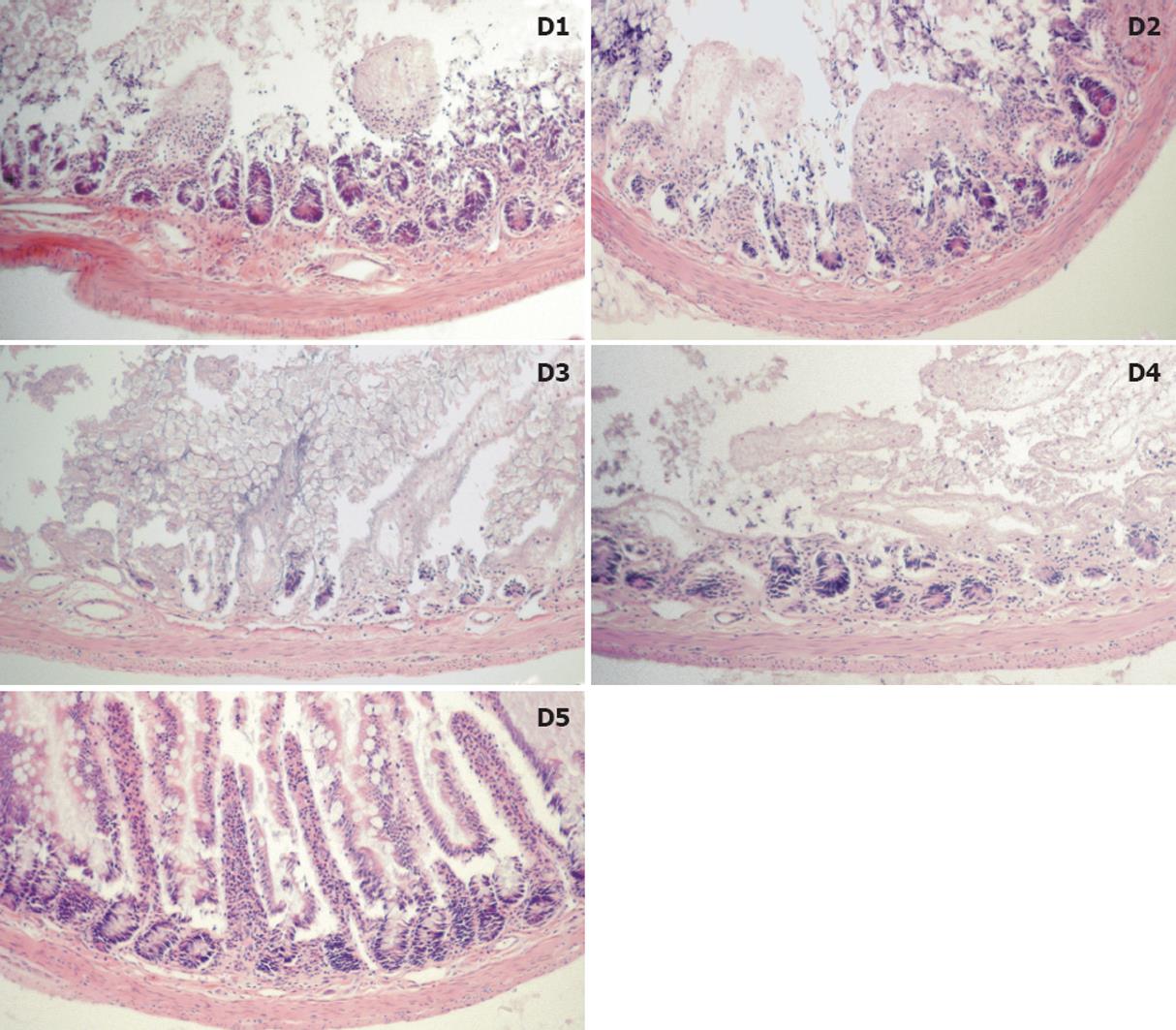
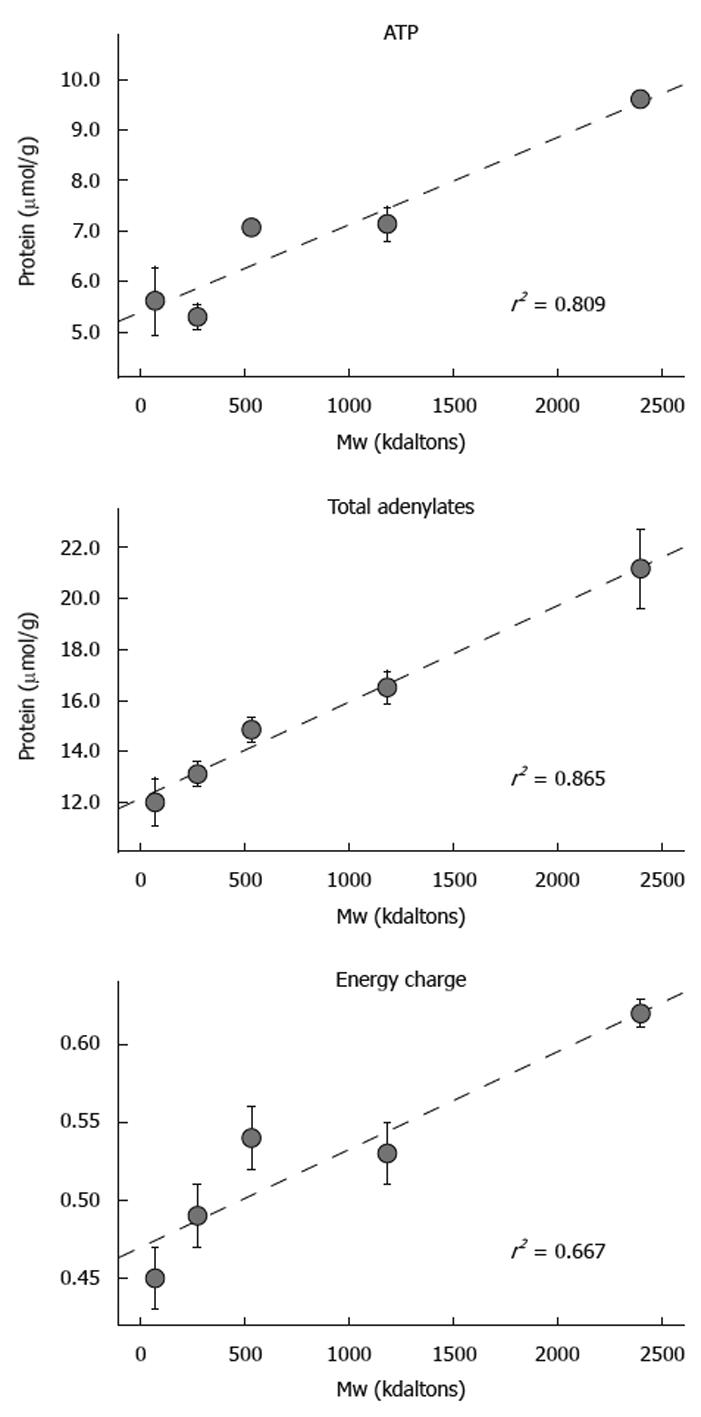
RESULTS: Using FITC-labeled dextrans, fluorescent micrographs demonstrated varying degrees of mucosal infiltration; lower Mw (groups D1-D3: 73-534 kdal) dextrans penetrated the mucosa as early as 2 h, whereas the largest dextran (D5: 2400 kdal) remained captive within the lumen and exhibited no permeability even after 12 h. After 12 h, median injury grades ranged from 6.5 to 7.5 in groups D1-D4 (73-1185 kdal) representing injury of the regenerative cryptal regions and submucosa; this was in contrast to group D5 (2400 kdal) which exhibited villus denudation (with intact crypts) corresponding to a median injury grade of 4 (P < 0.05). Analysis of tissue energetics reflected a strong positive correlation between Mw and adenosine triphosphate (r2 = 0.809), total adenylates (r2 = 0.865) and energy charge (r2 = 0.667).
CONCLUSION: Our data indicate that dextrans of Mw > 2400 kdal act as true impermeant agents during 12 h ischemic storage when incorporated into an intraluminal preservation solution.
- Citation: Schlachter K, Kokotilo MS, Carter J, Thiesen A, Ochs A, Khadaroo RG, Churchill TA. Redefining the properties of an osmotic agent in an intestinal-specific preservation solution. World J Gastroenterol 2010; 16(45): 5701-5709
- URL: https://www.wjgnet.com/1007-9327/full/v16/i45/5701.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i45.5701
Small bowel transplantation (SBT) has become an essential treatment for patients with irreversible intestinal failure who do not succeed on parenteral supplementation[1]. The global frequency and success rates of such procedures have seen steady increases over the last decade[1]. While only 11 intestinal transplants were performed in 1990, 140 cases were reported in 2003; currently approximate 200 are performed annually across the world[1]. For those individuals receiving antibody-based induction therapy and tacrolimus-based maintenance immunosuppression, one year post-transplant survival rates are comparable to those of liver (> 80%)[1]. The majority of individuals do not receive these therapies, hence 5-year graft survival rates for SBT remain low (31%-69%; 48% weighted average for 2005 data) compared to other commonly transplanted organs[2].
Among the obstacles to successful SBT is the extreme susceptibility of the mucosal epithelium to even brief periods of ischemia[3]. For this reason, the ability to successfully preserve graft viability during the period of ischemic cold storage is critical. Preventing hypothermia-induced cellular swelling is a basic principle of successful organ preservation[4]. One major advancement in preservation solution technology and design was the development of the “gold standard” preservation solution, the University of Wisconsin (UW) solution. Improvements in organ quality and safe cold ischemic times can be largely attributed to the control of cellular edema. This was accomplished by including cell-impermeant molecules lactobionate, raffinose, and hydroxyethylstarch (HES)[5]. With respect to the intestine, studies have shown that net fluid shifts contributing to mucosal injury can originate from the vasculature or lumen[6,7]. Since standard intestinal procurement involves a common intra-aortic flush of all abdominal organs, current preservation strategies do not address fluid shifts of a luminal origin.
Throughout the last several years, our laboratory has developed a novel nutrient-rich preservation solution (containing a large amino acid component) tailored to the specific metabolic requirements of the small intestine; termed AA solution. Numerous in vitro and in vivo models have documented superior maintenance of energetics, a reduction in oxidative stress, and a preservation of mucosal morphology and barrier function following intraluminal administration of the nutrient-rich solution[8-10]. We have demonstrated the benefits of using a high molecular weight (Mw) HES (2200 kdal) as an impermeant molecule in our novel solution[11,12], with no direct evidence that the starch molecule does not penetrate the mucosal barrier during ischemia. Recent data from our lab has demonstrated that dextran (Mw = 70 kdal) is not osmotically active when delivered as part of an intraluminal preservation solution; direct visualization of a fluorescently-labeled dextran-70 clearly showed the rapid migration of the dextran into the mucosa. This has raised the question as to the exact Mw characteristic of an effective impermeant agent in the realm of intestinal ischemia as it relates to a novel strategy of intraluminal preservation.
Although the UW solution contains HES on the basis of preventing interstitial edema incurred during cold ischemia[4], there exists controversy over its effectiveness for static organ storage. Some studies report that HES may be omitted from the UW solution without detrimental effects on overall graft quality[13-15], while others suggest a protective role of HES in stored tissues[16-20]. Because these previous studies deal solely with an intravascular delivery of UW solution, they do not provide information about the role of colloids in preservation solutions administered intraluminally for intestinal grafts. We suspect that during cold storage, alterations in the permeability characteristics of the mucosa will result in the infiltration of relatively large osmotic agents, thereby failing to effectively control tissue edema and mucosal viability. We hypothesized that there is a critical Mw for an effective osmotic agent to be used in an intraluminal preservation solution during cold ischemic storage.
Briefly, small intestines from rats were flushed intravascularly with UW solution, isolated and flushed intraluminally with a nutrient-rich preservation solution containing 5% dextran of varying Mw (73, 276, 534, 1185, 2200 kdal). Fluorescein isothiocyanate (FITC)-labeled dextrans to a final fluorescence of 10 × 106 fluorescence units/mL were incorporated into the solutions to aid in direct visualization of dextran infiltration. Intestines were stored at 4°C and samples were taken over a 12 h time-course for subsequent analysis.
Male Sprague-Dawley rats (200-250 g) were obtained from the University of Alberta and used as organ donors. All experiments were conducted in accordance with Canadian Council on Animal Care policies. Animals were fasted overnight and provided water ad libitum. Rats were induced with pentobarbital (65 mg/250 g; IP), followed by inhalational isoflurane (0.5%-2%) to maintain anesthesia. Following a midline laparotomy, the aorta was exposed infrarenally and at the celiac trunk. The supraceliac aorta was clamped and 2-4 mL modified UW solution was administered via the infrarenal aorta. The vena cava was transected to facilitate the outflow of blood and perfusate. The entire jejunum and ileum was subsequently harvested. A nutrient-rich preservation solution (AA solution), developed in our lab, was used to flush and clear the lumen of its contents (40 mL; approximate 2.0 mL/g). Sixteen centimeter-long sections of ileum were measured out. The sections were filled at the proximal end with 4-5 mL nutrient-rich preservation solution containing 5% dextran of varying Mw (fluorescence was standardized to 10 × 106 fluorescence units/mL and each end ligated with 3-0 silk). The preservation solution contained (values in brackets are mmol/L): Glutamine (35), Lactobionate (20), Glutamate (20), Aspartate (20), Glucose (20), BES [N,N-Bis(2-hydroxyethyl)taurine] (15), Arginine (10), Glycine (10), Asparagine (10), Threonine (10), Lysine (10), Valine (10), Serine (10), Methionine (5), Leucine (5), Isoleucine (5), Histidine (5), Ornithine (5), Proline (5), Adenosine (5), Cysteine (5), β-Hydroxybutyrate (3), Tyrosine (1), Tryptophan (1), Trolox (1), 3-Aminobenzamide (1), Allopurinol (1); plus the inclusion of 5% Dextran. The pH of each solution was adjusted to 7.40 with sodium hydroxide; measured osmolarity was 320 mOsm.
Group designations were as described in Table 1; briefly the dextran Mw for the 5 groups (D1-D5) were: 73 (D1); 276 (D2); 534 (D3); 1185 (D4); 2400 (D5) kdal.
| Group | Mp | Mw | Mn | PDI | IV |
| D1 | 62 900 | 72 700 | 50 700 | 1.43 | 0.263 |
| D2 | 238 000 | 275 900 | 204 600 | 1.35 | 0.463 |
| D3 | 490 000 | 534 000 | 371 000 | 1.43 | 0.633 |
| D4 | 1 050 000 | 1 185 000 | 705 000 | 1.67 | 0.862 |
| D5 | 2 000 000 | 2 400 000 | 1 600 000 | 1.50 | 0.803 |
Tissues were stored on ice at 4°C in standard AA solution and sampled at 2, 4, and 12 h; at each time-point a 4 cm length of intestine was sampled; half was used for histology and half for metabolite analysis. To arrest metabolic activity, samples were snap frozen in liquid nitrogen, and stored at -65°C until processed.
Dextrans used in this study were obtained from American Polymer Standards Corporation, Ohio, US; these dextrans were of the highest purity available and characteristics are provided in Table 1. Briefly, peak average molecular weight (Mp), weight average molecular weight (Mw), number average molecular weight (Mn), and polydispersity index (PDI) describe molecular distribution curves. The key parameter defining dextran purity is the PDI with a value of 1.0 representing absolute purity. PDI values of less than 3 represent high purity fractions; hence values in the current study of 1.35 to 1.67 are of extremely pure dextran fractions. The procedure used to FITC-label the dextrans from the above solutions was based on the original procedure used by De Belder and Granath[21]. The dextran of interest together with fluorescein 5-isothiocyanate (FITC) were dissolved in dimethyl sulphoxide, pyridine and dibutyltin dilaurate and heated to 95°C for 2 h. The mixture was then put on ice and the dextran was precipitated out with cold 100% ethanol, filtered and dried in an oven at 40°C. Labeling efficiency was 0.015-0.020; this corresponds to 1 fluorescein molecule per 50-67 glucose units. FITC-labeled dextran accounted for 8.5% ± 0.6% of total dextran in each solution; fluorescence was standardized. Fluorescence for FITC-dextrans was evaluated at an excitation wavelength = 485 nm/emission wavelength = 535 nm.
When labeling fluorescent moieties to target molecules, one of the key concerns that must be considered for subsequent experimentation to be valid is that the fluorescent label must remain bound to the target and does not spontaneously detach over time when in aqueous solution. In this study, a dilute solution (approximate 0.1%) of each dextran was incubated at 4°C for 24 h. Following the incubation period, FITC-dextrans were precipitated with cold 100% ethanol, centrifuged, and the supernatant (containing free FITC) was assessed for fluorescence. When solutions were incubated at 4°C, there were no statistically significant decreases in FITC-bound dextrans; this indicated a stable conjugation for all high-purity dextrans used in this study.
Frozen small bowel samples were weighed and then extracted 1:5 weight/volume in perchloric acid containing 1 mmol/L ethylenediamine tetra-acetic acid. The precipitated protein was removed by centrifugation (20 min at 20 000 ×g). Acid extracts were neutralized by the addition of 3 mol/L KOH/0.4 mol/L Tris/0.3 mol/L KCl and then recentrifuged (20 min at 14 000 ×g). Aliquots of neutralized extracts were immediately processed via standard enzyme-linked metabolite assays[22]. Spectrophotometric analysis was then performed to measure the absorbance of NADH at 340 nm, providing quantification of adenosine triphosphate (ATP), total adenylates [ATP + adenosine diphosphate (ADP) + adenosine monophosphate (AMP)] and energy charge [(ATP + 0.5 ADP)/total adenylates] and malondialdehyde[22,23]. Values are reported as μmol per gram protein. Protein was measured according to the method of Lowry et al[24].
Bowel samples were fixed in alcohol formalin solution, processed to paraffin wax, embedded, and sections cut at 5 μm. Sections were dewaxed then mounted with Prolong Gold anti-fade reagent with 4’,6-diamidino-2-phenylindole (DAPI; Invitrogen) and sealed with nail polish. Fluorescent microscopy was used to view FITC and DAPI fluorescence in tissues; wavelengths were Ex = 485/Em = 535 and Ex = 360/Em = 460, respectively. Fluorescent images were digitally captured using AxioVision software. All photos shown reflect representative findings. A second set of sections were stained with hematoxylin and eosin and graded according to a modified Park’s classification for intestinal injury[25] as follows.
Grade 0: Normal mucosa; grade 1: Subepithelial space at villus tip; grade 2: Moderate subepithelial space; grade 3: Epithelial lifting along villus sides; grade 4: Denuded villi; grade 5: Loss of villus tissue; grade 6: Crypt layer injury; grade 7: Transmucosal injury; and grade 8: Transmural injury.
Metabolite data were reported as mean ± SE for each group. Statistical differences between groups were determined using analysis of variance, followed by Student-Newman-Keuls’. Analysis of relationships between Mw and ATP, total adenylates, or energy charge was performed with a linear regression analysis for parametric data. Differences in histology grades were assessed by a non-parametric Kruskal-Wallis test.
The lower Mw dextrans tested (D1, D2, D3) consistently demonstrated infiltration of the fluorescent label at all time-points. At the earlier time-points, 2 and 4 h, fluorescence intensity was greatest in the epithelium or in sloughed epithelial cells. After 12 h storage, there was significant infiltration into the cryptal regions, the lamina propria, as well as the vascular epithelium. Mucosal injury was greatest in these groups (D1, D2, D3), ranging from development of subepithelial clefts at 2 h to complete denudation at the later time-points, leaving the underlying tissue vulnerable to further dextran penetration.
Interestingly, the intermediate Mw dextran, D4, exhibited no extensive penetration of label into the epithelial layer and largely remained within the layer of mucous coating the surface of the villi. Several goblet cells and the apices of some villi did have a minor amount of fluorescence. However, by 4 h, there was considerable infiltration of the dextran label throughout the underlying tissue. Limited mucosal injury was apparent even after 12 h storage, at which time epithelial clefting had developed. Treatment with the highest Mw tested, D5, resulted in no observable infiltration into the epithelium or lamina propria; this was evident at all time-points (Figure 1).
Histologic injury was evident early on during cold storage, particularly for the lower Mw dextrans after only 2 h. Most notably, in groups D1 and D2, 50% of specimens exhibited a significant degree of crypt infarction, compared to no evidence of crypt infarction in groups D3, D4, and D5 (P = 0.06).
By 12 h, 75% (12/16) of specimens in groups D1-D4 had an injury grade of 6 or greater (damage to the crypts, mucosa, or transmural injury). Conversely, 75% of specimens in Group D5 exhibited clefting where the epithelium had lifted off the underlying lamina propria (grade 3) and at times had dissociated from the villus proper (grade 4) (P < 0.05). A summary of injury grading and median grades are presented in Table 2. Representative fields of injury are presented in Figure 2 micrographs.
| Time (h) | Group | Grade | Median | Significance | Event |
| 2 | D1 | 0, 2, 6, 7 | 4 | Denuded villi | |
| D2 | 3, 4, 6, 6 | 5 | Loss of villi | ||
| D3 | 1, 2, 2, 3 | 2 | D3, D4, D5 vs D1, D2, P = 0.06 | Moderate clefting | |
| D4 | 1, 2, 3, 5 | 2.5 | Moderate-extensive clefting | ||
| D5 | 1, 2, 3, 4 | 2.5 | Moderate-extensive clefting | ||
| 12 | D1 | 0, 6, 7, 8 | 6.5 | Injury to crypts and submucosa | |
| D2 | 2, 6, 7, 7 | 6.5 | Injury to crypts and submucosa | ||
| D3 | 3, 7, 8, 8 | 7.5 | Injury to crypts, submucosa and muscularis | ||
| D4 | 3, 6, 8, 8 | 7 | Injury to crypts and submucosa | ||
| D5 | 0, 4, 4, 5 | 4 | D5 vs D1-D4, P < 0.05 | Clefting and denuded villi |
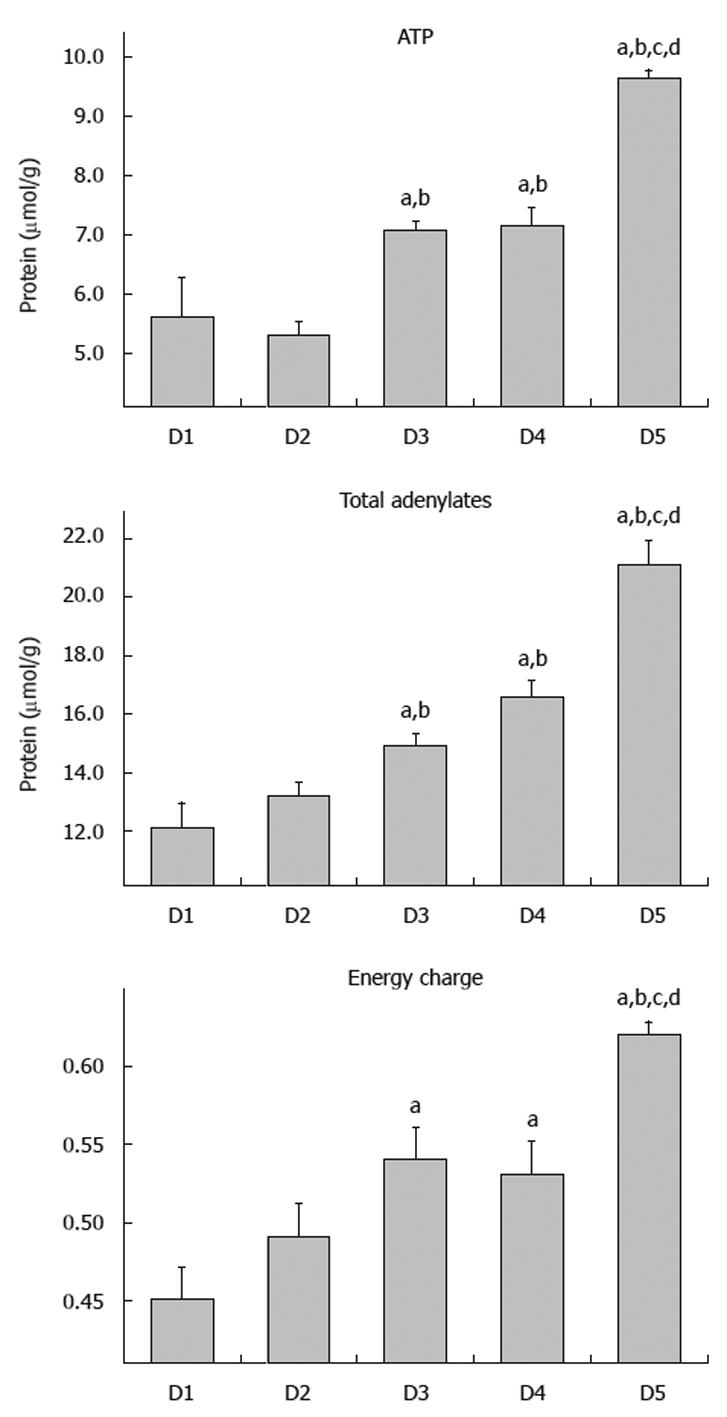
Following 12 h cold storage, ATP levels ranged from 5.3 to 9.6 μmol/g, with D1 and D2 having the lowest levels (5.3-5.6 μmol/g). Intermediate Mw groups, D3 and D4, exhibited intermediate ATP levels, both significantly different from D1 and D2 (P < 0.05). ATP progressively increased to a maximum of 9.6 μmol/g in D5; this group had significantly greater levels than all other groups (P < 0.05). Levels of total adenylates (ATP + ADP + AMP) closely resembled those of ATP. Levels in groups D2 and D3 were significantly higher than in D1 and D2, P < 0.05. Similarly, D5 level was significantly greater than all groups, P < 0.05. Atkinson described another useful measure of tissue energetics, “energy charge” as: EC = (ATP + ADP/2)/total adenylates[26]. In illustrating the significance of this measurement, Pegg used the analogy of a battery in describing the energy charge as being a measure of the “charged up” state of the adenylate pool[27]. As ATP levels decline relative to ADP and AMP levels, energy charge also drops, indicating that less of the total adenylate pool exists in a form that is immediately available for cellular work. Energy charge ratios showed similar trends to those of ATP and total adenylates. Values increased as Mw of the dextran increased, starting at 0.45 in D1 and reaching 0.62 in D5; D5 value was significantly different than all values in groups D1-D4 (P < 0.05) (Figure 3).
With respect to dextran Mw, there was a strong positive and statistically significant correlation with ATP (r2 = 0.809); total adenylates (r2 = 0.865); and energy charge (r2 = 0.667). Overall, group D5 was the most effective in preserving the three parameters of tissue energetics over the 12 h period of cold ischemic storage, while lower Mw groups exhibited poor conservation of energetics. Superior maintenance of energetic parameters supported the preservation of mucosal viability as determined by direct histologic observation of mucosal morphology as well as penetration of the FITC-labeled dextrans (Figure 4).
No significant differences were detected among groups D1-D5 with respect to the parameter of oxidative stress (malondialdehyde) after 12 h storage; values varied between 236 and 274 nmol/g protein in all 5 experimental groups (data not shown).
The major clinical application of dextrans, in addition to other starches and albumin, has been for their plasma volume expansion properties. These molecules exert their osmotic effects by binding water within the vasculature and creating an osmotic force to extract water from edematous tissues. The Mw of a molecule required to remain captive within the vasculature is very low as demonstrated by the effectiveness of an intravascular flush during organ procurement with the UW solution (osmotic agents, lactobionate and raffinose; Mw = 358 and 504, respectively). With respect to osmotic support and the application of an intraluminal preservation solution, the Mw permeability limit of the epithelial barrier during ischemic storage has not been clearly defined.
In the realm of organ preservation, osmotic agents are required to counteract the metabolic events that result in the influx of water into the cells/tissue. Under normal circumstances, cells have an extracellular environment rich in Na+ and low in K+[5]. The Na+/K+ transporter maintains the respective gradients of these ions, relying on the use of ATP as its energy source. Essentially, this pump makes Na+ an impermeant outside of the cell, creating an osmotic force that counteracts the osmotic pressure exerted by proteins and impermeable anions in the cell interior. Together, these intracellular proteins and impermeable ions require an osmotic force of 110-140 mOsm/kg to offset fluid flow into the cell[5]. In ischemic tissue under cold storage, the above situation is quite different. Hypothermic conditions coupled with aerobic metabolism collectively work to inhibit Na+/K+ ATPase activity, decreasing the cell membrane potential. As a result, Na+ and Cl- flow down their concentration gradients into the cell, and water follows, causing the cell to swell; cells eventually become damaged and may lyse[5,19]. Cell impermeant molecules are critically important in regulating fluid movement into the cell; there is a fundamental requirement of 110-140 mmol/L of osmotic agents in order to counteract cell swelling[5].
When utilizing an intraluminal preservation solution, inadequate osmotic support will culminate in direct injury to the epithelium. This leads to the compromise of cellular integrity and consumption of essential energy reserves in an attempt to repair damaged tissue. Depletion of cellular energy reserves (ATP and total adenylates) results in an inability of the mucosal epithelium to preserve its barrier function, compromising epithelial integrity during organ storage[20,28]. The barrier consists of numerous tight junctions located toward the apical surface of intestinal epithelial cells[8]. A number of proteins form the tight junctions, which are active, energy-consuming structures[8]. The transepithelial flow of both macromolecules and enteric bacteria are thought to be caused by tight junction dilation[3], and the consequences are twofold: (1) an increased likelihood of sepsis and (2) a need to consider the permeability of molecules used for osmotic support during preservation. Sepsis may occur due to bacterial translocation[29,30], and continues to account for the majority of deaths after intestinal transplantation (46.0% between April 1985 and May 2003)[1]. Increased permeability of the intestine during ischemic conditions should influence the critical Mw limit for molecules added to the intraluminal preservation solution for impermeant support. Inclusion of the appropriate agent capable of acting as a true osmotic will improve the quality of the intestinal graft and ultimately influence success of the transplant.
Hydrostatic and impermeant forces regulate the movement of fluids between intracellular and extracellular compartments. Hydrostatic forces have a predominant role in continuous perfusion methods; whereas, impermeant forces are the sole contributors governing net fluid flux in the setting of static cold storage, the current standard for most intra-abdominal organs. Shifts in net fluid flow during cold storage are proposed to originate from the intestinal vasculature and/or lumen[6,7]. In the current study, fluid shifts of vascular origin were controlled by delivering a standard intra-aortic vascular flush with UW solution to all treatment groups, as would happen in the clinical situation. Keeping such fluid shifts constant was essential, allowing us to attribute any inter-group differences of luminal permeability to the specific dextran size of interest. Based on the data presented in the current communication, there was a definitive inverse relationship between dextran Mw and its ability to cross the epithelial barrier during cold storage. Several relatively large Mw dextrans (73-534 kdal) exhibited a definitive pattern of penetration of the mucosal layer within 2 h cold storage, eventually infiltrating the submucosa and muscularis propria after 12 h. The only effective dextran fraction tested that exhibited impermeant characteristics by remaining captive within the lumen even after 12 h cold ischemia was one of Mw = 2400 kdal. This study is the first to establish the critical permeability limit of the mucosal layer during intestinal preservation and to delineate the requisite Mw of a true osmotic impermeant in the lumen.
The relationship between Mw (size) and permeability determined in this study was somewhat expected based on the results from various animal and human intestinal permeability studies[15,17,18]. An inverse relationship between Mw and mucosal-to-serosal permeability has been established previously for rodents[17]. In a comprehensive report addressing comparative intestinal permeability in pig, rat, and human models, Nejdfors et al[15] found an inverse relationship between the Mw of marker molecules and their mucosal permeability across all species, irrespective of location in the gastrointestinal tract. The power of such a study comes from the fact that identical standardized methods of evaluating molecular permeability and a wide range of Mw were used for each species; this included 14C-mannitol, FITC-dextrans 4.4/70 kdal and several proteinaceous macromolecules (α-lactalbumin, 65 kdal and ovalbumin, 45 kdal).
In an animal study assessing the uptake of FITC-dextran (Mw 70 kdal) by the small intestinal epithelium, Ekström et al[29] found that during neonatal development (< 30-d old) there were negligible amounts of FITC-dextran in the serum with no enterocyte infiltration. One should note that this experiment was conducted in healthy fully oxygenated tissues without any prior ischemic insult. Based on such reports, our nutrient-rich solution has in the past contained a dextran fraction with similar Mw for impermeant support[8,30,31]. There is no reason to suspect that a macromolecule which is impermeant in the lumen of a healthy bowel will do so during ischemic conditions, even at reduced temperatures. As the number of tight junctions and the magnitude of the dilatations increase, the potential for infiltration and translocation of large macromolecules and eventually bacteria also increases. As a consequence of these impermeant characteristics, the lack of osmotic support contributes directly to tissue edema and its negative sequelae. In tissues lacking sufficient impermeant support during hypothermic storage, edema becomes a major concern.
In the current study, parameters of cellular energetics (ATP, total adenylates and energy charge) consistently reflected strong and significant positive correlations with respect to dextran Mw. Although the value of ATP to the cell is obvious, higher Total Adenylate levels reflect reduced rates of purine catabolism, potentially leading to the accumulation of hypoxanthine and the production of uric acid and superoxide (a highly reactive oxygen free radical)[32]. A second consequence is that greater amounts of purines are available for ATP regeneration (once carbon exits the total adenylate pool, reconversion is not enzymatically possible in this pathway). These biochemical parameters of tissue integrity all indicate that the 2400 kdal dextran was maximally effective in protecting the mucosa from ischemic insult during cold storage.
In conclusion, the permeability characteristics of the rat small bowel during cold ischemia are very different than those of intestinal tissue that is not limited with respect to oxygen and nutrients. In order for a molecule to behave as a true osmotic impermeant, the Mw must be much greater than that established for intravascular preservation solutions. From the data presented in this study, the size of dextran showing the lowest degree of epithelial or mucosal infiltration, superior morphology and minimal disruption to cellular energetics was 2400 kdal. These findings clearly delineate the critical Mw limit that must be addressed in the development of an effective intraluminal preservation solution.
An effective osmotic agent is one of the fundamental requirements of organ preservation for transplantation. Commonly used preservation solutions that are typically administered via an intravascular route utilize low molecular weight (Mw) impermeant compounds to maintain tissue water balance. Our laboratory has developed a novel strategy for intestinal preservation involving the intraluminal delivery of a nutrient-rich preservation solution; however, an appropriate impermeant molecule has not been clearly defined.
Although organ preservation methods have existed for decades, luminal administration of a preservation solution tailored to the metabolic requirements of the small bowel is a novel concept.
This study reveals for the first time that a large Mw impermeant molecule is a fundamental requirement of an intraluminal preservation solution during cold, static storage of the small intestine. Interestingly, only the highest Mw dextran (2400 kdal)
remained within the intestinal lumen throughout the entire 12 h period of cold storage, resulting in superior graft energy status and tissue morphology.
The current study focused on defining a suitable osmotic agent for use in our intraluminal preservation solution, a key factor affecting intestinal viability during organ storage. As a back-table luminal flush that does not interfere with the clinical vascular preservation method, our AA solution has the potential to revolutionize intestinal preservation, and therefore improve patient outcomes following transplantation.
The manuscript submitted by Schlachter et al represents a methodical and well written study investigating the protective effects of different Mw dextrans in a novel intestinal preservation solution. The findings of the study are potentially important and widely applicable to the intestinal transplantation field.
Peer reviewers: Tor C Savidge, PhD, Associate Professor, Department of Gastroenterology and Hepatology, Galveston, TX 77555, United States; Dr. Thomas Wild, MD, Department of Surgery, Paracelsus Medical University, Feldgasse 88, Kapellerfeld, 2201, Austria
S- Editor Tian L L- Editor Logan S E- Editor Lin YP
| 1. | Grant D, Abu-Elmagd K, Reyes J, Tzakis A, Langnas A, Fishbein T, Goulet O, Farmer D. 2003 report of the intestine transplant registry: a new era has dawned. Ann Surg. 2005;241:607-613. |
| 2. | UNOS database. Data obtained from intestinal graft survival based on recipient age. Transplants performed in 1997-2000, data from survival after 2002-2005. Available from: http://www.unos.org. |
| 3. | Tsukamoto T, Nigam SK. Tight junction proteins form large complexes and associate with the cytoskeleton in an ATP depletion model for reversible junction assembly. J Biol Chem. 1997;272:16133-16139. |
| 4. | Southard JH, van Gulik TM, Ametani MS, Vreugdenhil PK, Lindell SL, Pienaar BL, Belzer FO. Important components of the UW solution. Transplantation. 1990;49:251-257. |
| 5. | Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation. 1988;45:673-676. |
| 6. | Black-Schaffer B, Gall EP, Shimizu RT, Esparza HS. The pathogenesis of the intestinal lesion of deep hypothermia and a proposed relationship to that of irreversible shock, including a note on a mechanism for the normal turnover of intestinal epithelium. Surgery. 1967;61:904-914. |
| 7. | Sandritter W. Shock-induced lesions of the small intestine in experimental animals. A Symposium of the Fifth Congress of the International Academy of Pathology. Philadelphia: FA Davis Co 1965; . |
| 8. | Salehi P, Zhu JZ, Castillo EG, Avila J, Lakey J, Churchill TA. Preserving the mucosal barrier during small bowel storage. Transplantation. 2003;76:911-917. |
| 9. | Fujimoto Y, Olson DW, Madsen KL, Zeng J, Jewell LD, Kneteman NM, Bigam DL, Churchill TA. Defining the role of a tailored luminal solution for small bowel preservation. Am J Transplant. 2002;2:229-236. |
| 10. | Olson DW, Jijon H, Madsen KL, Al-Saghier M, Zeng J, Jewell LD, Bigam DL, Churchill TA. Human small bowel storage: the role for luminal preservation solutions. Transplantation. 2003;76:709-714. |
| 11. | Kokotilo MS, Carter J, Thiesen A, Chen MH, Ochs A, Khadaroo RG, Churchill TA. Optimizing the concentration of hydroxyethylstarch in a novel intestinal-specific preservation solution. Cryobiology. 2010;Epub ahead of print. |
| 12. | Salehi P, Bigam DL, Ewaschuk JB, Madsen KL, Sigurdson GT, Jewell LD, Churchill TA. Alleviating intestinal ischemia-reperfusion injury in an in vivo large animal model: developing an organ-specific preservation solution. Transplantation. 2008;85:878-884. |
| 13. | Paull J. A prospective study of dextran-induced anaphylactoid reactions in 5745 patients. Anaesth Intensive Care. 1987;15:163-167. |
| 14. | Treib J, Baron JF, Grauer MT, Strauss RG. An international view of hydroxyethyl starches. Intensive Care Med. 1999;25:258-268. |
| 15. | Nejdfors P, Ekelund M, Jeppsson B, Weström BR. Mucosal in vitro permeability in the intestinal tract of the pig, the rat, and man: species- and region-related differences. Scand J Gastroenterol. 2000;35:501-507. |
| 17. | Pantzar N, Weström BR, Luts A, Lundin S. Regional small-intestinal permeability in vitro to different-sized dextrans and proteins in the rat. Scand J Gastroenterol. 1993;28:205-211. |
| 18. | Menzies IS. Transmucosal passage of inert molecules in health and disease. Intestinal absorption and secretion. Falk Symposium 36. Lancaster: MTP Press 1984; 527-543. |
| 19. | Churchill TA. Organ preservation for transplantation. Functional Metabolism: Regulation and Adaptation. Hoboken: Wiley-Liss 2004; 383-413. |
| 20. | Fuller BJ. The effects on cooling on mammalian cells. Clinical applications of cryobiology. London: CRC Press 1991; . |
| 21. | De Belder AN, Granath K. Preparation and properties of fluorescein labeled dextrans. Carbohyd Res. 1973;30:375-378. |
| 22. | Passonneau JV, Lowry OH. Enzymatic Analysis: A Practical Guide. Totowa: Humana Press 1993; . |
| 23. | Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351-358. |
| 24. | Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275. |
| 25. | Park PO, Haglund U, Bulkley GB, Fält K. The sequence of development of intestinal tissue injury after strangulation ischemia and reperfusion. Surgery. 1990;107:574-580. |
| 26. | Atkinson DE. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968;7:4030-4034. |
| 27. | Pegg DE. The biology of cell survival in vitro. Organ Preservation For Transplantation. New York and Basel: Marcel Dekker Inc 1981; 705. |
| 28. | Juurlink BH. Therapeutic potential of dietary phase 2 enzyme inducers in ameliorating diseases that have an underlying inflammatory component. Can J Physiol Pharmacol. 2001;79:266-282. |
| 29. | Ekström GM, Weström BR, Telemo E, Karlsson BW. The uptake of fluorescein-conjugated dextran 70,000 by the small intestinal epithelium of the young rat and pig in relation to macromolecular transmission into the blood. J Dev Physiol. 1988;10:227-233. |
| 30. | Salehi P, Madsen K, Zhu J, Castillo E, Avila J, Lakey JR, Churchill TA. Alleviating ischemia-reperfusion injury in small bowel. Am J Transplant. 2004;4:728-737. |
| 31. | Salehi P, Zhu LF, Sigurdson GT, Jewell LD, Churchill TA. Nutrient-related issues affecting successful experimental orthotopic small bowel transplantation. Transplantation. 2005;80:1261-1268. |