Published online Nov 28, 2010. doi: 10.3748/wjg.v16.i44.5611
Revised: June 9, 2010
Accepted: June 15, 2010
Published online: November 28, 2010
AIM: To track intravascularly transplanted mesenchymal stem cells (MSCs) labeled with superparamagnetic iron oxide (SPIO) by using magnetic resonance imaging (MRI) in an experimental rabbit model of hepatic failure.
METHODS: Human MSCs labeled with FDA-approved SPIO particles (Feridex) were transplanted via the mesenteric vein into rabbits (n = 16) with carbon tetrachloride-induced hepatic failure. Magnetic resonance (MR) examinations were performed with a 3.0 T clinical scanner immediately before and 2 h and 1, 3, and 7 d after transplantation. Signal intensity (SI) changes on T2*-weighted MRI were measured, and correlation between MR findings and histomorphologic findings was also investigated.
RESULTS: SI on T2*-weighted MRI decreased significantly in the liver 2 h after injection of human MSCs and returned gradually to the levels found before injection in 7 d. Changes in SI in the liver at 2 h, 1, 3, and 7 d were 41.87% ± 9.63%, 10.42% ± 4.3%, 5.12% ± 1.9%, 3.75% ± 1.2%, respectively (P < 0.001). Histologic analyses confirmed the presence of MSCs in the liver, localized mainly in the sinusoids in early period (2 h and 1 d) and concentrated to the border zone in late period (3 and 7 d). The number of iron-positive cells in the liver at 2 h and on 1, 3 and 7 d after transplantation was 29.2 ± 4.8, 10.1 ± 3.7, 6.7 ± 2.2, and 5.8 ± 2.1, respectively (P = 0.013).
CONCLUSION: Intravascularly injected SPIO-labeled MSCs in an experimental rabbit model of hepatic failure can be detected and followed with MRI.
- Citation: Son KR, Chung SY, Kim HC, Kim HS, Choi SH, Lee JM, Moon WK. MRI of magnetically labeled mesenchymal stem cells in hepatic failure model. World J Gastroenterol 2010; 16(44): 5611-5615
- URL: https://www.wjgnet.com/1007-9327/full/v16/i44/5611.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i44.5611
As a potential treatment, liver cell transplantation provides an effective strategy for the treatment of liver failure[1]. Transdifferentiation of stem cells of various types into functional hepatocytes has been demonstrated, including embryonic stem cells, hepatoblasts, hepatic oval cells, pancreatic progenitor cells, bone marrow hematopoietic stem cells, and mesenchymal stem cells (MSCs)[2-7]. The use of MSCs as a potent potential source for hepatocytic transdifferentiation has been tried[8,9] and bone marrow-derived MSCs protected against carbon tetrachloride-induced liver fibrosis in rats[10].
In the past several years, a great deal of research has been focused on stem cell therapy. However, there remain many important issues to be addressed regarding cell therapy, and noninvasive and repeated monitoring of in vivo transplanted stem cells is an important topic in these days. Therefore, more recent research activities have focused on in vivo real-time tracking and detecting the fate of transplanted stem cells by using appropriate imaging technologies[11,12].
The use of magnetic resonance imaging (MRI) is well suited to evaluate the ability of cells to migrate and engraft to target organs, as MRI can provide a detailed anatomy of target organs with excellent spatial resolution. Paramagnetic or modified dextran-coated superparamagnetic iron oxide (SPIO) contrast agents have been used to label cells, allowing investigators to monitor cellular migration using MRI[12,13]. However, few studies have been undertaken to determine the feasibility of the use of in vivo MRI of cell therapy in models of hepatic failure. The aim of the present study is to assess in vivo MRI with the use of a clinical 3-Tesla MRI unit for the depiction of SPIO-labeled MSCs in a rabbit model of hepatic failure.
The method of cell culture and labeling was previously reported[14] and is only briefly described here. Human MSCs (Bio-Whittaker, Walkersville, MD) were grown in mesenchymal stem cell basal medium (MSCBM, Bio-Whittaker) at 37°C and in a 95% air, 5% CO2 atmosphere. Human MSCs were cocultured in MSCBM containing FDA-approved SPIO particles (Feridex; Berlex, Wayne, NJ). The iron concentrations of the SPIO preparations were 125 μg/mL. Poly-L-Lysine (PLL) (Sigma-Aldrich, St. Louis, MO) was used as a transfection agent.
All animal work was conducted in accordance with the guidelines provided by the Institutional Animal Control and Utilization Committee. The experiments were performed with 24 New Zealand white rabbits weighing 2.5-3.2 kg. The average age of rabbits was 10 wk. The rabbits were allowed food and water ad libitum and were kept in 44 cm × 68 cm × 39 cm sized cage with 22 ± 2°C of temperature and 40%-60% of humidity.
Hepatic fibrosis was induced by intragastric administration of carbon tetrachloride (CCl4) twice a week for 2 wk with 0.1 mL/kg in olive oil (at a 1:1 ratio). One day after the fourth administration of CCl4, rabbits were anesthetized with intramuscular injection of 25-50 mg/kg ketamine hydrochloride (Yuhan Yanghang) and 10-20 mg/kg 2% xylazine hydrochloride (Bayer). After the abdomens of the rabbits were opened to expose the mesenteric vein, 1 × 106 SPIO-labeled human MSCs were slowly injected into the mesenteric vein in the experimental group (16 rabbits). One milliliter normal saline was injected into the mesenteric vein in control group (8 rabbits).
In all 24 rabbits MRI was performed with a 3.0 T MRI scanner (Signa Excite; GE Healthcare, Milwaukee, WI) with a knee coil. MRI of the rabbit liver was carried out immediately before, and 2 h, and 1, 3, and 7 d after injection of these stem cells. Transverse T2*-weighted gradient-echo (repetition time msec/echo time msec, 600/20; flip angle, 30°; section thickness, 3 mm; FOV, 18 cm; matrix, 256 × 160; number of signals acquired, two) sequences were employed.
Changes in signal intensity (SI) were characterized by use of region of interest (ROI) analysis on a well-centered slice with an area of 1 mm2. A minimum of 15 pixels was required per region as displayed on a picture archiving and communication system (PACS) workstation (Maroview; Marotech). The value of the ROI was determined as the mean ± SD after an estimated three times, and was normalized to that of back muscle[15]. SI changes in each rabbit were calculated according to the use of the following formula: [SI (pre) - SI (post)]/SI (pre) × 100; SI (pre) and SI (post) are the normalized SI values as compared to back muscle before and after labeled cell injection.
Two hours, and 1, 3, and 7 d after MRI, 4 rabbits each time in the experimental group and 2 rabbits each time in the control group were sacrificed for histological examination. Rabbit liver tissue blocks were fixed in 4% paraformaldehyde and were processed for paraffin embedding. Microsections (4 μm) were prepared with a microtome and were used for Prussian blue staining to detect labeled iron particles in the cells. An experienced board-certified pathologist calculated the number of Prussian blue stain positive cells per high power field (HPF) in each group. Prussian blue stain positive cells were counted in at least three HPFs per section and a minimum of six sections were examined.
The Kruskall-Wallis test was used to evaluate differences in SI changes and Prussian blue stain positive cells. P value of less than 0.05 was considered to indicate a statistical significance, and statistical computer software (SPSS 12.0; SPSS, Chicago, Ill) was used.
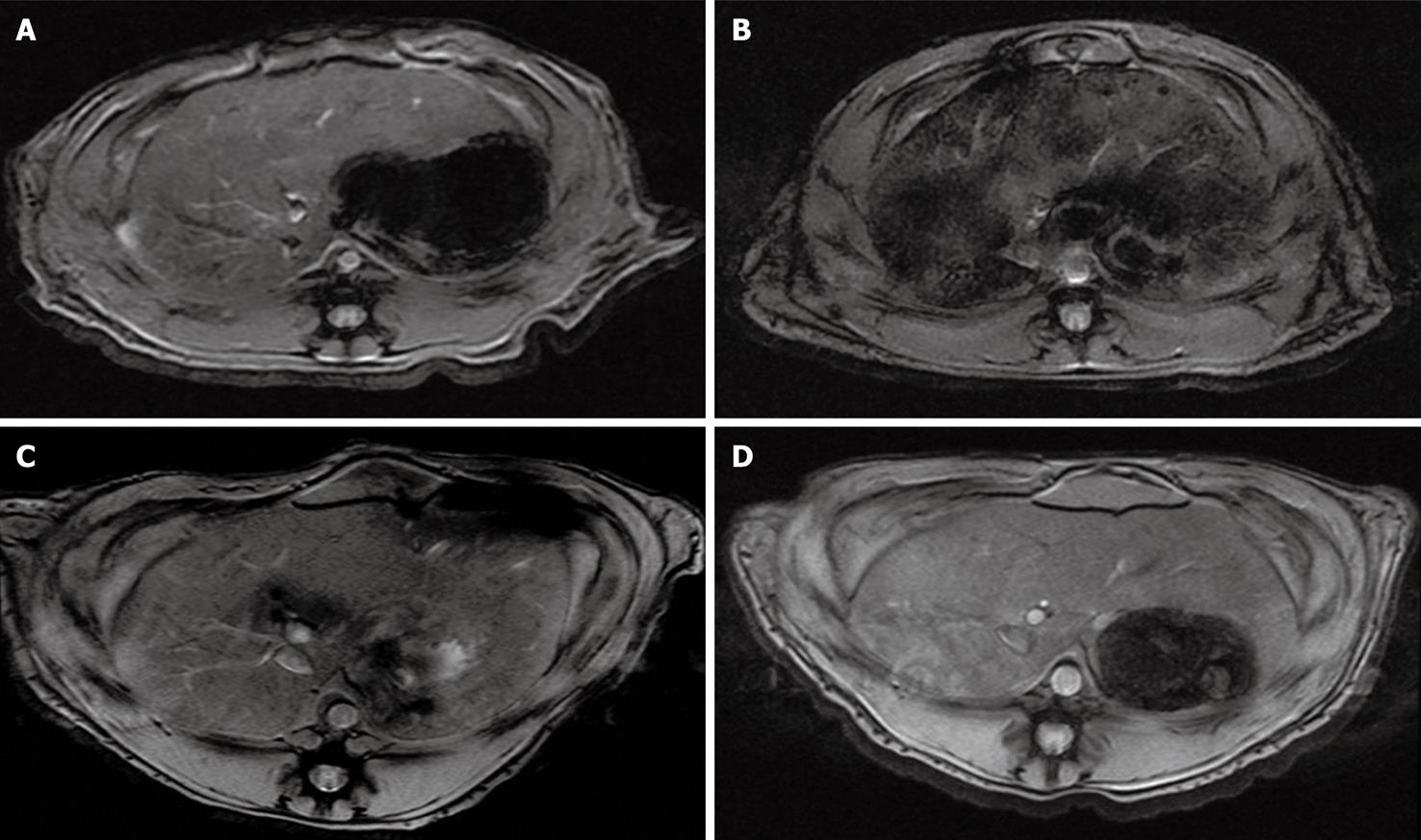
In the experimental group (n = 16), axial T2*-weighted images of the liver demonstrated a significant signal loss of fine granular signal voids 2 h after intravascular administration of SPIO-labeled cells in comparison to baseline (Figure 1). The change in SI of the liver on T2*-weighted images peaked at 2 h after injection and subsequently declined. Changes in SI in the liver at 2 h, 1, 3 and 7 d were 41.87% ± 9.63%, 10.42% ± 4.3%, 5.12% ± 1.9%, 3.75% ± 1.2%, respectively (P < 0.001). In the control group (n = 8), no overt signal changes of the liver were observed.
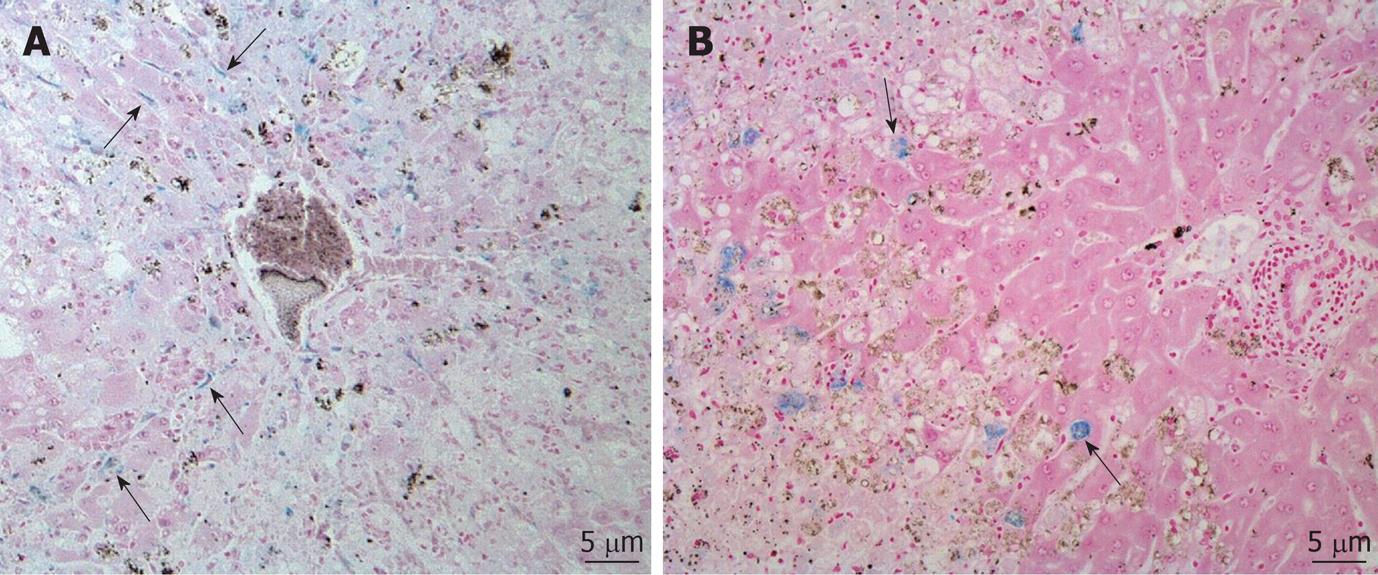
CCl4 treatment of rabbits induced typical histopathological changes indicative of persistent damage and fibrotic changes in the liver. Prussian blue staining studies showed that the iron-containing cells were mainly distributed in the portal triad regions throughout the liver 2 h and 1 d after injection of human MSCs. These cells showed amorphous shapes with positive iron staining in their cytoplasms and tended to localize along the sinusoids (Figure 2A). However, the iron-positive cells in the experimental group mainly appeared in the border zone between normal liver parenchyma and hepatic injury areas 3 and 7 d after injection (Figure 2B). The number of iron-positive cells in the experimental group at 2 h and on 1, 3 and 7 d after injection was 29.2 ± 4.8, 10.1 ± 3.7, 6.7 ± 2.2, and 5.8 ± 2.1, respectively (P = 0.013). In the control group, Prussian blue positively stained cells were absent from the liver.
It is widely accepted that efficient monitoring of the distribution, migration, and differentiation of transplanted stem cells holds the key to the development of effective methods in stem cell therapy[16]. Because of its high resolution and sensitivity, MRI has been regarded as a method of first choice compared to other imaging modalities. MRI can be broadly defined as a non-invasive and repetitive modality of imaging for targeted cells and cellular processes[17], which entails proper labeling of cells with appropriate magnetic resonance (MR) contrast agents.
In most reports that have described MRI of grafted stem cells, local implantation has been used as the route for cell grafting into the rat brain and spine[18,19]. After implantation, these cells are concentrated in a localized area rather than dispersed throughout the target organ, as occurs after intravascular injection. These locally implanted stem cells were observed to migrate only at a rate of a few millimeters a week[18-20]. This stereotactic method can hardly be applied in a hepatic failure model because liver damage often has a widespread distribution. Thus, we injected MSCs into the mesenteric vein, as a large number of MSCs could be directly transferred to the whole liver.
Our results and others[21] demonstrate that MSCs are particularly present along the sinusoids where the cells can be mechanically trapped after injection into the portal vein. Recently, the process of migration of stem cells from the spleen to the liver in rats was tracked in vivo by labeling stem cells with superparamagnetic particles[12], which showed that the majority of iron-positive cells appeared in the liver at 3 h of post-transplantation. This is similar to our findings. The results together suggest that direct intravascular transplantation is an efficient way of delivering MSCs to the liver.
The histological data obtained in the present study showed that Prussian blue stain-positive cells were found mainly in the border zone between normal liver parenchyma and the hepatic injury areas 3 and 7 d after injection. The exact reason why MSCs were concentrated in the border zone in liver damage is not known. This phenomenon may be caused by secreted cytokines such as hepatocyte growth factor which is secreted by mesenchymal cells and has a major role in adult organ regeneration and in wound healing[22]. We speculate MSCs might contribute the healing process of liver damage.
SI on T2*-weighted images decreased substantially in the liver 2 h after injection of MSCs and gradually returned to the levels found before injection. The gradual increase of SI in the liver may be the result of mobilization of labeled cells out of the liver, dilution of intracellular iron as a result of cell division, or cell death resulting from immune rejection[12]. In our study, SI decreased more quickly than that reported in other study[12] and this was probably due to the use of human MSCs in our study that induces more potent immune rejection than previous study.
There are some limitations to this study. First, we used only commercially available human MSCs, and not rabbit MSCs, because we focused on the validity of MRI for the depiction of SPIO-labeled MSCs in hepatic failure model rather than to access recovery of hepatic function. Second, we did not perform immunohistochemical analysis on human MSCs under the conditions that we tested with the rabbit model, and therefore could not confirm whether Prussian blue stained positive cells were associated with SPIO-labeled MSCs in vivo.
In conclusion, SPIO-labeled MSCs could be detected in vivo by the use of a clinically available 3-Tesla MR unit after intravascular injection in a rabbit model of hepatic failure.
Stem cell transplantation provides an effective strategy for the treatment of liver failure. Noninvasive and repeated monitoring of in vivo transplanted stem cells is one of important topics in these days.
In vivo real-time tracking and detecting the fate of transplanted stem cells by magnetic resonance imaging (MRI) would be a good way to evaluate the ability of cells to migrate and engraft to target organs. However, few studies have been undertaken to determine the feasibility of the use of in vivo MRI of cell therapy in models of hepatic failure.
This study showed the possibility of stem cell tracking by the use of a clinically available 3-Tesla magnetic resonance unit.
In vivo real-time tracking transplanted stem cells by MRI might facilitate clinical use of stem cell.
Mesenchymal stem cells: multipotent stem cells that can differentiate into a variety of cell types including osteoblasts (bone cells), chondrocytes (cartilage cells) and adipocytes (fat cells).
It is a well-written but moderately designed study, with interesting and important scientific merit.
Peer reviewer: Dr. Ferenc Sipos, 2nd Department of Internal Medicine, Semmelweis University, Szentkirályi u. 46. Budapest, 1088, Hungary
S- Editor Wang YR L- Editor O’Neill M E- Editor Zheng XM
| 1. | Tuñón MJ, Alvarez M, Culebras JM, González-Gallego J. An overview of animal models for investigating the pathogenesis and therapeutic strategies in acute hepatic failure. World J Gastroenterol. 2009;15:3086-3098. |
| 2. | Possamai LA, Antoniades CG, Anstee QM, Quaglia A, Vergani D, Thursz M, Wendon J. Role of monocytes and macrophages in experimental and human acute liver failure. World J Gastroenterol. 2010;16:1811-1819. |
| 3. | Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168-1170. |
| 4. | Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229-1234. |
| 5. | Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291-1302. |
| 6. | Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490-1494. |
| 7. | Shupe TD, Piscaglia AC, Oh SH, Gasbarrini A, Petersen BE. Isolation and characterization of hepatic stem cells, or "oval cells," from rat livers. Methods Mol Biol. 2009;482:387-405. |
| 8. | Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, Chen JR, Chen YP, Lee OK. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275-1284. |
| 9. | Sato Y, Araki H, Kato J, Nakamura K, Kawano Y, Kobune M, Sato T, Miyanishi K, Takayama T, Takahashi M. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005;106:756-763. |
| 10. | Zhao DC, Lei JX, Chen R, Yu WH, Zhang XM, Li SN, Xiang P. Bone marrow-derived mesenchymal stem cells protect against experimental liver fibrosis in rats. World J Gastroenterol. 2005;11:3431-3440. |
| 11. | Modo M, Cash D, Mellodew K, Williams SC, Fraser SE, Meade TJ, Price J, Hodges H. Tracking transplanted stem cell migration using bifunctional, contrast agent-enhanced, magnetic resonance imaging. Neuroimage. 2002;17:803-811. |
| 12. | Ju S, Teng GJ, Lu H, Zhang Y, Zhang A, Chen F, Ni Y. In vivo MR tracking of mesenchymal stem cells in rat liver after intrasplenic transplantation. Radiology. 2007;245:206-215. |
| 13. | Frank JA, Miller BR, Arbab AS, Zywicke HA, Jordan EK, Lewis BK, Bryant LH Jr, Bulte JW. Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology. 2003;228:480-487. |
| 14. | Jung SI, Kim SH, Kim HC, Son KR, Chung SY, Moon WK, Kim HS, Choi JS, Moon MH, Sung CK. In vivo MR imaging of magnetically labeled mesenchymal stem cells in a rat model of renal ischemia. Korean J Radiol. 2009;10:277-284. |
| 15. | Firbank MJ, Coulthard A, Harrison RM, Williams ED. A comparison of two methods for measuring the signal to noise ratio on MR images. Phys Med Biol. 1999;44:N261-N264. |
| 16. | Bulte JW. In vivo MRI cell tracking: clinical studies. AJR Am J Roentgenol. 2009;193:314-325. |
| 17. | Modo M, Hoehn M, Bulte JW. Cellular MR imaging. Mol Imaging. 2005;4:143-164. |
| 18. | Bulte JW, Zhang S, van Gelderen P, Herynek V, Jordan EK, Duncan ID, Frank JA. Neurotransplantation of magnetically labeled oligodendrocyte progenitors: magnetic resonance tracking of cell migration and myelination. Proc Natl Acad Sci USA. 1999;96:15256-61. |
| 19. | Bulte JW, Douglas T, Witwer B, Zhang SC, Strable E, Lewis BK, Zywicke H, Miller B, van Gelderen P, Moskowitz BM. Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat Biotechnol. 2001;19:1141-1147. |
| 20. | Hoehn M, Küstermann E, Blunk J, Wiedermann D, Trapp T, Wecker S, Föcking M, Arnold H, Hescheler J, Fleischmann BK. Monitoring of implanted stem cell migration in vivo: a highly resolved in vivo magnetic resonance imaging investigation of experimental stroke in rat. Proc Natl Acad Sci USA. 2002;99:16267-16272. |
| 21. | Bos C, Delmas Y, Desmoulière A, Solanilla A, Hauger O, Grosset C, Dubus I, Ivanovic Z, Rosenbaum J, Charbord P. In vivo MR imaging of intravascularly injected magnetically labeled mesenchymal stem cells in rat kidney and liver. Radiology. 2004;233:781-789. |
| 22. | Funakoshi H, Nakamura T. Hepatocyte growth factor: from diagnosis to clinical applications. Clin Chim Acta. 2003;327:1-23. |