Published online Nov 21, 2010. doi: 10.3748/wjg.v16.i43.5467
Revised: August 27, 2010
Accepted: September 4, 2010
Published online: November 21, 2010
AIM: To analyze plasma osteopontin levels and liver stiffness using transient elastography in postoperative biliary atresia (BA) children compared with healthy controls.
METHODS: Thirty children with postoperative BA and 10 normal controls were enrolled. The patients were categorized into two groups according to their jaundice status. Plasma levels of osteopontin were determined using commercially available enzyme-linked immunosorbent assay. Liver stiffness was measured by using transient elastography (Fibroscan). Ten validated Fibroscan measurements were performed in each patient and control with the result expressed in kilopascals (kPa).
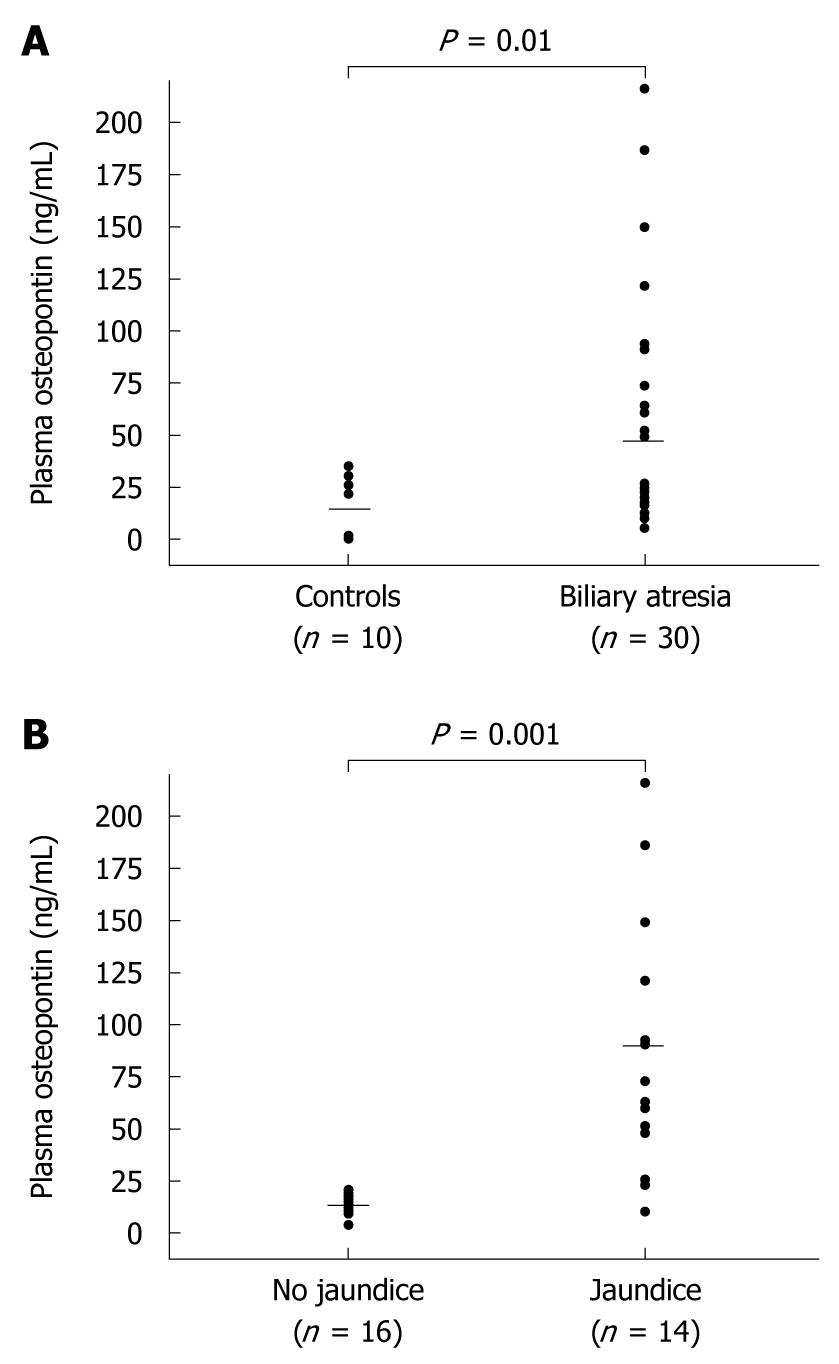
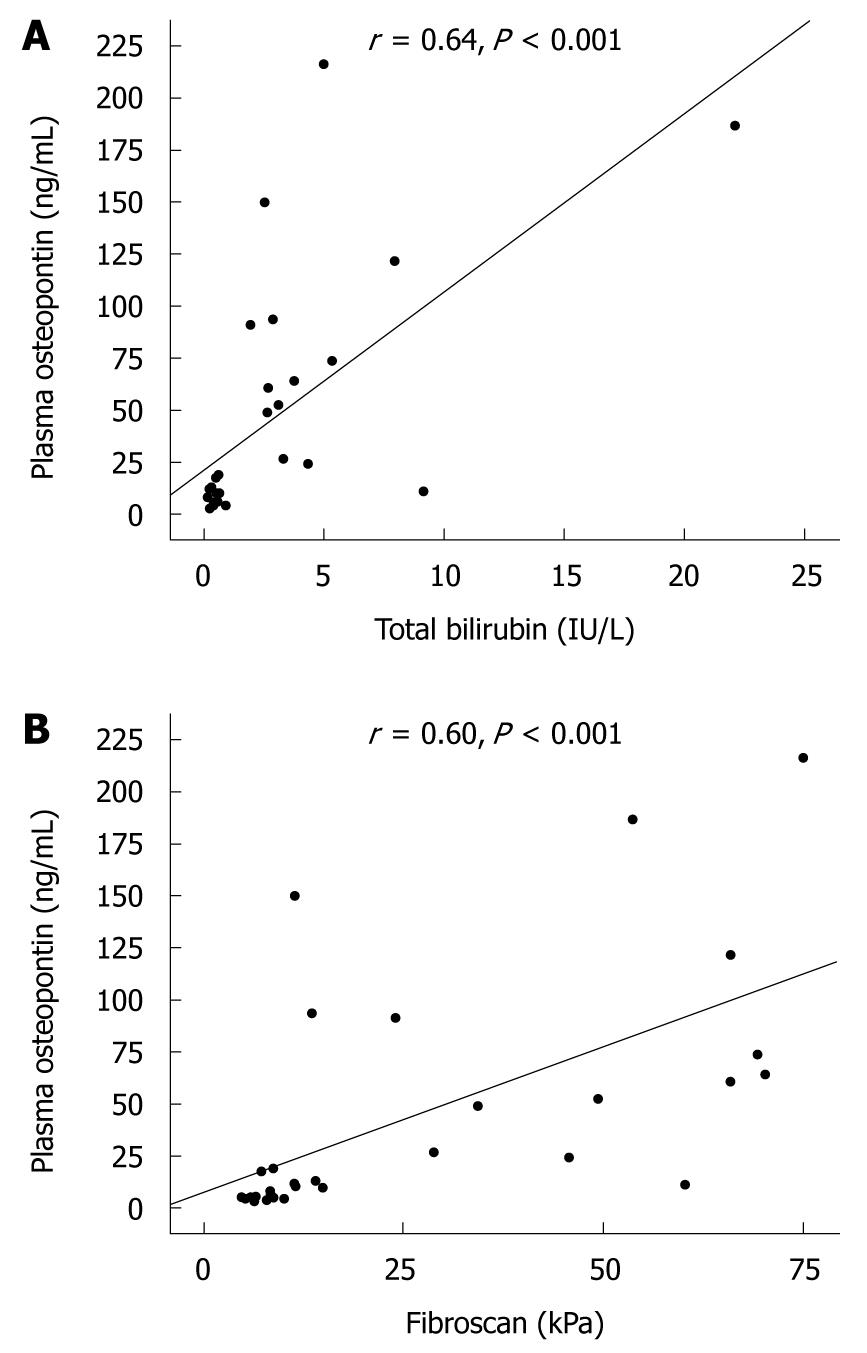
RESULTS: Plasma osteopontin was significantly elevated in BA children compared with that of healthy controls (47.0 ± 56.4 ng/mL vs 15.1 ± 15.0 ng/mL, P = 0.01). The liver stiffness measurement was markedly elevated in the patients with BA compared with that of controls (26.9 ± 24.6 kPa vs 3.9 ± 0.7 kPa, P = 0.001). Subgroup analysis showed that the BA patients with jaundice had more pronounced plasma osteopontin levels than those without jaundice (87.1 ± 61.6 ng/mL vs 11.9 ± 6.1 ng/mL, P = 0.001). Furthermore, the mean liver stiffness was significantly greater in the jaundiced BA patients compared with non-jaundiced patients (47.7 ± 21.8 kPa vs 8.7 ± 3.0 kPa, P = 0.001). Additionally, plasma osteopontin was positively related to serum total bilirubin (r = 0.64, P < 0.001). There was also a correlation between plasma osteopontin and liver stiffness values (r = 0.60, P < 0.001).
CONCLUSION: High plasma osteopontin positively correlated with degree of hepatic fibrosis and could be used as a biochemical parameter reflecting disease severity in postoperative BA children.
- Citation: Honsawek S, Chayanupatkul M, Chongsrisawat V, Vejchapipat P, Poovorawan Y. Increased osteopontin and liver stiffness measurement by transient elastography in biliary atresia. World J Gastroenterol 2010; 16(43): 5467-5473
- URL: https://www.wjgnet.com/1007-9327/full/v16/i43/5467.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i43.5467
Biliary atresia (BA) is an intractable liver disease of unknown cause that affects hepatic bile ducts resulting in chronic cholestasis, hepatic fibrosis and biliary cirrhosis. BA children mostly present with a triad of obstructive jaundice, acholic stools, and hepatosplenomegaly. It is the most common indication for liver transplantation in infants and children[1]. Even after effective bile flow has been established by the Kasai operation, the disease still progresses to end-stage liver disease, in which patients will suffer from the complication of portal hypertension and hepatic dysfunction[2,3]. Several etiologies have been considered to account for the pathogenesis of BA, including neonatal viral infections, genetic insults, and abnormalities in immune response, but the precise mechanism of BA is poorly understood[4]. Although it has been documented that a variety of cytokines and growth factors, including bone morphogenetic protein 7[5], basic fibroblast growth factor[6], and stem cell factor[7], play essential roles in the pathophysiology of BA, published data regarding osteopontin expression in postoperative BA is currently limited.
Osteopontin, also known as early T-cell activation gene-1 (Eta-1), is a secreted phosphoprotein 1 (SPP1) that has been implicated in the pathogenesis of various inflammatory and fibrotic disorders. It stimulates T cell proliferation and induces T cells and macrophages to express other T helper type 1 (Th1) cytokines during inflammation[8]. Osteopontin also induces accumulation of extracellular matrix by binding to type I collagen, fibronectin, and osteocalcin, contributing to tissue fibrosis[9,10]. Osteopontin comprises multiple functional domains, with a high sialic acid content, an aspartate-rich domain, calcium-binding domain, thrombin cleavage site, and many residues with consensus for phosphorylation as well as an integrin-binding arginine-glycine-aspartate (RGD) motif, which play a key role in several inflammatory disorders[11]. High levels of circulating osteopontin have been demonstrated in patients with multiple sclerosis[12], osteoarthritis[13], hepatitis C viral infection[14], as well as gastric and liver cancer[15-19]. However, no detailed studies are presently available regarding the relationship between plasma osteopontin levels and the degree of liver stiffness in BA.
It has been recently reported that measurement of liver stiffness using transient elastography or Fibroscan (Echosens, Paris, France) reflects the degree of hepatic fibrosis, which is a principal factor determining the functional liver reserve[20]. In this study, we postulated that elevated plasma osteopontin levels might be associated with the severity of clinical outcomes and the liver stiffness in BA patients. Hence, the objective of this investigation was to analyze plasma osteopontin levels in postoperative BA patients and to evaluate the possible correlations of plasma osteopontin with the disease severity.
This investigation was approved by the Institutional Review Board of the Faculty of Medicine, Chulalongkorn University, and was conducted in agreement with the Declaration of Helsinki. All parents of children with BA and healthy controls were informed of the study’s objectives, and written informed consent was obtained from the parents prior to the children entering the study.
A total of 30 BA patients after Kasai procedure (13 males and 17 females; mean age 7.2 ± 3.4 years) who attended the follow-up visit at the Pediatric Liver Clinic, and 10 healthy children (4 males and 6 females; mean age 7.4 ± 3.9 years) from the Well Baby Clinic at King Chulalongkorn Memorial Hospital, were enrolled in this study. Children in the healthy control group had normal physical examinations.
Among the 30 BA patients in the present study, none had any symptoms and signs of infection or ascending cholangitis or clotting abnormalities at the time of blood sampling. None had received liver transplantation. To compare the clinical outcomes among BA patients, they were categorized according to their levels of serum total bilirubin (TB) into two groups: patients without jaundice (TB < 20 mg/L, n = 16) and patients with persistent jaundice (TB ≥ 20 mg/L, n = 14).
Liver stiffness was measured using Fibroscan (Echosens, Paris, France), a new medical device based on elastometry (or one-dimensional transient elastography). This technique is used to quantify hepatic fibrosis in a totally noninvasive and painless manner, with no contraindications. Details of the technique and the examination procedure have been described in a previous report[21]. Briefly, this system is operated with a probe, with an ultrasonic transducer mounted on the axis of a vibrator. A vibration of mild amplitude and low frequency is transmitted from the vibrator on to the tissue by the transducer itself, which induces propagation of an elastic shear wave through the tissue. A pulse-echo acquisition is performed at this time to follow the propagation of the shear wave and measure its velocity. This velocity is directly correlated to the stiffness of liver, which reflects the degree of fibrosis. Results are expressed in kilopascals (kPa).
All measurements were performed according to the manufacturer’s instructions. Patients were placed in supine decubitus position with the right arm in abduction. The probe was placed on the skin between 2 ribs at the level of the right lobe of the liver. The measurement area was located by A-mode images provided by the probe transducer. Ten validated measurements were performed for each patient. The median value was considered as representative of liver stiffness. A set of measurements was considered to be reliable if the success rate was at least 60% and the interquartile range was less than one third of the median liver stiffness value. Because normal values have not yet been established in healthy children without liver disease, a control group of 10 healthy gender- and age-matched children was recruited. Measurements were performed by the same operator under the same conditions as for the BA patients.
Samples of peripheral venous blood were collected from each patient and healthy control, centrifuged, and then stored at -80°C until analysis. Plasma osteopontin concentrations were measured using a commercially available enzyme-linked immunosorbent assay (Immuno-Biological Laboratories Co., Gunma, Japan) following the manufacturer’s recommendations. In brief, standards of recombinant human osteopontin and plasma samples were added to microtiter plates pre-coated with rabbit polyclonal antibody against osteopontin and incubated for 1 h at room temperature. The wells were then washed 7 times with washing buffer and incubated for 30 min at 4°C with a horseradish peroxidase-labeled mouse monoclonal antibody to human osteopontin. After washing thoroughly with washing buffer for 9 times, substrate solution was added to each well, and the plate was incubated for 30 min at room temperature in the dark. Finally, the reaction was stopped with the stop solution, and then absorbance was measured at 450 nm using an automated microtiter plate reader. Recombinant human osteopontin was used to generate a linear standard calibration curve (range 5-320 ng/mL). In addition, liver function tests, including TB, direct bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP), were assessed using a Hitachi 912 automated chemical analyzer at the central laboratory of our hospital. The aspartate aminotransferase to platelets ratio index (APRI) was calculated as follows: (AST/upper limit of normal) × 100/platelet count (109/L)[22].
Statistical analysis was performed using the statistical package for social sciences (SPSS) software, version 16.0 for Windows. All values are expressed as a mean ± SD. Comparisons of demographic data and biochemical parameters between groups were determined by unpaired t-test. Correlations between numerical data were acquired using the Pearson correlation coefficient (r). A P-value < 0.05 indicated statistical significance.
Plasma osteopontin levels were measured in 30 BA patients and 10 healthy controls. There were no statistically significant differences with respect to age (7.2 ± 3.4 years vs 7.4 ± 3.9 years) and gender (male:female, 13:17 vs 4:6) between the BA patients and healthy controls. The baseline characteristics of the BA patients and healthy controls are summarized in Table 1. Mean plasma osteopontin concentration of BA children was significantly higher in comparison with that of healthy controls (47.0 ± 56.4 ng/mL vs 15.1 ± 15.0 ng/mL, P = 0.01) (Figure 1A). Furthermore, the mean liver stiffness value was markedly elevated in the patients with BA compared with that of healthy controls (26.9 ± 24.6 kPa vs 3.9 ± 0.7 kPa, P = 0.001).
| Variables | Controls (n = 10) | BA patients (n = 30) | P-value |
| Age (yr) | 7.4 ± 3.9 | 7.2 ± 3.4 | 0.8 |
| Gender (M:F) | 4:6 | 13:17 | 0.5 |
| Albumin (g/L) | - | 43 ± 0.5 | NA |
| Total bilirubin (mg/L) | - | 28 ± 4.3 | NA |
| Direct bilirubin (mg/L) | - | 22 ± 4.3 | NA |
| AST (IU/L) | - | 129.4 ± 100.8 | NA |
| ALT (IU/L) | - | 119.4 ± 105.1 | NA |
| ALP (IU/L) | - | 469.9 ± 345.6 | NA |
| Platelet count (103/mm3) | - | 167.2 ± 96.3 | NA |
| APRI | - | 2.9 ± 2.9 | NA |
| Osteopontin (ng/mL) | 15.1 ± 15.0 | 47.0 ± 56.4 | 0.01 |
| Liver stiffness (kPa) | 3.9 ± 0.7 | 26.9 ± 24.6 | 0.001 |
The demographic data and biochemical parameters including liver function tests, plasma osteopontin levels and liver stiffness values based on jaundice status are given in Table 2. Subgroup analysis demonstrated that BA patients with persistent jaundice had significantly higher serum levels of AST, ALT, ALP, and APRI than those without jaundice. As shown in Figure 1B, the BA patients with persistent jaundice had more pronounced plasma osteopontin levels than those without jaundice (87.1 ± 61.6 ng/mL vs 11.9 ± 6.1 ng/mL, P = 0.001). There was no significant difference in plasma osteopontin between BA patients without jaundice and healthy controls. In addition, the mean liver stiffness was significantly greater in the jaundiced BA patients compared with non-jaundiced patients (47.7 ± 21.8 kPa vs 8.7 ± 3.0 kPa, P = 0.001). Moreover, plasma osteopontin was positively related to serum TB (r = 0.64, P < 0.001) (Figure 2A). There was also a correlation between plasma osteopontin and liver stiffness score (r = 0.60, P < 0.001) (Figure 2B).
| Variables | BA patients without jaundice (n = 16) | BA patients with jaundice (n = 14) | P-value |
| Age (yr) | 7.3 ± 3.7 | 7.0 ± 3.1 | 0.9 |
| Gender (M:F) | 7:9 | 6:8 | 0.5 |
| Albumin (g/L) | 46 ± 0.3 | 39 ± 0.4 | 0.001 |
| Total bilirubin (mg/L) | 5 ± 0.2 | 55 ± 5.5 | 0.001 |
| Direct bilirubin (mg/L) | 2 ± 0.1 | 46 ± 5.5 | 0.003 |
| AST (IU/L) | 64.9 ± 46.9 | 203.1 ± 95.5 | 0.001 |
| ALT (IU/L) | 73.6 ± 61.2 | 171.8 ± 121.5 | 0.01 |
| ALP (IU/L) | 287.9 ± 116.8 | 677.9 ± 404.1 | 0.001 |
| Platelet count (103/mm3) | 215.8 ± 81.3 | 111.6 ± 81.0 | 0.002 |
| APRI | 0.8 ± 0.8 | 5.1 ± 2.7 | < 0.001 |
| Osteopontin (ng/mL) | 11.9 ± 6.1 | 87.1 ± 61.6 | 0.001 |
| Liver stiffness (kPa) | 8.7 ± 3.0 | 47.7 ± 21.8 | 0.001 |
BA is one of the most serious digestive tract disorders characterized by progressive, fibrosclerotic cholangiopathy affecting both intrahepatic and extrahepatic bile ducts. It may lead to obstruction or discontinuity of the biliary tract at any point between the porta hepatis and the duodenum. Without medical and surgical treatment, the majority of BA patients will develop severe cholestasis, hepatic fibrosis, and eventually die within a few years[1]. It is accepted that Kasai hepatoportoenterostomy is the first line of surgical treatment. Despite early diagnosis and successful Kasai operation, more than half of the BA patients inevitably develop biliary cirrhosis, portal hypertension, and end-stage liver disease[2]. Alternatively, liver transplantation is an effective treatment modality when the Kasai hepatoportoenterostomy has failed and serious complications occur such as recurrent ascending cholangitis, persistent jaundice, cirrhosis, progressive ascites, and bleeding esophageal varices[3]. Nonetheless, the exact pathophysiology of liver fibrosis or cirrhosis in BA children remains unknown.
Osteopontin is a highly phosphorylated acidic glycoprotein that is not only present in bone, but also is produced by a number of cell types including chondrocytes, immune cells, smooth muscle cells, epithelial cells, and endothelial cells[23,24]. It is now considered as a potent chemo-attractive and pro-inflammatory mediator which is involved in a variety of physiologic and pathologic events, such as cell adhesion, proliferation, migration, inflammation, apoptosis, vascular remodeling, and wound healing[25,26]. The role of osteopontin in liver diseases has not been completely elucidated. Carbon tetrachloride administration has been shown to increase osteopontin expression in the rat liver where it was localized mostly to activated Kupffer cells, hepatic macrophages, and stellate cells[27,28]. Recombinant osteopontin also activated rat hepatic macrophage migration in vitro, whereas in vivo, the levels of macrophage infiltration in injured liver were reduced in osteopontin-knockout mice[28]. These findings indicate that osteopontin may be involved in the initiation of inflammatory reactions in the liver and activation of hepatic fibrosis.
In recent years, the upregulation of osteopontin expression has been documented by using gene expression microarray and Northern blot analysis of liver samples from BA patients[29]. It has been shown in previous studies that gene expression of hepatic osteopontin is highly upregulated in BA. This finding suggests that human bile duct epithelial cells are able to synthesize osteopontin[30,31]. These observations prompted us to speculate that osteopontin may be responsible for the pathogenesis of BA. However, plasma osteopontin level at various clinical stages of BA and its possible role in BA patients has not received much attention.
In the present study, we demonstrated that plasma osteopontin levels in BA patients were significantly elevated compared to those in healthy controls. Further analysis revealed that plasma osteopontin levels were markedly higher in BA patients with persistent jaundice than those without jaundice. High plasma osteopontin was positively correlated with serum TB in postoperative BA patients. These findings suggest that plasma osteopontin is associated with jaundice status in postoperative BA patients. Additionally, jaundice status in BA patients is likely to be a parameter for intrahepatic biliary obstruction. Hence, these results suggest that osteopontin plays a possible role in the pathogenesis of hepatocellular damage in BA, and that it seems to be correlated with the degree of biliary obstruction.
To the best of our knowledge, this study is the first to show that plasma osteopontin is elevated in BA patients compared with healthy controls, and that osteopontin concentration is associated with clinical outcome (status of jaundice, hepatic dysfunction, and hepatic fibrosis) in BA. Increased plasma osteopontin has been documented in a variety of liver disorders, including acute hepatic dysfunction, chronic hepatitis, liver cirrhosis, hepatocellular carcinoma, and primary biliary cirrhosis[16-19]. In accordance with our study, plasma osteopontin was shown to be significantly elevated in cirrhosis patients, and was correlated with the severity of hepatic injury[17]. Kim et al[19] also revealed an elevation of plasma osteopontin with advancing degree of hepatocellular carcinoma. These findings suggest that high plasma osteopontin is associated with hepatic damage and hence reflects liver dysfunction. Accordingly, our results also showed that plasma osteopontin was positively associated with degree of liver stiffness determined using transient elastography or Fibroscan. Liver stiffness measures are well correlated with advanced stages of hepatic fibrosis and cirrhosis in adults and children[20,32,33]. These findings suggest that plasma osteopontin could serve as a potential biochemical parameter for measuring progression of liver impairment and development of hepatic fibrosis in postoperative BA patients and, therefore, may be predictive of prognosis with respect to the progression of liver dysfunction.
Several possible mechanisms could contribute to high plasma osteopontin in BA. Firstly, production of osteopontin in the damaged liver may lead to elevation of plasma osteopontin. Secondly, elevated osteopontin levels could be attributed to an imbalance between osteopontin production and osteopontin clearance. In advanced BA stages, reduced osteopontin clearance could be responsible for increased circulating osteopontin levels. Additionally, because other organs apart from the liver can synthesize and secrete osteopontin, the main sources of elevated plasma osteopontin in the present study might be extrahepatic organs. Further research will be necessary to clarify this observation.
Inevitably, we are aware of some limitations in our study. Firstly, the sample size of patients enrolled in this study was small and could preclude us from making a strong conclusion. Secondly, incomplete assessment of potential confounding factors (age, sex, medical comorbidities) needs to be taken into consideration. Lastly, as this study was designed as a cross-sectional study, a definite cause and effect relationship cannot be concluded. In order to overcome these limitations, a well-designed, well-controlled, randomized study of a large population will be needed to draw a more definite conclusion. However, with the supporting evidence from other studies regarding the potential role of osteopontin expression and the degree of systemic inflammatory response in BA[29-31], it is likely that the high plasma osteopontin levels observed in postoperative BA patients may be involved in the pathophysiology of hepatocellular injury and development of hepatic fibrosis. Biochemical parameters and clinical characteristics of some BA subjects have been reported recently[34]. We have not previously reported liver stiffness measurement or the relationship of liver stiffness with osteopontin in BA.
In conclusion, this investigation showed that BA patients had significantly elevated concentrations of plasma osteopontin in comparison with healthy controls. Plasma osteopontin and liver stiffness values were remarkably higher in the BA patients with persistent jaundice than in those without jaundice. Moreover, there was a positive correlation between plasma osteopontin, TB and liver fibrosis. Delineation of the mechanisms underlying bile duct injury will be advantageous in the development of new potential therapeutic treatments for this serious pediatric disorder.
Biliary atresia (BA) is an intractable liver disease of unknown cause that affects hepatic bile ducts resulting in chronic cholestasis, hepatic fibrosis and biliary cirrhosis. Human bile duct epithelial cells are able to synthesize osteopontin, a highly phosphorylated acidic glycoprotein.
Osteopontin has been implicated in the pathogenesis of various inflammatory and fibrotic disorders. However, no detailed studies are presently available on the relationship between plasma osteopontin levels and the degree of liver stiffness in BA. In this study, the authors demonstrate that elevated plasma osteopontin levels could be associated with the severity of clinical outcomes and the liver stiffness in BA patients.
Recent reports have highlighted the importance of hepatic osteopontin expression in chronic liver disease, including BA. This is the first study to report that plasma osteopontin is elevated in BA patients compared with healthy controls, and that osteopontin concentration is associated with status of jaundice and hepatic dysfunction in BA.
High plasma osteopontin levels positively correlate with the degree of hepatic fibrosis in postoperative BA children. Therefore, plasma osteopontin may be used as a biochemical parameter reflecting disease severity and for monitoring the progression of liver fibrosis in BA patients after Kasai operation.
Osteopontin, also known as early T-cell activation gene-1, is a secreted phosphoprotein 1 that comprises multiple functional domains, with a high sialic acid content, an aspartate-rich domain, calcium-binding domain, thrombin cleavage site, and many residues with consensus for phosphorylation as well as an integrin-binding arginine-glycine-aspartate motif, which play a key role in several inflammatory disorders.
Nice article about BA. I am unsure what the mechanism of the osteopontin levels are, but further research should surely tell us.
Peer reviewer: Michael E Zenilman, MD, Clarence and Mary Dennis Professor and Chairman, Department of Surgery, SUNY Downstate Medical Center, Box 40, 450 Clarkson Avenue, Brooklyn, NY 11202, United States
S- Editor Sun H L- Editor Logan S E- Editor Lin YP
| 2. | Bassett MD, Murray KF. Biliary atresia: recent progress. J Clin Gastroenterol. 2008;42:720-729. |
| 3. | Erlichman J, Hohlweg K, Haber BA. Biliary atresia: how medical complications and therapies impact outcome. Expert Rev Gastroenterol Hepatol. 2009;3:425-434. |
| 4. | A-Kader HH, Abdel-Hameed A, Al-Shabrawi M, Mohsen N, El-Karaksy H, Hassanein B, Elsayed B, Abdel-Khalik MK, Karjoo M. Is biliary atresia an autoimmune disease? Eur J Gastroenterol Hepatol. 2003;15:447. |
| 5. | Chayanupatkul M, Honsawek S, Vejchapipat P, Chongsrisawat V, Poovorawan Y. Elevated serum bone morphogenetic protein 7 levels and clinical outcome in children with biliary atresia. Eur J Pediatr Surg. 2009;19:246-250. |
| 6. | Honsawek S, Chongsrisawat V, Vejchapipat PI, Thawornsuk N, Poovorawan Y. High levels of serum basic fibroblast growth factor in children with biliary atresia. Hepatogastroenterology. 2008;55:1184-1188. |
| 7. | Honsawek S, Chongsrisawat V, Vejchapipat P, Thawornsuk N, Tangkijvanich P, Poovorawan Y. Elevation of serum stem-cell factor in postoperative biliary atresia. Pediatr Int. 2007;49:888-893. |
| 8. | Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, Rittling SR, Denhardt DT, Glimcher MJ, Cantor H. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:860-864. |
| 9. | McKee MD, Nanci A, Khan SR. Ultrastructural immunodetection of osteopontin and osteocalcin as major matrix components of renal calculi. J Bone Miner Res. 1995;10:1913-1929. |
| 10. | Suzuki H, Amizuka N, Oda K, Li M, Yoshie H, Ohshima H, Noda M, Maeda T. Histological evidence of the altered distribution of osteocytes and bone matrix synthesis in klotho-deficient mice. Arch Histol Cytol. 2005;68:371-381. |
| 12. | Comabella M, Pericot I, Goertsches R, Nos C, Castillo M, Blas Navarro J, Río J, Montalban X. Plasma osteopontin levels in multiple sclerosis. J Neuroimmunol. 2005;158:231-239. |
| 13. | Honsawek S, Tanavalee A, Sakdinakiattikoon M, Chayanupatkul M, Yuktanandana P. Correlation of plasma and synovial fluid osteopontin with disease severity in knee osteoarthritis. Clin Biochem. 2009;42:808-812. |
| 14. | Huang W, Zhu G, Huang M, Lou G, Liu Y, Wang S. Plasma osteopontin concentration correlates with the severity of hepatic fibrosis and inflammation in HCV-infected subjects. Clin Chim Acta. 2010;411:675-678. |
| 15. | Wu CY, Wu MS, Chiang EP, Wu CC, Chen YJ, Chen CJ, Chi NH, Chen GH, Lin JT. Elevated plasma osteopontin associated with gastric cancer development, invasion and survival. Gut. 2007;56:782-789. |
| 16. | Arai M, Yokosuka O, Kanda T, Fukai K, Imazeki F, Muramatsu M, Seki N, Miyazaki M, Ochiai T, Hirasawa H. Serum osteopontin levels in patients with acute liver dysfunction. Scand J Gastroenterol. 2006;41:102-110. |
| 17. | Zhao L, Li T, Wang Y, Pan Y, Ning H, Hui X, Xie H, Wang J, Han Y, Liu Z. Elevated plasma osteopontin level is predictive of cirrhosis in patients with hepatitis B infection. Int J Clin Pract. 2008;62:1056-1062. |
| 18. | Matsui A, Mochida S, Ohno A, Nagoshi S, Hirose T, Fujiwara K. Plasma osteopontin levels in patients with fulminant hepatitis. Hepatol Res. 2004;29:202-206. |
| 19. | Kim J, Ki SS, Lee SD, Han CJ, Kim YC, Park SH, Cho SY, Hong YJ, Park HY, Lee M. Elevated plasma osteopontin levels in patients with hepatocellular carcinoma. Am J Gastroenterol. 2006;101:2051-2059. |
| 20. | Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48-54. |
| 21. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. |
| 22. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. |
| 23. | Bautista DS, Saad Z, Chambers AF, Tonkin KS, O'Malley FP, Singhal H, Tokmakejian S, Bramwell V, Harris JF. Quantification of osteopontin in human plasma with an ELISA: basal levels in pre- and postmenopausal women. Clin Biochem. 1996;29:231-239. |
| 24. | Denhardt DT, Giachelli CM, Rittling SR. Role of osteopontin in cellular signaling and toxicant injury. Annu Rev Pharmacol Toxicol. 2001;41:723-749. |
| 25. | Giachelli CM, Steitz S. Osteopontin: a versatile regulator of inflammation and biomineralization. Matrix Biol. 2000;19:615-622. |
| 26. | Sodek J, Ganss B, McKee MD. Osteopontin. Crit Rev Oral Biol Med. 2000;11:279-303. |
| 27. | Lorena D, Darby IA, Gadeau AP, Leen LL, Rittling S, Porto LC, Rosenbaum J, Desmoulière A. Osteopontin expression in normal and fibrotic liver. altered liver healing in osteopontin-deficient mice. J Hepatol. 2006;44:383-390. |
| 28. | Kawashima R, Mochida S, Matsui A, YouLuTuZ Y, Ishikawa K, Toshima K, Yamanobe F, Inao M, Ikeda H, Ohno A. Expression of osteopontin in Kupffer cells and hepatic macrophages and Stellate cells in rat liver after carbon tetrachloride intoxication: a possible factor for macrophage migration into hepatic necrotic areas. Biochem Biophys Res Commun. 1999;256:527-531. |
| 29. | Bezerra JA, Tiao G, Ryckman FC, Alonso M, Sabla GE, Shneider B, Sokol RJ, Aronow BJ. Genetic induction of proinflammatory immunity in children with biliary atresia. Lancet. 2002;360:1653-1659. |
| 30. | Whitington PF, Malladi P, Melin-Aldana H, Azzam R, Mack CL, Sahai A. Expression of osteopontin correlates with portal biliary proliferation and fibrosis in biliary atresia. Pediatr Res. 2005;57:837-844. |
| 31. | Huang L, Wei MF, Feng JX. Abnormal activation of OPN inflammation pathway in livers of children with biliary atresia and relationship to hepatic fibrosis. Eur J Pediatr Surg. 2008;18:224-229. |
| 32. | Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835-847. |
| 33. | de Lédinghen V, Le Bail B, Rebouissoux L, Fournier C, Foucher J, Miette V, Castéra L, Sandrin L, Merrouche W, Lavrand F. Liver stiffness measurement in children using FibroScan: feasibility study and comparison with Fibrotest, aspartate transaminase to platelets ratio index, and liver biopsy. J Pediatr Gastroenterol Nutr. 2007;45:443-450. |
| 34. | Honsawek S, Vejchapipat P, Chongsrisawat V, Thawornsuk N, Poovorawan Y. Association of circulating osteopontin levels with clinical outcomes in postoperative biliary atresia. Pediatr Surg Int. 2010;Epub ahead of print. |