Published online Nov 21, 2010. doi: 10.3748/wjg.v16.i43.5416
Revised: August 2, 2010
Accepted: August 9, 2010
Published online: November 21, 2010
AIM: To monitor the early responses to irradiation in primary and metastatic colorectal cancer (CRC) with 18F-fluorothymidine (18F-FLT) and 18F-fluorodeoxyglucose (18F-FDG) small-animal position emission tomography (micro-PET).
METHODS: The primary and metastatic CRC cell lines, SW480 and SW620, were irradiated with 5, 10 and 20 Gy. After 24 h, the cell cycle phases were analyzed. A dual-tumor-bearing mouse model of primary and metastatic cancer was established by injecting SW480 and SW620 cells into mice. micro-PET with 18F-FLT and 18F-FDG was performed before and 24 h after irradiation with 5, 10 and 20 Gy. The region of interest (ROI) was drawn over the tumor and background to calculate the ratio of tumor to non-tumor (T/NT) in tissues. Immunohistochemical assay and Western blotting were used to examine the levels of integrin β3, Ki-67, vascular endothelial growth factor receptor 2 (VEGFR2) and heat shock protein 27 (HSP27).
RESULTS: The proportion of SW480 and SW620 cells in the G2-M phase was decreased with an increasing radiation dose. The proportion of SW480 cells in the G0-G1 phase was increased from 48.33% ± 4.55% to 87.09% ± 7.43% (P < 0.001) and that of SW620 cells in the S-phase was elevated from 43.57% ± 2.65% to 66.59% ± 7.37% (P = 0.021). In micro-PET study, with increasing dose of radiation, 18F-FLT uptake was significantly reduced from 3.65 ± 0.51 to 2.87 ± 0.47 (P = 0.008) in SW480 tumors and from 2.22 ± 0.42 to 1.76 ± 0.45 (P = 0.026) in SW620 tumors. 18F-FDG uptake in SW480 and SW620 tumors was reduced but not significantly (F = 0.582, P = 0.633 vs F = 0.273, P = 0.845). Dose of radiation was negatively correlated with 18F-FLT uptake in both SW480 and SW620 tumors (r = -0.727, P = 0.004; and r = -0.664, P = 0.009). No significant correlation was found between 18F-FDG uptake and radiation dose in SW480 or SW620 tumors. HSP27 and integrin β3 expression was higher in SW480 than in SW620 tumors. The T/NT ratio for 18F-FLT uptake was positively correlated with HSP27 and integrin β3 expression (r = 0.924, P = 0.004; and r = 0.813, P = 0.025).
CONCLUSION: 18F-FLT is more suitable than 18F-FDG in monitoring early responses to irradiation in both primary and metastatic lesions of colorectal cancer.
- Citation: Wang H, Liu B, Tian JH, Xu BX, Guan ZW, Qu BL, Liu CB, Wang RM, Chen YM, Zhang JM. Monitoring early responses to irradiation with dual-tracer micro-PET in dual-tumor bearing mice. World J Gastroenterol 2010; 16(43): 5416-5423
- URL: https://www.wjgnet.com/1007-9327/full/v16/i43/5416.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i43.5416
Radiation therapy has long been used for curative or palliative management of colorectal cancer (CRC) patients[1,2]. However, some patients undergoing radiotherapy might present primary CRC lesions as well as metastatic lymph nodes. The current method for assessing the response of a solid tumor to radiotherapy is to measure the change in tumor size on anatomical imaging modalities. It takes weeks to months to detect the change, so it is difficult to evaluate early responses to therapy via morphological means. Noninvasive methods for monitoring early responses to radiotherapy would be of great value in individualized treatment.
Positron emission tomography (PET) is a quantitative molecular imaging technique that allows for noninvasive in vivo imaging and quantification of biological processes[3-5]. 18F-fluorodeoxyglucose (18F-FDG) is the most widely used PET tracer and has become an indispensable staging modality for many types of cancer. However, 18F-FDG may be unsuitable for monitoring the response after radiotherapy, because increased uptake can appear in inflammatory lesions and fibrosis[6-8].
18F-fluorothymidine (18F-FLT) is a pyrimidine analogue that uses the salvage pathway of DNA synthesis to reveal cell proliferation. 18F-FLT has been found useful for non-invasive assessment of the proliferation rate of several types of cancer, such as colorectal, esophageal, breast and laryngeal cancer. Imaging and measurement of proliferation with 18F-FLT-PET could be a noninvasive tool to monitor the response to anticancer treatment[9,10].
Recently, many studies claimed that PET has a special promise as a biomarker for anticancer treatment, and can be used longitudinally and provide information on the whole body or tumor. Early identification of cancer patients who are responding or resistant to radiotherapy may lead to individualized therapeutic approaches and improved clinical outcomes[11]. Yang et al[12] found that tumor uptake of 18F-FLT was reduced significantly at 24 h after radiation with 10 Gy and 20 Gy compared with 18F-FDG. At 48 h after irradiation, 18F-FLT uptake was further reduced, but 18F-FDG uptake was reduced slightly. So, 18F-FLT-PET may be a promising imaging modality for monitoring the early effects of radiation therapy.
Bearing those in mind, we wondered whether 18F-FLT could be used to reflect the early response to irradiation and compared 18F-FLT and 18F-FDG-PET in a possible early response in CRC primary or metastatic lesions. We chose two kinds of human CRC cells, SW480 and SW620[13-15], derived from CRC primary and lymph-node metastatic lesions, respectively, in the same patient to create a dual-tumor-bearing model. PET with 18F-FLT and 18F-F DG was performed before and 24 h after increasing doses of irradiation. The radioactivity uptake in SW480 and SW620 tumors was investigated with small-animal (micro)-PET.
RPMI1640, Leibovitz’s L15 medium, and fetal bovine serum (FBS) were obtained from PAA Laboratories GmbH, Linz, Austria. All the other chemicals were of reagent grade. Cell cycle and cell apoptosis kits were from Naniing Keygen Biotechnology. Antibodies against K-i67, a cell proliferation antigen, and anti-integrin β3 were from Santa Cruz Biotechnology. The antibodies anti-vascular endothelial growth factor receptor 2 (VEGFR2) and anti-heat shock protein (HSP) 27 were from Abcam. The diaminobenzidine (DAB) kit was obtained from Zhongshan Biotechnology Co., Beijing, China.
The human CRC cell lines SW480 and SW620 were from the Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China). SW480 cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (10 000 IU/mL), at 5% CO2 in a humidified atmosphere at 37°C. SW620 cells were cultured in L15 medium supplemented with 10% FBS and 1% penicillin/streptomycin (10 000 IU/mL) without CO2 in a humidified atmosphere at 37°C.
Eighteen male Balb/C nude mice (6 wk old, 20 g) were obtained from the Animal Laboratory of the Chinese Academy of Sciences. Two tumors per animal were generated by inoculating 5 × 106 SW480 viable cells into nude mice on the lateral side of the left front leg and the same amount of SW620 cells on the right front leg. Mice were kept under sterile conditions with a standard light/dark cycle and had free access to food and water. Tumor size in the front legs was determined by caliper measurement at least twice a week by the formula V = 1/2 (l × w × h) (l, length; w, width; h, height of the tumor). Micro-PET/CT scans of 18F-FDG and 18F-FLT uptake were performed for tumors with volumes between 100 and 500 mm3.
Local external beam radiation was applied using a clinical X-ray therapy unit (Precise ELEKDA, 6 MV X-ray, at a dose rate of 388 MU/min). The mice were anesthetized using 1% chloral hydrate (0.45 mg/g body weight) and positioned prone on the scanning table. The dual tumors were locally irradiated and the other parts of the mouse body were protected from irradiation with lead shielding. For homogeneous dose distributions, antero-posterior and postero-anterior external beam radiation fields were used. When SW480 and SW620 cells arrived at 50% confluence and when tumor size was about 100-500 mm3, cells and tumors underwent single-dose irradiation at 5 Gy (n = 6 wells or mice), 10 Gy (n = 6) and 20 Gy (n = 6), respectively. After 24 h, tumors underwent micro-PET/CT scanning. All animal experiments were carried out in accordance with the Dutch Law on Animal Experimentation and approved by the institutional committee on animal experimentation of our institution.
At 24 h after irradiation, SW480 and SW620 cells were washed twice with phosphate-buffered saline (PBS), detached with 0.25% trypsin and fixed with 75% ethanol and stored at 4°C. Cells were centrifuged to remove 75% ethanol before cell cycle determination, washed twice with PBS and resuspended in 0.5 mL PBS. After cells were stained with propidium iodide in the dark for 10 min, DNA content was measured by flow cytometry (FACScalibur, Becton Dickinson) to obtain the percentage of cells in each phase.
PET images of tumors in dual-tumor-bearing mice were obtained using the small animal micro-PET/CT (Explore VISTA micro-PET/CT, GE). At 24 h after irradiation, 3 mice in each group underwent 18F-FLT-PET and 3 mice underwent 18F-FDG-PET. The mice were anesthetized and positioned prone in the scanner, and 18F-FLT or 18F-FDG was injected via the tail vein at 20 ± 1.84 MBq in 0.25 mL saline. Image data were acquired for 10 min at 1 h after injection. For image reconstruction, list-mode data were sorted into 3-D sinograms, then underwent Fourier rebinning and 2-D ordered-subset expectation maximization reconstruction with 2 iterations and 50 subsets. Image pixel size was 0.385 mm × 0.385 mm × 0.335 mm. For quantitation of tumor uptake of 18F-FLT or 18F-FDG, image software was used to analyze the region of interest (ROI) in reconstructed images. Three consecutive coronal slice images containing tumors were selected visually, and ROIs were drawn on the tumor and lung as background, and the ratio of tumor to background (T/NT) uptake was calculated.
The staining procedure has been described elsewhere[10]. Sections of two kinds of tumors were stained with hematoxylin and eosin and antibodies against anti-integrin β3 (sc-52685), anti-Ki67 (sc-52685), anti-HSP27 (ab2790), and anti-VEGFR2 (ab3968) antibody (1:100). For a negative control, the primary antibody was omitted and replaced with PBS. Specimens were examined under light microscopy. The number of integrin β3-, Ki-67-, HSP27-, and VEGFR2-positive and HE-positive cells in adjacent sections was counted in 5 randomly selected fields per section. Western blotting analysis of 150 μg protein from SW480 and SW620 tumors was performed as described earlier[15]. The antibodies were the same as described in the immunocytochemical assay. Integrin β3, Ki-67, HSP27, and VEGFR2 expression was described by gray scale analysis with the Labworks software.
Data analysis was performed using SPSS v11.5 (SPSS Inc., Chicago, IL). Percentages of cells in each cell phase after irradiation were compared by one-way ANOVA. Differences in radiotracer uptake before and after irradiation in each mouse were compared by independent-samples t test. Linear regression analysis was used to determine the correlation between radiotracer uptake and radiation dose or cell cycle phase. All data were expressed as mean ± SD. P < 0.05 was considered statistically significant.
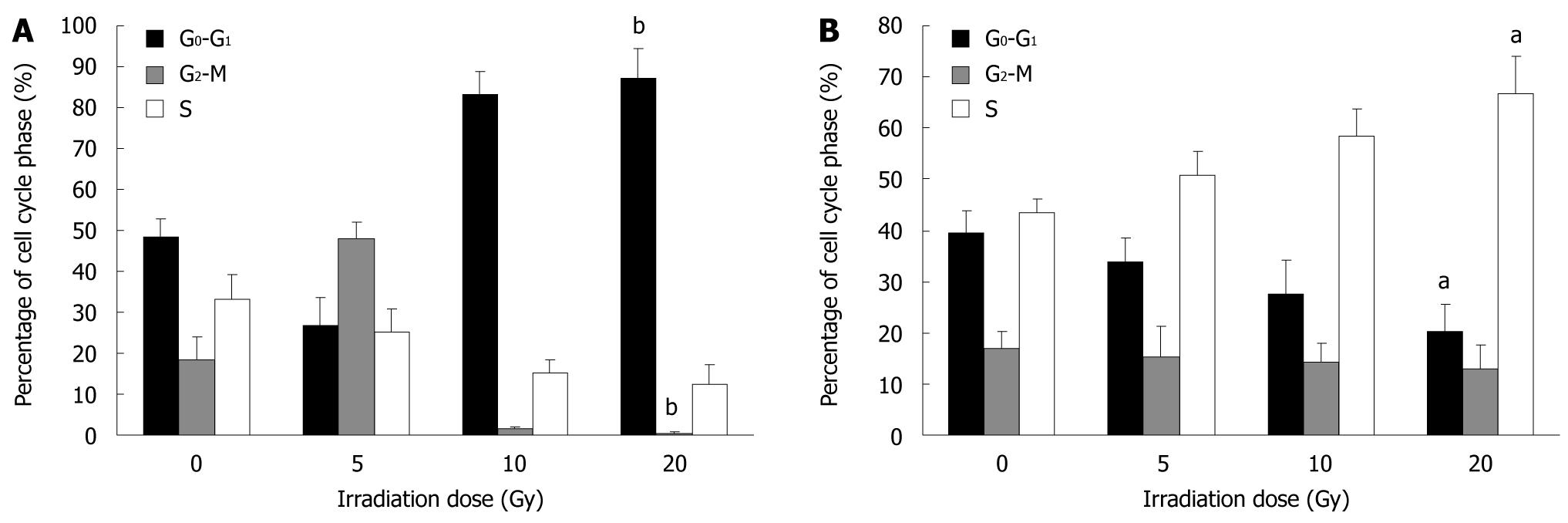
We examined the effects of irradiation on cell cycle distribution by flow cytometry. At 24 h after irradiation, the proportion of SW480 cells in the G0-G1 phase decreased from 48.33% ± 4.55% at 0 Gy to 26.70% ± 7.09% at 5 Gy, then increased to 87.09% ± 7.43% at 20 Gy, the proportion in the S phase decreased from 33.23% ± 6.09% at 0 Gy to 12.44% ± 4.60% at 20 Gy; and that in the G2-M phase decreased from 18.44% ± 5.67% at 0 Gy to 0.47% ± 0.34% at 20 Gy (Figure 1A). At 0-20 Gy, the proportion of SW620 cells in the G0-G1 phase decreased from 39.37% ± 4.37% to 20.39% ± 5.12%, and that in the S phase increased from 43.57% ± 2.65% to 66.59% ± 7.37%. The proportion in G2-M phase decreased from 17.07% ± 3.09% to 13.02% ± 4.55% (Figure 1B).
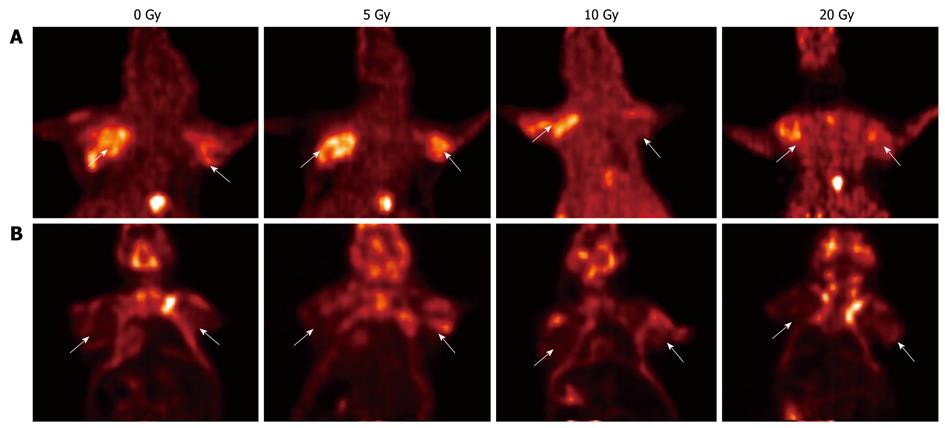
Micro-PET/CT scanning results of 18F-FLT and 18F-FDG uptake in SW480 and SW620 tumors irradiated with increasing doses are shown in Table 1 and Figure 2. Before irradiation, the T/NT ratio in the ROI for 18F-FLT was higher in SW480 (3.65 ± 0.51) than in SW620 tumors (2.22 ± 0.42). At 24 h after irradiation with 20 Gy, the T/NT ratio for 18F-FLT uptake was significantly decreased in both SW480 (2.87 ± 0.47, P = 0.008) and SW620 cells (1.76 ± 0.45, P = 0.026) (Figure 2A).
Before irradiation, the T/NT ratio for 18F-FDG in SW480 and SW620 tumors was 2.69 ± 0.98 and 3.09 ± 1.26, respectively (P = 0.524). At 24 h after irradiation at 20 Gy, the T/NT ratio for 18F-FDG uptake was reduced but not significantly (2.40 ± 0.52 and 2.89 ± 0.29, both P > 0.05) (Figure 2B).
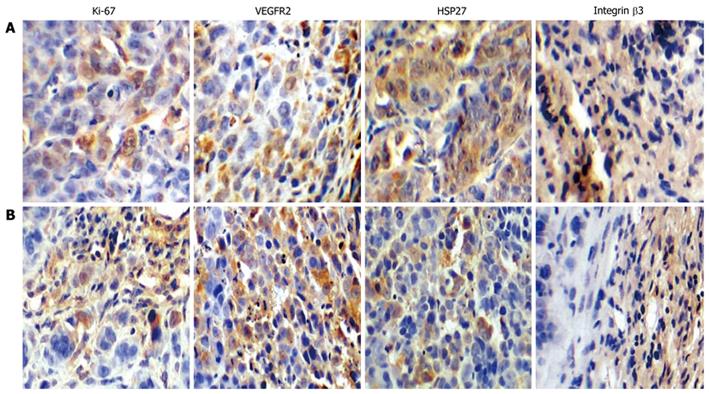
Integrin β3, HSP27, Ki-67 and VEGFR2 proteins were all overexpressed in SW480 and SW620 tumors (Figure 3). SW480 cells showed more intense staining for integrin β3 and HSP27 protein in cytoplasm or nucleus than did SW620 tumors. Integrin β3 protein was also overexpressed in the tumor matrix near vasculature. Staining for VEGFR2 and especially Ki-67 expression was lower in SW480 tumors than in SW620 tumors.
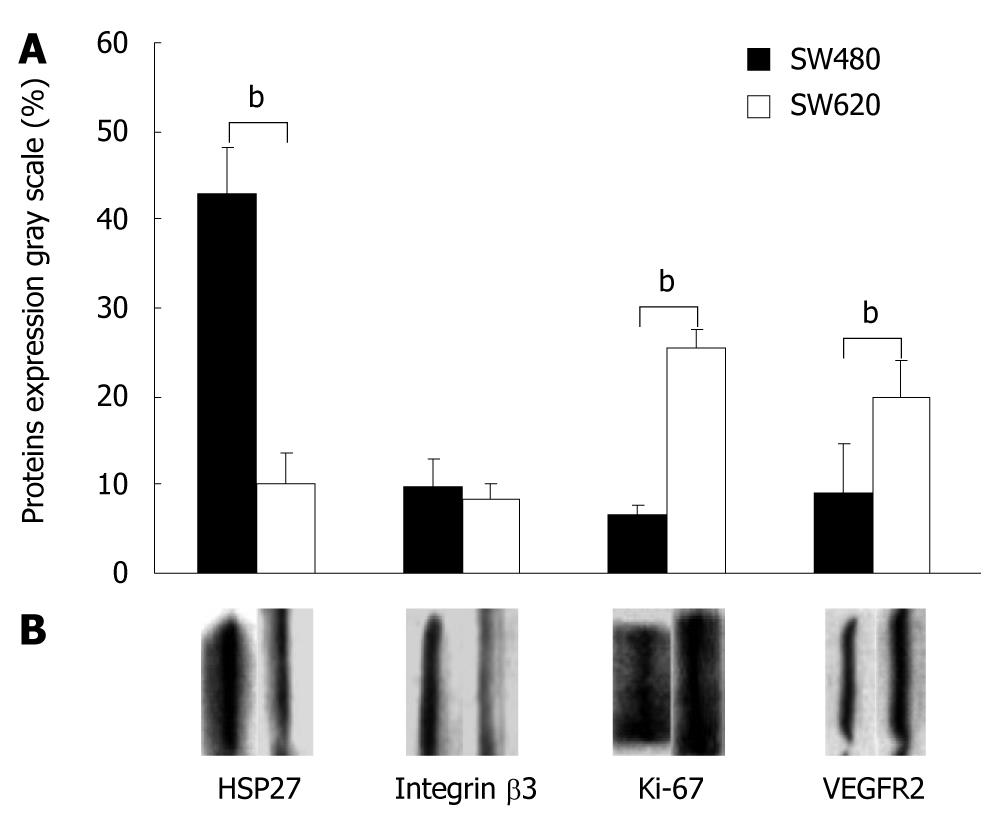
Western blotting analysis revealed that HSP27 and integrin β3 expression was higher in SW480 than in SW620 tumors (42.86% ± 5.15% vs 10.10% ± 3.50%, for Hsp27, P = 0.002; and 9.61% ± 3.20% vs 8.43% ± 1.85% for integrin β3, P = 0.164). The expression of K-i67 and VEGFR2 protein was less pronounced in SW480 than in SW620 tumors (6.5% ± 1.25% and 9.00% ± 2.38% for K-i67, P = 0.009; and 25.33% ± 5.59% and 19.96% ± 4.20% for VEGFR2, P < 0.001) (Figure 4A and B).
We found a significant negative correlation between dose of radiation and 18F-FLT uptake in SW480 and SW620 tumors (r = -0.727, P = 0.004, and r = -0.664, P = 0.009, respectively). Dose of radiation was positively but not significantly correlated with 18F-FDG uptake in SW480 tumors (r = 0.401, P = 0.098) and positively but significantly correlated with 18F-FDG uptake in SW620 tumors (r = 0.640, P = 0.013). Dose of radiation was negatively correlated with proportion of SW480 cells in the G2-M phase (r = -0.798, P = 0.001) and negatively but not significantly correlated with proportion of SW620 cells in the G2-M phase (r = -0.184, P = 0.283). Dose of radiation was positively correlated with proportion of SW620 cells in the S phase (r = 0.870, P < 0.001) and negatively correlated with proportion of SW620 cells in the G0-G1 phase (r = -0.6730, P = 0.008). The T/NT ratio for 18F-FLT uptake was positively correlated with integrin β3 and HP27expression (r = 0.813, P = 0.025, and r = 0.924, P = 0.004), but not with Ki-67 or VEGFR2 expression. Similarly, the T/NT ratio for 18F-FDG uptake was not significantly correlated with integrin β3, HSP27, Ki-67 and or VEGFR2 expression.
Therapy monitoring plays a major role in the evaluation of therapeutic approaches[16-18]. 18F-FDG-PET is clinically used for the diagnosis, staging and re-staging of a wide variety of tumors. However, the technique contains several shortcomings in reflecting changes in tumors after treatment, especially radiotherapy[19-22]. Several research groups have suggested that 18F-FLT, as a cell proliferation tracer, is a more cancer-specific tracer than 18F-FDG[23-27], but some results are contradictory[28]. In the present study, we investigated 18F-FLT-PET as a potential tool for monitoring the early response to irradiation in a mouse model of dual-tumor-bearing CRC. In clinical practice, we have often found primary lesions and metastatic lymph nodes together in the same patient. A model of dual tumors created with CRC SW480 and SW620 cells is similar to clinical practice, so we compared the uptake of 18F-FLT and18F-FDG in response to irradiation in the two kinds of CRC tumors.
In a micro-PET study, we found a higher uptake of 18F-FLT than 18F-FDG in SW480 and SW620 tumors. After irradiation for 24 h, the uptake of 18F-FLT in SW480 or SW620 tumors increased at a low dose (5 Gy), then reduced gradually with increasing radiation dose. A statistical difference was found in both tumor groups although 18F-FLT uptake reduced more significantly in SW480. Whereas the 18F-FDG uptake was increased at a low dose (5 Gy) and reduced slightly at a high dose (20 Gy) without a significant difference. Liang et al[13] did not find a dose-dependent decrease in uptake of 18F-FLT in tumors. However, in the current study, with a dose greater than 5 Gy, the T/NT ratio for 18F-FLT uptake was dose-dependently decreased in both SW480 and SW620 tumors. A significantly negative correlation was found between dose of radiation and 18F-FLT uptake in SW480 and SW620 tumors. No correlation was found between dose of radiation and 18F-FDG uptake. So 18F-FLT uptake can be more sensitive and accurate than 18F-FDG to monitor the response to irradiation after 24 h.
Recent studies have shown that a decrease in cellular proliferation rate is one of the early events in response to tumor treatment. In the present study, after 24 h irradiation, cell cycle redistribution was found, and proliferation inhibition of the two kinds of CRC cells occurred in a dose-dependent manner, and the response to seam dose was different. G2-M phase decrease and G0-G1 phase arrest were found earlier in SW480 than that in SW620 cells. We also found a decrease in the proportion of SW480 and SW620 cells in the S phase with the increasing radiation dose, and the dose of irradiation was negatively correlated with proportion of G2-M phase in both kinds of cells. T/NT ratio for 18F-FLT was negatively correlated with proportion of SW480 or SW620 cells in the G2-M phase. The proportion of cells decreased in the G0-G1 arrest and S phases may not be important for the 18F-FLT uptake decrease in SW480 and SW620 tumors.
A previous study with another tumor cell line also showed that tumor uptake of 18F-FDG was decreased at 24 h after irradiation[29]. The mechanism of the increased 18F-FDG and 18F-FLT uptake after 24 h irradiation at a low dose (5Gy) has remained unclear. It may be due to the G2-M or S phase arrest enhancing metabolism after irradiation in a short time. After 24 h irradiation, the two kinds of tumors were excised immediately. No necrosis formation occurred in the tumors possibly due to the short time (24 h) after radiotherapy.
In our dual-tumor-bearing animal study, the reason for the difference in 18F-FLT and 18F-FDG uptake in response to irradiation is not clear. The different response may be related to different biological characteristics, such as tumor marker expression. A large number of different proteins are expressed in SW480 and SW620 cell lines. In this study, we selected 4 tumor biomarkers, integrin β3, Ki67, HSP27 and VEGFR2, known as tumor invasion, proliferation, apoptosis, angiogenesis markers, respectively[30-32]. VEGFR2 or Ki-67 expression was stronger in SW620 than in SW480 cells. In contrast, HSP27 and integrin β3 expression was more intense in SW480 cells. The T/NT ratio for 18F-FLT was significantly correlated with HSP27 and integrin β3 expression. Tumor biomarkers play a major role in indicating tumor characteristics, exploring possible mechanisms, and in evaluating and suggesting new therapeutic approaches. The results suggest that other factors, besides proliferation, may influence the response to irradiation.
Although our study population is small, differences were found in the 18F-FLT and 18F-FDG uptake response in CRC to radiotherapy on PET. 18F-FLT PET may be a useful noninvasive imaging modality to assess early response to irradiation in both primary and metastatic CRC lesions. 18F-FLT-PET is also quick and effective in monitoring the response to irradiation in primary tumors. The capacity of 18F-FLT to reveal response to irradiation within 24 h may be useful for individualizing therapy. Additional studies are being carried out to investigate other radiation doses and the mechanism among different tumor cells.
In conclusion, compared with 18F-FDG-PET, 18F-FLT-PET might be better in monitoring the response to 24 h irradiation in both primary and metastatic CRC lesions with increasing radiation doses.
Colorectal cancer is one of the most frequently encountered malignancies in China and is associated with a high mortality rate. Irradiation therapy has long been used for curative or palliative management in colorectal cancer (CRC). When irradiation was performed, primary CRC and metastatic lymph node metastatic lesions often appear in the same patient.As it takes weeks to months to detect the change, it is difficult to evaluate the early responses to therapy via morphological means. Noninvasive methods for monitoring early responses to radiotherapy would be of great value in individualized treatment. So there are some questions to answer: Weather 18F-fluorodeoxyglucose (18F-FDG) or 18F-fluorothymidine (18F-FLT) could depict the difference earlier, and which is more suitable for doing so? This study was designed to investigate whether 18F-FLT-PET could be used to reflect the early effect of irradiation in both CRC primary and lymph node metastatic lesions as compared with 18F-FDG-PET.
Recently, many studies claimed that PET has particular promise as a biomarker for anticancer therapies, can be used longitudinally and provides information on the patient or tumor. Early identification of cancer patients who are responding or resistant to radiotherapy may lead to individualized therapeutic approaches and improved clinical outcomes. 18F-FLT-PET may be a promising imaging modality for monitoring the early effects of radiation therapy.
18F-FLT PET may be used as a useful noninvasive imaging modality to monitor early response to irradiation for different CRCs. This is the first study to report in a new angle that 18F-FLT is more helpful than18F-FDG in reflecting the early effects of irradiation in CRC primary lesions or lymph metastatic lesions.
SW480 and SW620 tumors, either primary or from metastatic lymph nodes have different responses to irradiation at early phase; 18F-FLT response to irradiation is more sensitive than 18F-FDG. Evaluation of the response to irradiation would be helpful for individualizing treatment and improving outcomes of CRC patients in clinical practice.
18F-FDG is the most widely used PET tracer, but it has several shortcomings in reflecting changes in tumors after treatment, especially radiotherapy. 18F-FLT is a pyrimidine analogue and believed to be an agent for imaging cellular proliferation via the salvage pathway of DNA synthesis, which is closely associated with cellular proliferation. The 4 kinds of tumor biomarkers, HSP27, Integrinβ3, VEGFR2 and Ki67, are related to tumor differentiation, invasion, angiogenesis and proliferation respectively.
The authors investigated whether 18F-FLT-PET or 18F-FDG-PET could be used to reflect the early effects of irradiation in CRC primary lesions or lymph metastatic lesions. The results revealed that 18F-FLT-PET might be better in monitoring the response to 24 h irradiation in both primary and metastatic CRC lesions with increasing radiation doses. The results are interesting and helpful for individualized treatment in clinical practice.
Peer reviewer: Paul E Sijens, PhD, Associate Professor, Radiology, UMCG, Hanzeplein 1, 9713GZ Groningen, The Netherlands
S- Editor Cheng JX L- Editor Ma JY E- Editor Ma WH
| 1. | Mathis KL, Nelson H, Pemberton JH, Haddock MG, Gunderson LL. Unresectable colorectal cancer can be cured with multimodality therapy. Ann Surg. 2008;248:592-598. |
| 2. | Mohiuddin M, Marks J, Marks G. Management of rectal cancer: short- vs long-course preoperative radiation. Int J Radiat Oncol Biol Phys. 2008;72:636-643. |
| 3. | Low G, Tho LM, Leen E, Wiebe E, Kakumanu S, McDonald AC, Poon FW. The role of imaging in the pre-operative staging and post-operative follow-up of rectal cancer. Surgeon. 2008;6:222-231. |
| 4. | Herrmann K, Wieder HA, Buck AK, Schöffel M, Krause BJ, Fend F, Schuster T, Meyer zum Büschenfelde C, Wester HJ, Duyster J. Early response assessment using 3’-deoxy-3’-[18F]fluorothymidine-positron emission tomography in high-grade non-Hodgkin’s lymphoma. Clin Cancer Res. 2007;13:3552-3558. |
| 5. | Rajendran JG, Mankoff DA. Beyond detection: novel applications for PET imaging to guide cancer therapy. J Nucl Med. 2007;48:855-856. |
| 6. | Leyton J, Latigo JR, Perumal M, Dhaliwal H, He Q, Aboagye EO. Early detection of tumor response to chemotherapy by 3’-deoxy-3’-[18F]fluorothymidine positron emission tomography: the effect of cisplatin on a fibrosarcoma tumor model in vivo. Cancer Res. 2005;65:4202-4210. |
| 7. | Molthoff CF, Klabbers BM, Berkhof J, Felten JT, van Gelder M, Windhorst AD, Slotman BJ, Lammertsma AA. Monitoring response to radiotherapy in human squamous cell cancer bearing nude mice: comparison of 2’-deoxy-2’-[18F]fluoro-D-glucose (FDG) and 3’-[18F]fluoro-3’-deoxythymidine (FLT). Mol Imaging Biol. 2007;9:340-347. |
| 8. | Kasper B, Egerer G, Gronkowski M, Haufe S, Lehnert T, Eisenhut M, Mechtersheimer G, Ho AD, Haberkorn U. Functional diagnosis of residual lymphomas after radiochemotherapy with positron emission tomography comparing FDG- and FLT-PET. Leuk Lymphoma. 2007;48:746-753. |
| 9. | Chao KS. 3’-deoxy-3’-(18)F-fluorothymidine (FLT) positron emission tomography for early prediction of response to chemoradiotherapy--a clinical application model of esophageal cancer. Semin Oncol. 2007;34:S31-S36. |
| 10. | Apisarnthanarax S, Alauddin MM, Mourtada F, Ariga H, Raju U, Mawlawi O, Han D, Bornmann WG, Ajani JA, Milas L. Early detection of chemoradioresponse in esophageal carcinoma by 3’-deoxy-3’-3H-fluorothymidine using preclinical tumor models. Clin Cancer Res. 2006;12:4590-4597. |
| 11. | Stephen RM, Gillies RJ. Promise and progress for functional and molecular imaging of response to targeted therapies. Pharm Res. 2007;24:1172-1185. |
| 12. | Yang YJ, Ryu JS, Kim SY, Oh SJ, Im KC, Lee H, Lee SW, Cho KJ, Cheon GJ, Moon DH. Use of 3’-deoxy-3’-[18F]fluorothymidine PET to monitor early responses to radiation therapy in murine SCCVII tumors. Eur J Nucl Med Mol Imaging. 2006;33:412-419. |
| 13. | Liang L, Qu L, Ding Y. Protein and mRNA characterization in human colorectal carcinoma cell lines with different metastatic potentials. Cancer Invest. 2007;25:427-434. |
| 14. | Duranton B, Holl V, Schneider Y, Carnesecchi S, Gossé F, Raul F, Seiler N. Polyamine metabolism in primary human colon adenocarcinoma cells (SW480) and their lymph node metastatic derivatives (SW620). Amino Acids. 2003;24:63-72. |
| 15. | Zhao L, Liu L, Wang S, Zhang YF, Yu L, Ding YQ. Differential proteomic analysis of human colorectal carcinoma cell lines metastasis-associated proteins. J Cancer Res Clin Oncol. 2007;133:771-782. |
| 16. | Hicks RJ. The role of PET in monitoring therapy. Cancer Imaging. 2005;5:51-57. |
| 17. | Roels S, Slagmolen P, Nuyts J, Lee JA, Loeckx D, Maes F, Stroobants S, Penninckx F, Haustermans K. Biological image-guided radiotherapy in rectal cancer: is there a role for FMISO or FLT, next to FDG? Acta Oncol. 2008;47:1237-1248. |
| 18. | Okazumi S, Dimitrakopoulou-Strauss A, Schwarzbach MH, Strauss LG. Quantitative, dynamic 18F-FDG-PET for the evaluation of soft tissue sarcomas: relation to differential diagnosis, tumor grading and prediction of prognosis. Hell J Nucl Med. 2009;12:223-228. |
| 19. | Tan MC, Linehan DC, Hawkins WG, Siegel BA, Strasberg SM. Chemotherapy-induced normalization of FDG uptake by colorectal liver metastases does not usually indicate complete pathologic response. J Gastrointest Surg. 2007;11:1112-1119. |
| 20. | Yamamoto Y, Nishiyama Y, Kimura N, Ishikawa S, Okuda M, Bandoh S, Kanaji N, Asakura M, Ohkawa M. Comparison of (18)F-FLT PET and (18)F-FDG PET for preoperative staging in non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2008;35:236-245. |
| 21. | Kameyama R, Yamamoto Y, Izuishi K, Takebayashi R, Hagiike M, Murota M, Kaji M, Haba R, Nishiyama Y. Detection of gastric cancer using 18F-FLT PET: comparison with 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2009;36:382-388. |
| 22. | Yamamoto Y, Nishiyama Y, Ishikawa S, Gotoh M, Bandoh S, Kanaji N, Asakura M, Ohkawa M. 3’-Deoxy-3’-18F-fluorothymidine as a proliferation imaging tracer for diagnosis of lung tumors: comparison with 2-deoxy-2-18f-fluoro-D-glucose. J Comput Assist Tomogr. 2008;32:432-437. |
| 23. | Been LB, Suurmeijer AJ, Cobben DC, Jager PL, Hoekstra HJ, Elsinga PH. [18F]FLT-PET in oncology: current status and opportunities. Eur J Nucl Med Mol Imaging. 2004;31:1659-1672. |
| 24. | Molthoff CF, Klabbers BM, Berkhof J, Felten JT, van Gelder M, Windhorst AD, Slotman BJ, Lammertsma AA. Monitoring response to radiotherapy in human squamous cell cancer bearing nude mice: comparison of 2’-deoxy-2’-[18F]fluoro-D-glucose (FDG) and 3’-[18F]fluoro-3’-deoxythymidine (FLT). Mol Imaging Biol. 2007;9:340-347. |
| 25. | Yang YJ, Ryu JS, Kim SY, Oh SJ, Im KC, Lee H, Lee SW, Cho KJ, Cheon GJ, Moon DH. Use of 3’-deoxy-3’-[18F]fluorothymidine PET to monitor early responses to radiation therapy in murine SCCVII tumors. Eur J Nucl Med Mol Imaging. 2006;33:412-419. |
| 26. | Sugiyama M, Sakahara H, Sato K, Harada N, Fukumoto D, Kakiuchi T, Hirano T, Kohno E, Tsukada H. Evaluation of 3’-deoxy-3’-18F-fluorothymidine for monitoring tumor response to radiotherapy and photodynamic therapy in mice. J Nucl Med. 2004;45:1754-1758. |
| 27. | Murayama C, Harada N, Kakiuchi T, Fukumoto D, Kamijo A, Kawaguchi AT, Tsukada H. Evaluation of D-18F-FMT, 18F-FDG, L-11C-MET, and 18F-FLT for monitoring the response of tumors to radiotherapy in mice. J Nucl Med. 2009;50:290-295. |
| 28. | Bading JR, Shields AF. Imaging of cell proliferation: status and prospects. J Nucl Med. 2008;49 Suppl 2:64S-80S. |
| 29. | Robertson JH, Iga AM, Sales KM, Winslet MC, Seifalian AM. Integrins: a method of early intervention in the treatment of colorectal liver metastases. Curr Pharm Des. 2008;14:296-305. |
| 30. | Yamamoto Y, Nishiyama Y, Ishikawa S, Nakano J, Chang SS, Bandoh S, Kanaji N, Haba R, Kushida Y, Ohkawa M. Correlation of 18F-FLT and 18F-FDG uptake on PET with Ki-67 immunohistochemistry in non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2007;34:1610-1616. |
| 31. | Kushlinsky NE, Trapeznikova MF, Gershtein ES, Glibin PA, Kazantceva IA, Kilichbekov MB. Vascular endothelial growth factor and its type 2 receptor in tumors and serum of patients with renal cancer. Bull Exp Biol Med. 2008;145:744-747. |
| 32. | Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86-103. |