Published online Nov 21, 2010. doi: 10.3748/wjg.v16.i43.5405
Revised: September 7, 2010
Accepted: September 14, 2010
Published online: November 21, 2010
After surgery for Crohn’s disease (CD), early endoscopic lesions are frequently observed despite no symptomatic recurrence. The severity of lesions found at postoperative endoscopy is reported to be a strong predictive factor for future clinical recurrence. If endoscopic lesions in the early postoperative period can be reduced with medications, symptomatic recurrence will likely be delayed and decreased. Before the introduction of biologic therapies, various medications were used for the maintenance of clinical remission after surgery; however, few demonstrated consistent efficacy. Infliximab is a recombinant anti-tumor necrosis factor-α antibody. Although infliximab is one of the most effective medications in the management of CD, its efficacy for early endoscopic lesions after surgery has not yet been assessed. The author and colleagues recently conducted a prospective study in order to investigate the impact of infliximab on early endoscopic lesions after resection for CD. We found that infliximab therapy showed clear suppressive effects on clinical and endoscopic disease activity in patients with early endoscopic lesions after resection.
- Citation: Yamamoto T. Prevention of recurrence after surgery for Crohn’s disease: Efficacy of infliximab. World J Gastroenterol 2010; 16(43): 5405-5410
- URL: https://www.wjgnet.com/1007-9327/full/v16/i43/5405.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i43.5405
Crohn’s disease (CD) is a chronic relapsing, remitting inflammatory bowel disease, the cause of which remains unknown. Nearly 80% of patients with CD require surgery during their lifetime[1]. CD can be palliated but not cured by surgery because inflammation tends to return in areas adjacent to those that were previously removed. Postoperative recurrence is common, and many patients require repeat operations. Reoperation rates for recurrence are reported to be 10%-35% at 5 years, 20%-45% at 10 years and 45%-55% at 20 years[1].
Although postoperative recurrence is common in CD, the determinants of disease recurrence remain speculative. Several patients with CD experience frequent recurrences while others have prolonged periods of remission after surgery. Identifying risk factors for postoperative recurrence would be useful to identify patients at a high risk of progressive recurrence and to determine strategies for medical therapy after surgery.
The author and colleagues have conducted systematic reviews and meta-analyses of clinical trials reporting on surgical outcomes for CD in order to determine risk factors for postoperative recurrence[1-5].
The most significant factor affecting postoperative recurrence of CD is smoking. In a meta-analysis[2], 16 studies encompassing 2962 patients including 1425 non-smokers (48.1%), 1393 smokers (47.0%) and 137 ex-smokers (4.6%) were investigated. Smokers had significantly higher clinical recurrence than non-smokers [odds ratio (OR): 2.15, 95% confidence interval (CI): 1.42-3.27]. Smokers were also more likely to experience surgical recurrence within 5 years (OR: 1.06, 95% CI: 0.32-3.53) and 10 years (OR: 2.56, 95% CI: 1.79-3.67) of follow-up compared to non-smokers. There was no significant difference between ex-smokers and non-smokers in the reoperation rate at 10 years or in the rate of postoperative acute relapses. In other clinical trials[6,7], quitting smoking reduced the recurrence rate in patients with CD.
In one meta-analysis[3], 13 studies reported on 3044 patients, 1337 (43.9%) of whom had perforating disease (perforation, fistula or abscess) and 1707 (56.1%) had non-perforating indications for surgery. The probability of reoperation for recurrence was found to be significantly higher in patients with perforating indications compared to those with non-perforating indications [hazard ratio (HR): 1.50, 95% CI: 1.16-1.93]. The indication for reoperation in CD tends to be the same as the primary operation, i.e. perforating disease tends to re-present as perforating disease, and non-perforating as non-perforating.
In a recent meta-analysis[4], 21 studies reported 2236 patients with CD, of whom 1050 (47.0%) had granulomas (granulomatous group) and 1186 (53.0%) had no granulomas (non-granulomatous group). The number of recurrences and reoperations was found to be significantly higher in the granulomatous group compared to the non-granulomatous group (OR: 1.37, 95% CI: 1.02-1.84 and OR: 2.38, 95% CI: 1.43-3.95, respectively).
A number of studies have shown a higher risk of postoperative recurrence when the duration of the disease before surgery was short[1]. There were, however, different definitions of “short” among the studies.
The author and colleagues conducted a meta-analysis to investigate the impact of anastomotic type on the incidence of perianastomotic recurrence[5]. Eight studies reporting on 661 patients who underwent 712 anastomoses, of which 383 (53.8%) were sutured end-to-end anastomosis and 329 (46.2%) were other anastomotic configurations were included. There was no significant difference between the anastomotic configurations in perianastomotic recurrence and reoperation for perianastomotic recurrence. Furthermore, a recent randomized controlled trial (RCT)[8] compared endoscopic and symptomatic recurrence rates between patients who had stapled side-to-side anastomosis and hand-sewn end-to-end anastomosis. The endoscopic recurrence rate was 42.5% in the end-to-end anastomosis group compared with 37.9% in the side-to-side anastomosis group (not significant). The symptomatic recurrence rate was 21.9% in the end-to-end anastomosis group compared with 22.7% in the side-to-side anastomosis group (not significant). Based on these results, the anastomotic technique following bowel resection does not seem to affect postoperative recurrence.
The following factors do not seem to be predictive of postoperative recurrence: age at onset of disease, sex, family history of CD, anatomical site of disease, length of resected bowel, blood transfusions and postoperative complications[1].
In summary, risk factors for postoperative recurrence are smoking, perforating disease and granuloma in the resection specimen.
Rutgeerts et al[9] reported that recurrent lesions were observed endoscopically in the neo-terminal ileum (the proximal site of the ileocolonic anastomosis) within 1 year of resection in 73% of patients, although only 20% of the patients had symptoms. Three years after surgery, the endoscopic recurrence rate increased to 85% and symptomatic recurrence occurred in 34%. In their study[9], patients with severe endoscopic lesions within 1 year after resection developed early clinical recurrence. In contrast, patients with no or mild endoscopic lesions had a low frequency of subsequent clinical recurrence. The severity of the endoscopic inflammation in the neo-terminal ileum during the first year after resection was found to be a reliable predictive risk factor for future clinical recurrence.
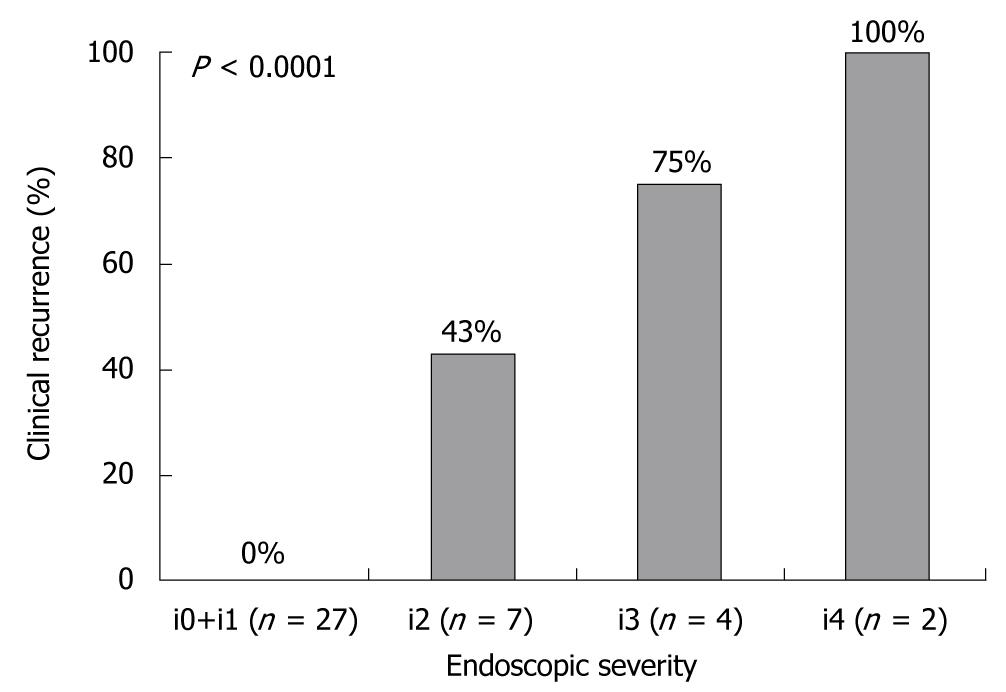
The Rutgeerts score is a well-established endoscopic scoring system based on examination of the neo-terminal ileum; i0: no lesions; i1: < 5 aphthous lesions; i2: > 5 aphthous lesions with normal mucosa between lesions, or skip areas of larger lesions or lesions confined to < 1 cm from the ileocolonic anastomosis; i3: diffuse aphthous ileitis with diffusely inflamed mucosa; and i4: diffuse inflammation with larger ulcers, nodules, and/or narrowing[9]. This scoring system is widely used in clinical practice, and endoscopic recurrence after resection for CD is defined as a score of i2, i3, or i4.
The author and colleagues[10] conducted a prospective study to investigate the relationship between endoscopic findings in the neo-terminal ileum (the proximal site of the anastomosis) and subsequent clinical recurrence rates following ileocolonic resection for CD. Forty patients who had maintained clinical remission defined as CD activity index (CDAI)[11] < 150 with 3 g/d mesalamine during 6 mo after ileocolonic resection were studied. Six months after surgery, ileo colonoscopy was performed, and the endoscopic activity score in the neo-terminal ileum was determined according to the Rutgeerts score. All patients were regularly monitored, and clinical disease activity was assessed. Clinical recurrence was defined as CDAI ≥ 150. Corticosteroids, immunosuppressants or tumor necrosis factor (TNF)-α blocking agents were not given unless there was clinical recurrence. Six months after surgery, the endoscopic scores were i0 or i1 in 27 patients, i2 in 7 patients, i3 in 4 patients, and i4 in 2 patients. There was a significant positive correlation between the endoscopic severity of the neo-terminal ileum 6 mo after surgery and the clinical recurrence rate during the following 1 year (Figure 1). From these results, the assessment of endoscopic lesions in the neo-terminal ileum appeared to be valuable for predicting subsequent clinical recurrence after ileocolonic resection for CD. Patients who postoperatively develop early endoscopic lesions despite mesalamine therapy do not benefit from continuing mesalamine. For such patients, more aggressive therapies such as TNF-α blocking agents should be considered. Thus, the early endoscopic inflammation in the neo-terminal ileum after ileocolonic resection is a suitable model to investigate the pathogenesis of CD, and also to evaluate new therapeutic modalities for prevention of progressive recurrence.
A Cochrane systematic review[12] was conducted to investigate the efficacy of medical therapies for the prevention of postoperative recurrence of CD. Twenty-three RCTs that compared medical therapy with placebo or other medical agents for the prevention of recurrence were included. Mesalamine therapy was associated with a significantly reduced risk of clinical recurrence [relative risk (RR): 0.76, 95% CI: 0.62-0.94, number needed to treat (NNT) = 12], and severe endoscopic recurrence (RR: 0.50, 95% CI: 0.29-0.84, NNT = 8) when compared with placebo. Nitroimidazole antibiotics appeared to reduce the risk of clinical recurrence (RR: 0.23, 95% CI: 0.09-0.57, NNT = 4) and endoscopic recurrence (RR: 0.44, 95% CI: 0.26-0.74, NNT = 4) when compared with placebo. However, these agents were associated with a higher risk of serious adverse events (RR: 2.39, 95% CI: 1.5-3.7). Azathioprine/6-mercaptopurine (6-MP) was also associated with a significantly reduced risk of clinical recurrence (RR: 0.59, 95% CI: 0.38-0.92, NNT = 7) and severe endoscopic recurrence (RR: 0.64, 95% CI: 0.44-0.92, NNT = 4) when compared with placebo. Neither agent had a higher risk than placebo of serious adverse events. When compared to azathioprine/6-MP, mesalamine was associated with a higher risk of any endoscopic recurrence (RR: 1.45, 95% CI: 1.03-2.06), but a lower risk of serious adverse events (RR: 0.51, 95% CI: 0.30-0.89).
The author and colleagues[13] conducted a prospective non-RCT to investigate the impact of enteral nutritional therapy with elemental diet on postoperative recurrence of CD. After resection, 20 patients continuously received enteral nutritional therapy (EN group), and 20 had neither nutritional therapy nor food restriction (non-EN group). In the EN group, an elemental diet was infused through a nasogastric tube in the night-time, and low fat foods were taken in the daytime. The clinical recurrence rate during 1-year follow-up was significantly lower in the EN group than in the non-EN group (5% vs 35%). One year after surgery, the endoscopic recurrence rate was also significantly lower in the EN group than in the non-EN group (30% vs 70%). We found that enteral nutrition significantly reduced postoperative clinical and endoscopic recurrences. However, an RCT with a larger number of patients is necessary to assess the definite efficacy of enteral nutritional therapy for the prevention of postoperative recurrence.
Infliximab is a recombinant anti-TNF-α antibody, and it reduces intestinal inflammation in patients with CD by binding to and neutralizing TNF-α on the cell membrane and in the blood, and by destroying TNF-α producing cells. Infliximab is indicated for treatment of moderately to severely active CD for the reduction of signs and symptoms in patients who have an inadequate response to conventional medications. In maintenance studies[14-16], a re-treatment regimen of infliximab can provide long-term suppression of disease activity in patients with CD. At the present time, infliximab is one of the most effective medications in the management of CD. However, the impact of infliximab on recurrence in the postoperative setting had not yet been reported.
Recently, Sorrentino et al[17] conducted a prospective pilot study, in which infliximab was administered after surgery along with low-dose methotrexate, while controls were treated with mesalamine alone. Infliximab was given as an intravenous infusion (5 mg/kg), with an intravenous 100 mg bolus of hydrocortisone starting from 2 wk after surgery, followed by standard maintenance treatment (2, 6, and then every 8 wk) and therapy with low-dose methotrexate (10 mg/wk, orally). Patients in the control group were given mesalamine-coated tablets, 800 mg 3 times daily, starting from 2 wk after surgery. In the group treated with infliximab and low-dose methotrexate, none of 7 patients had endoscopic or clinical recurrence during 2 years after surgery. In contrast, in the group treated with mesalamine, only 4 of the 16 patients (25%) were disease free during 2 years after surgery. Among 12 patients with recurrent disease, 7 had endoscopic recurrence, while 5 had both endoscopic and clinical recurrences.
Subsequently, Regueiro et al[18] conducted a randomized, double-blind, placebo-controlled trial. Twenty-four patients with CD who had undergone ileocolonic resection were allocated to receive intravenous infliximab (5 mg/kg, n = 11), administered within 4 wk of surgery and continued for 1 year, or placebo (n = 13). The primary endpoint was endoscopic recurrence (Rutgeerts score of i2, i3, or i4) at 1 year. Secondary end points were clinical recurrence (CDAI > 150) and histologic recurrence (scoring system according to D’Haens[19]). The endoscopic recurrence rate at 1 year was significantly lower in the infliximab group (9.1%) compared with the placebo group (84.6%). There was a non-significantly lower proportion of patients with clinical recurrence in the infliximab group (20.0%) compared with the placebo group (46.2%). The histologic recurrence rate at 1 year was significantly lower in the infliximab group (27.3%) compared with the placebo group (84.6%). From these results, 1-year infliximab treatment after surgery was effective for preventing endoscopic and histologic recurrences of CD. Albeit a small sample size, this study provides the strongest evidence for the efficacy of postoperative infliximab therapy.
Infliximab may be useful for the prevention of postoperative recurrence of CD[17,18]. However, this new agent cannot be recommended for all patients after surgery because of potential adverse events and high medical costs. A number of patients can maintain remission without medications or with mesalamine after surgery as shown in our study[10]. Infliximab should be used for patients at high risk of postoperative recurrence. Although smoking and perforating disease are risk factors for recurrence[1-3], these parameters are not so useful and are of limited use in clinical practice. A more practical predictor of postoperative recurrence is the severity of endoscopic inflammation in the early postoperative period[9,10].
Recently, the author and colleagues[20] conducted a prospective pilot study to investigate the efficacy of infliximab on early endoscopic recurrence after ileocolonic resection. Twenty-six patients maintaining clinical remission (CDAI < 150) with mesalamine (Pentasa® 3 g/d) after surgery showed endoscopic recurrence (Rutgeerts score of i2, i3, or i4) in the neo-terminal ileum at 6 mo postoperatively (study baseline). Patients were then allocated to one of 3 treatment groups. In Japan, azathioprine and infliximab maintenance therapy was approved by the Ministry of Health, Labor and Welfare quite recently (in June 2006, and November 2007, respectively). During the first study period (from January 2005 to June 2006), patients were continuously treated with mesalamine (Pentasa® 3 g/d) (mesalamine group, n = 10) for the following 6 mo. During the second period (from June 2006 to June 2007), azathioprine therapy (Imuran® 50 mg/d) was used (azathioprine group, n = 8), and during the last period (from June 2007 to May 2008), infliximab therapy (Remicade® 5 mg/kg, every 8 wk) was introduced (infliximab group, n = 8). In each treatment group, administration of azathioprine or infliximab was started at 6 mo after surgery, and then continued for 6 mo. At baseline and 6 mo later, endoscopic examination was conducted for all patients. During the 6-mo observation, no patients in the infliximab group, 3 (38%) in the azathioprine group, and 7 (70%) in the mesalamine group developed clinical recurrence (CDAI ≥ 150, P = 0.01). At 6 mo, endoscopic inflammation was improved in 75% of patients in the infliximab group, 38% in the azathioprine group, and 0% in the mesalamine group (P = 0.006). Complete mucosal healing was achieved in 38% of patients in the infliximab group, 13% in the azathioprine group, and 0% in the mesalazine group (P = 0.10). These results clearly showed that infliximab therapy reduced clinical and endoscopic disease activity in patients with early endoscopic recurrence after surgery.
Our infliximab therapy was started at 6 mo after surgery when endoscopic recurrence was confirmed[20]. However, our clinical and endoscopic disease activity at 1 year after surgery was similar to that in Regueiro’s study[18]. These results indicate that a 6-mo postoperative observation period does not adversely affect the efficacy of infliximab. It is reasonable to identify patients who require infliximab treatment according to endoscopic findings at 6 mo after surgery.
Sorrentino et al[21] studied 12 consecutive patients who maintained clinical and endoscopic remission with maintenance infliximab (5 mg/kg) for 24 mo after surgery, and whose infliximab treatment was discontinued. At 4 mo after discontinuation of infliximab, 10 of the 12 patients (83%) developed endoscopic recurrence (Rutgeerts score of i2, i3, or i3). The 10 patients were treated again with infliximab (3 mg/kg, every 8 wk), and then mucosal integrity was restored and maintained for 1 year. From their findings, long-term maintenance therapy with infliximab is required to maintain mucosal integrity in patients after surgery for CD. However, a dose of 3 mg/kg (a 40% reduction from the standard dose) was sufficient to avoid endoscopic recurrence.
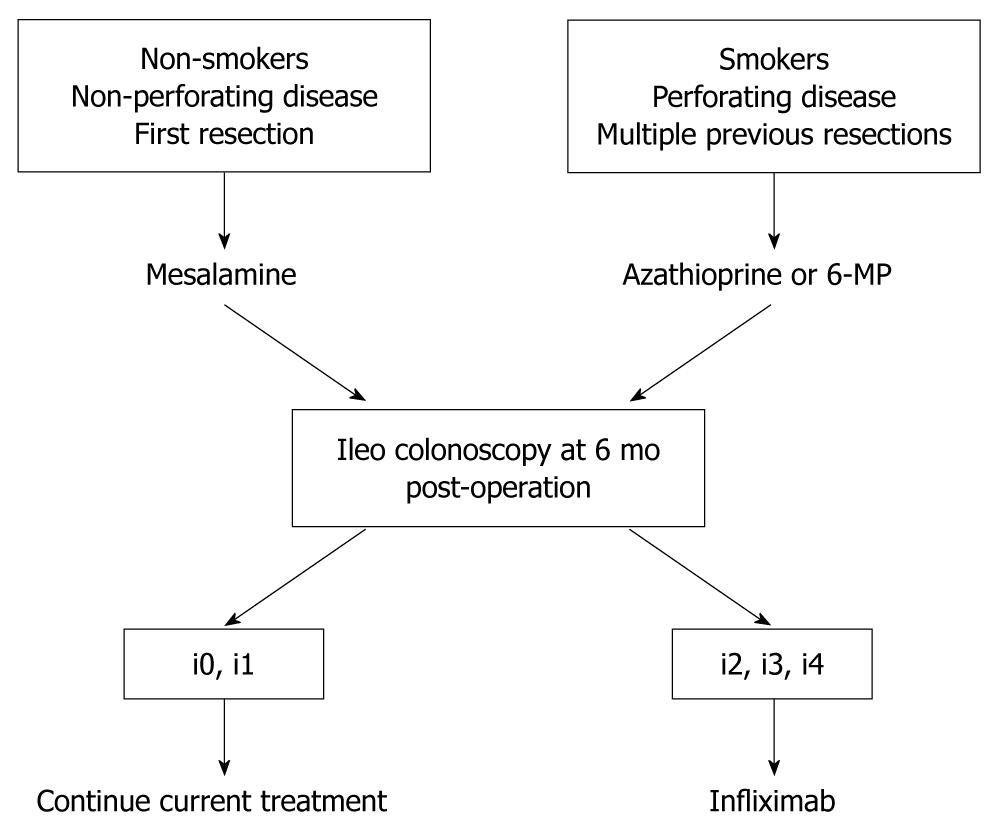
Our management strategy for the prevention of recurrence after ileocolonic resection is presented in Figure 2. As a Cochrane review[12] has shown that azathioprine and 6-MP are effective for preventing post-operative recurrence, for patients with known risk factors for recurrence such as smoking, perforating disease, and multiple previous resections, treatment with azathioprine or 6-MP should be started soon after surgery. Patients without these risk factors are treated with mesalamine. At 6 mo after surgery, ileo colonoscopy should be conducted in all patients, and the severity of macroscopic inflammation in the neo-terminal ileum assessed. In patients with no or mild endoscopic lesions (Rutgeerts score of i0 or i1), current management can be continued. However, we recommend repeat endoscopic examination 6 mo later for early detection of endoscopic lesions. For patients who develop early endoscopic lesions (Rutgeerts score of i2, i3, or i4) at 6 mo after surgery despite optimal mesalamine or immunosuppressant therapy, infliximab treatment should be considered.
Infliximab therapy showed clear suppressive effects on clinical and endoscopic disease activity in patients with early endoscopic lesions after ileocolonic resection for CD. These data strongly suggest that infliximab can delay the timing of postoperative recurrence and reduce recurrence rates by improving endoscopic inflammation. Patients with early endoscopic lesions are good candidates for infliximab therapy after surgery. However, RCTs with a larger number of patients and a longer duration of follow-up are necessary to more accurately evaluate the efficacy of infliximab in early endoscopic lesions after surgery for CD.
Peer reviewer: Patricia Sylla, MD, General and Colorectal Surgery, Massachusetts General Hospital, WACC 460, 15 Parkman Street, Boston, MA 02114, United States
S- Editor Sun H L- Editor Cant MR E- Editor Ma WH
| 1. | Yamamoto T. Factors affecting recurrence after surgery for Crohn's disease. World J Gastroenterol. 2005;11:3971-3979. |
| 2. | Reese GE, Nanidis T, Borysiewicz C, Yamamoto T, Orchard T, Tekkis PP. The effect of smoking after surgery for Crohn's disease: a meta-analysis of observational studies. Int J Colorectal Dis. 2008;23:1213-1221. |
| 3. | Simillis C, Yamamoto T, Reese GE, Umegae S, Matsumoto K, Darzi AW, Tekkis PP. A meta-analysis comparing incidence of recurrence and indication for reoperation after surgery for perforating versus nonperforating Crohn's disease. Am J Gastroenterol. 2008;103:196-205. |
| 4. | Simillis C, Jacovides M, Reese GE, Yamamoto T, Tekkis PP. Meta-analysis of the role of granulomas in the recurrence of Crohn disease. Dis Colon Rectum. 2010;53:177-185. |
| 5. | Simillis C, Purkayastha S, Yamamoto T, Strong SA, Darzi AW, Tekkis PP. A meta-analysis comparing conventional end-to-end anastomosis vs. other anastomotic configurations after resection in Crohn's disease. Dis Colon Rectum. 2007;50:1674-1687. |
| 6. | Cosnes J, Beaugerie L, Carbonnel F, Gendre JP. Smoking cessation and the course of Crohn's disease: an intervention study. Gastroenterology. 2001;120:1093-1099. |
| 7. | Ryan WR, Allan RN, Yamamoto T, Keighley MR. Crohn's disease patients who quit smoking have a reduced risk of reoperation for recurrence. Am J Surg. 2004;187:219-225. |
| 8. | McLeod RS, Wolff BG, Ross S, Parkes R, McKenzie M. Recurrence of Crohn's disease after ileocolic resection is not affected by anastomotic type: results of a multicenter, randomized, controlled trial. Dis Colon Rectum. 2009;52:919-927. |
| 9. | Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn's disease. Gastroenterology. 1990;99:956-963. |
| 10. | Yamamoto T, Umegae S, Matsumoto K, Saniabadi AR. The relationship between endoscopic findings at the proximal site of anastomosis and subsequent clinical relapse following ileal/ileocolonic resection for Crohn’s disease: a prospective endoscopic cohort study. Gut. 2009;58:A175. |
| 11. | Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70:439-444. |
| 12. | Doherty G, Bennett G, Patil S, Cheifetz A, Moss AC. Interventions for prevention of post-operative recurrence of Crohn's disease. Cochrane Database Syst Rev. 2009;CD006873. |
| 13. | Yamamoto T, Nakahigashi M, Umegae S, Kitagawa T, Matsumoto K. Impact of long-term enteral nutrition on clinical and endoscopic recurrence after resection for Crohn's disease: A prospective, non-randomized, parallel, controlled study. Aliment Pharmacol Ther. 2007;25:67-72. |
| 14. | Rutgeerts P, D'Haens G, Targan S, Vasiliauskas E, Hanauer SB, Present DH, Mayer L, Van Hogezand RA, Braakman T, DeWoody KL. Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn's disease. Gastroenterology. 1999;117:761-769. |
| 15. | Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541-1549. |
| 16. | Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, Kamm MA, Korzenik JR, Lashner BA, Onken JE. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004;350:876-885. |
| 17. | Sorrentino D, Terrosu G, Avellini C, Maiero S. Infliximab with low-dose methotrexate for prevention of postsurgical recurrence of ileocolonic Crohn disease. Arch Intern Med. 2007;167:1804-1807. |
| 18. | Regueiro M, Schraut W, Baidoo L, Kip KE, Sepulveda AR, Pesci M, Harrison J, Plevy SE. Infliximab prevents Crohn's disease recurrence after ileal resection. Gastroenterology. 2009;136:441-450.e1; quiz 716. |
| 19. | D'Haens GR, Geboes K, Peeters M, Baert F, Penninckx F, Rutgeerts P. Early lesions of recurrent Crohn's disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology. 1998;114:262-267. |
| 20. | Yamamoto T, Umegae S, Matsumoto K. Impact of infliximab therapy after early endoscopic recurrence following ileocolonic resection of Crohn's disease: a prospective pilot study. Inflamm Bowel Dis. 2009;15:1460-1466. |
| 21. | Sorrentino D, Paviotti A, Terrosu G, Avellini C, Geraci M, Zarifi D. Low-dose maintenance therapy with infliximab prevents postsurgical recurrence of Crohn's disease. Clin Gastroenterol Hepatol. 2010;8:591-599.e1; quiz e78-e79. |