Published online Nov 14, 2010. doi: 10.3748/wjg.v16.i42.5306
Revised: June 25, 2010
Accepted: July 2, 2010
Published online: November 14, 2010
AIM: To investigate the effect of total salvianolic acid (TSA) on ischemia-reperfusion (I/R)-induced rat mesenteric microcirculatory dysfunctions.
METHODS: Male Wistar rats were randomly distributed into 5 groups (n = 6 each): Sham group and I/R group (infused with saline), TSA group, TSA + I/R group and I/R + TSA group (infused with TSA, 5 mg/kg per hour). Mesenteric I/R were conducted by a ligation of the mesenteric artery and vein (10 min) and subsequent release of the occlusion. TSA was continuously infused either starting from 10 min before the ischemia or 10 min after reperfusion. Changes in mesenteric microcirculatory variables, including diameter of venule, velocity of red blood cells in venule, leukocyte adhesion, free radicals released from venule, albumin leakage and mast cell degranulation, were observed through an inverted intravital microscope. Meanwhile, the expression of adhesion molecules CD11b/CD18 on neutrophils was evaluated by flow cytometry. Ultrastructural evidence of mesenteric venules damage was assessed after microcirculation observation.
RESULTS: I/R led to multiple responses in mesenteric post-capillary venules, including a significant increase in the adhesion of leukocytes, production of oxygen radicals in the venular wall, albumin efflux and enhanced mast cell degranulation in vivo. All the I/R-induced manifestations were significantly reduced by pre- or post-treatment with TSA, with the exception that the I/R-induced increase in mast cell degranulation was inhibited only by pre-treatment with TSA. Moreover, pre- or post-treatment with TSA significantly attenuated the expression of CD11b/CD18 on neutrophils, reducing the increase in the number of caveolae in the endothelial cells of mesentery post-capillary venules induced by I/R.
CONCLUSION: The results demonstrated that TSA protects from and ameliorates the microcirculation disturbance induced by I/R, which was associated with TSA inhibiting the production of oxygen-free radicals in the venular wall and the expression of CD11b/CD18 on neutrophils.
- Citation: Wang MX, Liu YY, Hu BH, Wei XH, Chang X, Sun K, Fan JY, Liao FL, Wang CS, Zheng J, Han JY. Total salvianolic acid improves ischemia-reperfusion-induced microcirculatory disturbance in rat mesentery. World J Gastroenterol 2010; 16(42): 5306-5316
- URL: https://www.wjgnet.com/1007-9327/full/v16/i42/5306.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i42.5306
Ischemia-reperfusion (I/R) injury occurs in a wide range of situations, including trauma, vascular reflow after contraction, percutaneous transluminal coronary angioplasty, thrombolysis treatment, organ transplantation, and hypovolemic shock with resuscitation[1], leading to systemic disorders with high morbidity and mortality. I/R-induced microcirculatory disturbance is the pathological basis of I/R injury, manifesting as a complex process that includes endothelial cell dysfunction, enhanced adhesion of leukocyte, macromolecular efflux, production of reactive oxygen species, and mast cell degranulation[2]. The insults triggered by I/R in microcirculation interplay with each other in various ways that creates a vicious circle that exaggerates the impact, among which, the explosively produced peroxides injure the vessels[2], promote expression of intercellular cell adhesion molecule-1 (ICAM-1)[3] and leukocyte adhesion molecules CD11b/CD18[4], leading to an increase in the adhesion of leukocytes to venules[5]. On the other hand, the leukocytes adhered to the venular wall, in turn, release peroxides and protease[6-9] that injure the endothelium and basement membrane of vessels, causing the leakage of serum[2]. Besides, the degranulated mast cell induced by I/R[2] release pro-inflammatory factors and vasoactive substances to attack the vessels from outside[8-10]. Clearly, an agent that targets the multiple insults simultaneously would be appealing for management of I/R-induced microcirculatory dysfunction. However, the study in this regard remains limited.
Total salvianolic acid (TSA) is the major water-soluble ingredient of Salvia miltiorrhiza (SM), including 3,4-dihydroxy-phenyl lactic acid (DLA), salvianolic acid A (Sal A), salvianolic acid B (Sal B) and other salvianolic acids[2]. SM and SM-containing preparations have been clinically used in the prevention and treatment of various vascular diseases[2]. Previous studies have shown that pre-treatment with Cardiotonic Pills (CP, the major ingredients of which are DLA and Sal B) attenuated the gut I/R-induced leukocyte adhesion in liver sinusoids and plasma tumor necrosis factor-α (TNF-α) and endotoxin[11], diminished the thrombi induced by photochemical stimulation in rat mesenteric venules[12], and prevented I/R-induced myocardial microcirculatory disturbance and myocardial damage in rats[13]. DLA attenuated the production of oxygen radicals and the expression of leukocyte adhesion molecules CD11b/CD18 in mesenteric microcirculatory disturbance induced by I/R[14]. Sal A has the potential of anti-oxidation[15-19], and may normalize membrane permeability[19]. Sal B could inhibit endotoxin-induced rat mesenteric microcirculation disturbance, inhibit the expression of adhesion molecule CD11b/CD18, production of hydrogen peroxide and negative oxygen anion[20-22]. However, the extraction process of DLA and Sal B is complex and the extraction conditions are hard to control, leading to a high cost for DLA and Sal B production. On the other hand, TSA, as the major water-soluble ingredient of SM, has the advantage that the extraction procedure is easily manipulated with a high output and low cost. However, whether TSA can improve microcirculatory disturbance induced by I/R is not clear. Therefore, in this study, we investigated the effect of TSA on I/R-induced rat mesenteric microcirculatory dysfunctions.
TSA was purchased from Kun Ming Feng-Shan-Jian Medical Company (Yun-Nan, China). Dihydrorhodamine 123 (DHR) was obtained from Molecular Probes Ltd. (Eugene, OR, USA). Fluorescein isothiocyanate (FITC)-albumin and toluidine blue were obtained from Sigma Chemical Co. (St Louis, MO, USA). FITC-labeled anti-CD11b, FITC-labeled anti-CD18 antibodies were from BD Biosciences Pharmingen (USA), all other chemicals used were of the highest grade available commercially.
Male Wistar rats, weighing 200-250 g, were obtained from the Animal Center of Peking University Health Science Center (Beijing, certificate No. SCXK 2002-0001), and randomly distributed into Sham group, I/R group, TSA group, TSA + I/R group and I/R + TSA group. The rats were fasted for 12 h before the experiment, allowing for free access to water. All animals were handled according to the guidelines of the Peking University Health Science Center Animal Research Committee, and the surgical procedures and experimental protocol were approved by Peking University Biomedical Ethics Committee Experimental Animal Ethics Branch.
Surgical procedure in the present study was almost the same as our previous study. Rats were anesthetized and the left jugular vein was cannulated for the infusion of TSA or saline. After the abdomen was opened, an ileocecal portion of the mesentery was gently mounted on a transparent plastic stage designed for the rat. The mesentery was kept warm and moist by continuous superfusion with saline solution at 37°C. The mesenteric microcirculation was observed by a transillumination method using an inverted microscope (DM-IRB, Leica, Germany). The mesentery was transilluminated with a 12-V, 100-W, direct current-stabilized light source. A color video camera (Jk-TU53H, Toshiba, Japan) was mounted on the microscope, and the image was transmitted onto a monitor (J2118A, TCL, Korea). The images were recorded with a Digital Video Disk (DVD) videocassette recorder (DVR-R25, Malata, China). Single unbranched venules with diameters ranging between 30 and 50 μm and length longer than 200 μm were selected for study[2].
After 10 min of basal observation of the hemodynamics in the rat mesenteric microvasculature, the I/R was accomplished by ligating the feeding branch of the anterior mesenteric artery and the corresponding vein simultaneously with a snare created by 2-0 silk suture for 10 min and subsequent release of the blood flow. Red blood cell velocity in the vessels during the ischemia was not zero because of the possible collateral perfusion to the observed area. Thus both artery and vein were ligated to stop blood supply and induce venule congestion to enhance ischemia. A previous study showed that 10-min ischemia followed by reperfusion was long enough to induce mesenteric microcirculatory disturbance with minimum intestinal tissue injury[5]. Sham-operated rats without I/R were used as control[14].
In the I/R group, the vehicle saline (8 mL/kg per hour) was infused via the left jugular vein catheter starting from 10 min before the ischemia and sustained until the end of the observation. The animals of the sham-operated group (Sham group) received the same infusion as those in the I/R group. The animals of the TSA group received only TSA (5 mg/kg per hour) until the end of the observation, without I/R surgery. In pre-treatment with TSA group (TSA + I/R group), the TSA (5 mg/kg per hour) was continuously infused via the left jugular vein catheter starting from 10 min before ischemia until the end of the observation. In post-treatment with TSA group (I/R + TSA group), the TSA (5 mg/kg per hour) was continuously infused via the left jugular vein catheter at the same doses as those in pre-treatment group but starting from 10 min after reperfusion until the end of the observation (Figure 1).
Venular diameter was measured on the recorded video images at baseline (before ischemia), 1, 10, 20, 30, 40, 50 and 60 min after reperfusion, using Image-Pro Plus 5.0 software (Media Cybernetic, USA). The diameter was presented as the mean of three measurements at one location[23].
The velocity of red blood cells (RBCs) in the venule was recorded at a rate of 1000 frames/s using a high-speed video camera system (Ektapro 1000, San Diego, CA, USA), and the recordings were replayed at a rate of 25 frames/s from the high-speed stored images. RBCs velocity in the venule was measured with Image-Pro Plus 5.0 software at baseline (before ischemia), 1, 10, 20, 30, 40, 50 and 60 min after reperfusion[23].
The number of leukocytes adhered to the venules was determined off-line during play-back of videotaped images. Leukocytes adhered to the venules were identified as cells that attached to the same site for more than 10 s judging from the replayed video images. The number of adherent leukocytes was counted along venules (30-50 μm in diameter, 200 μm in length) selected from the videotape images recorded and expressed as the number per 200 μm of venule length[23].
The oxidant-sensitive fluorescent probe dihydrorhodamine 123 (DHR; Molecular probes) was added to the mesenteric surface (10 μmol/L) to assess the oxidant stress in venular walls. The excitation light of 455 nm was irradiated from a mercury burner (100 W) to the inverted fluorescence microscope (DM-IRB, Leica, Germany) to observe the fluorescence. The fluorescent image was recorded at baseline (before ischemia), 1, 10, 20, 30, 40, 50 and 60 min after reperfusion, using CD recorder and the fluorescent intensity of venular walls and extravenular interstice was measured with Image-Pro Plus 5.0 software. The difference between the fluorescent intensity of venular wall and extravenular interstice at every time point was determined, and the ratio of the value at each time point to that at baseline was calculated[23].
In another set of experiments, to evaluate the albumin leakage across mesenteric venules, the animals were intravenously injected with 5 mg/kg body weight of FITC-labeled bovine serum albumin. After 10 min of basic observation, an excitation light (455 nm wavelength) was irradiated from a mercury burner (100 W) to the inverted fluorescence microscope (DM-IRB, Leica, Germany). At baseline (before ischemia), 1, 10, 20, 30, 40, 50 and 60 min after reperfusion, the image was recorded using a Compact Disk recorder. The fluorescence intensity of FITC-albumin inside the lumen of selected venules (Iv) and in the surrounding interstitial area (Ii) was estimated. The ratio of Ii/Iv was calculated and compared with the baseline as an indicator of albumin leakage[23].
Sixty minutes after reperfusion, the tissue was stained with 0.1% toluidine blue for 1 min and rinsed with saline. Degranulated mesenteric mast cells were identified by the presence of intracellular granules released into the surrounding tissue, and counted within each circular microscopic field of view with a 20 × objective lens. Five fields were evaluated along the microvasculature for each mesenteric window. The numbers of both non-degranulated and degranulated mast cells were scored, and the percentage of degranulated mast cells was thus calculated[23].
In another set of experiments, blood was taken from the abdominal aorta of rats and anticoagulated with heparin, and, afterward, incubated with FITC-labeled anti-CD11b antibody (5 μg/mL) or FITC-labeled anti-CD18 antibody (5 μg/mL) or corresponding FITC-labeled mouse isotype (5 μg/mL) for 20 min at room temperature. The erythrocyte lysis was accomplished using haemolysin according to the manufacturer’s instruction, and the cells were washed twice with PBS. The mean fluorescence intensity was calculated with flow cytometry (FACS Calibur; BD Company, USA). Neutrophils were then sorted by characteristic forward-/side-scatter expression as reported previously[23]. Five thousand neutrophils were evaluated for each sample.
The mesentery in each experiment condition was also prepared for electron microscopy. Briefly, immediately after 60 min perfusion, the rats, maintained under deep anesthesia, underwent perfusion through the left ventricle with physiological saline followed by 120 mL of phosphate-buffered 40 g/L paraformaldehyde plus 20 g/L glutaraldehyde at a speed of 3 mL/min. The mesentery tissues were then removed, localized and further fixed by immersion in phosphate-buffered 30 g/L glutaraldehyde for 1 h. The tissues were routinely processed for transmission electron microscopy and examined in JEM 1230 (JEOL, Japan)[24].
The data were analyzed by one-way ANOVA and Fisher’s post test. All values were expressed as mean ± SE of values from 6 rats. P < 0.05 was considered statistically significant.
No significant alteration was observed in the diameter of either arterioles or venules in mesenteric microcirculation during 60 min of observation, and the situation remained unchanged by continuous per- or post-treatment with TSA (data not shown).
I/R induced a significant decrease in the velocity of RBCs over the first 10 min after reperfusion. A similar alteration was observed in the TSA + I/R group and I/R + TSA group. The result demonstrated that pre- or post- treatment with TSA has no significant influence on the decrease of RBCs velocity induced by I/R (data not shown).
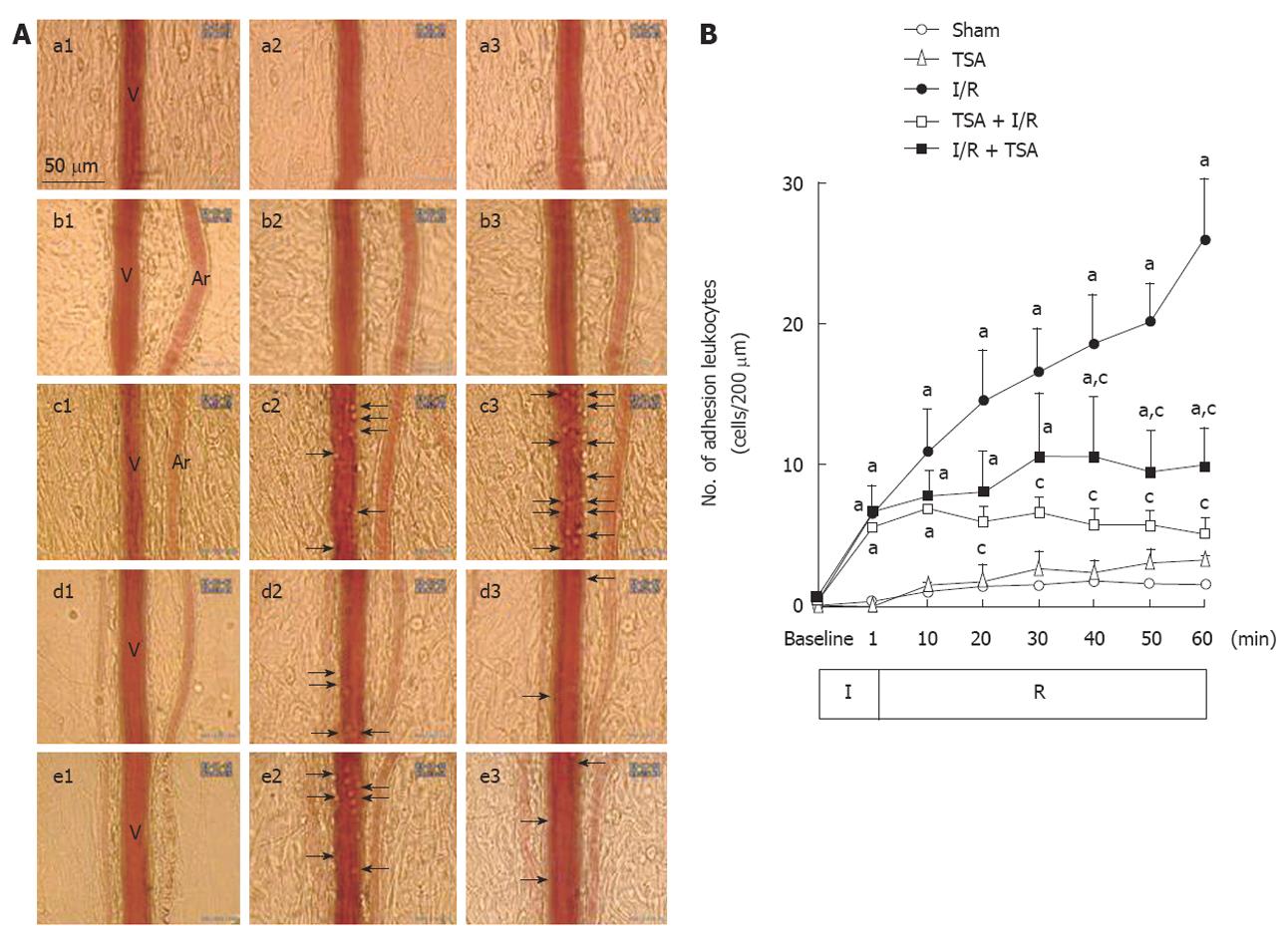
The images of leukocytes adhered to the venular walls in each group at baseline, 10 and 60 min after the reperfusion are illustrated in Figure 2A. No adherent leukocyte was observed before ischemia in each group (a1-e1). At 10 min after reperfusion, a small amount of adherent leukocytes were observed along the venular walls in the animals subjected to I/R (c2-e2). At 60 min after reperfusion, numerous adherent leukocytes were observed along the venular walls (c3) in I/R group. While in TSA+I/R group (d3) and I/R+TSA group (e3), only few of the adherent leukocytes were observed.
The time course of changes in the number of leukocytes adherent to venular walls was examined and the result is depicted in Figure 2B. Obviously, the number of adherent leukocytes in the Sham and TSA group increased only slightly during the whole period of observation. The number of adherent leukocytes was significantly increased from 10 min and further up to 60 min after reperfusion. Pre-treatment with TSA significantly decreased the number of adherent leukocytes at 20 min after reperfusion. Post-treatment with TSA significantly attenuated the further increase of adherent leukocytes from 40 min after reperfusion.
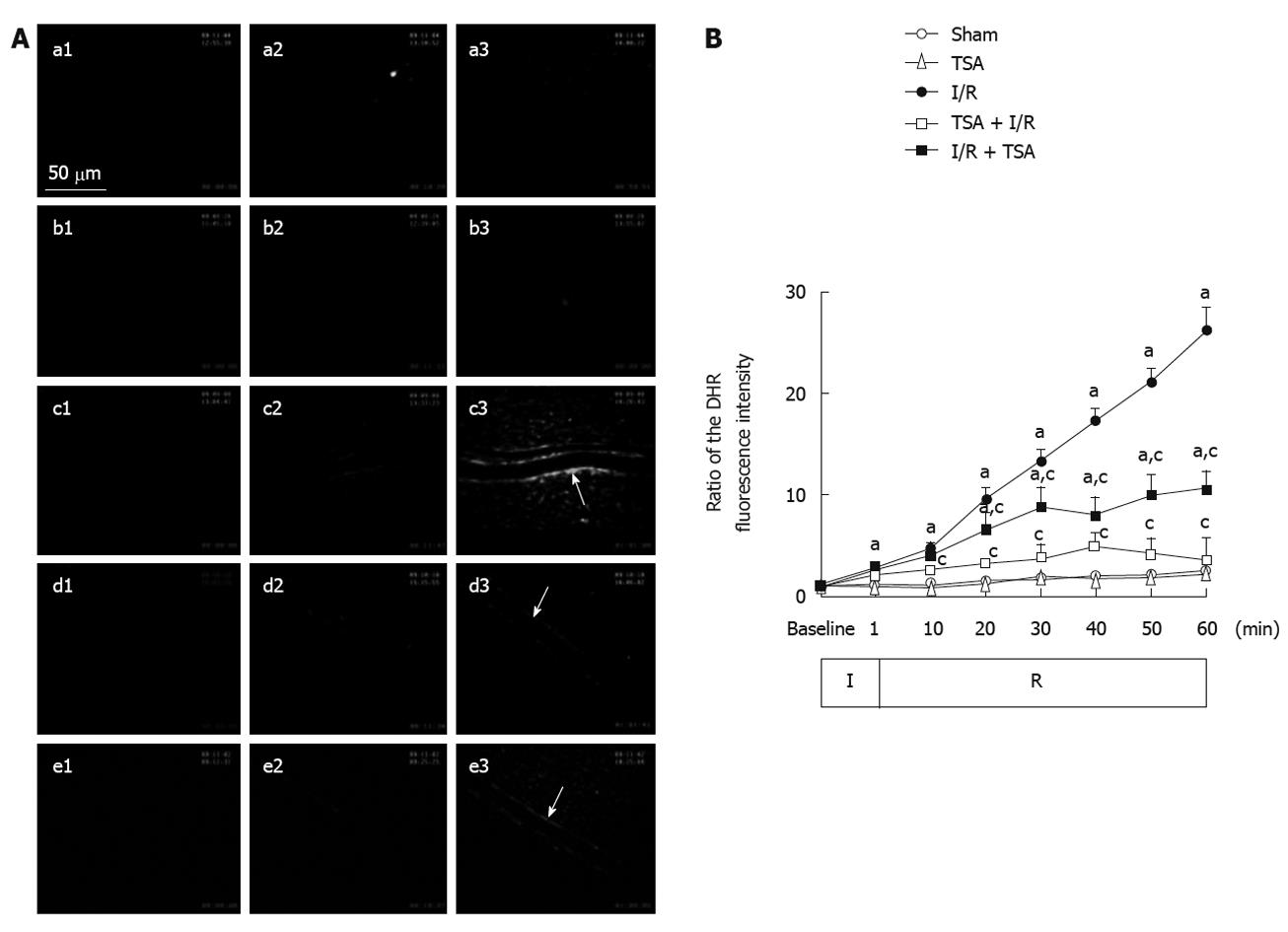
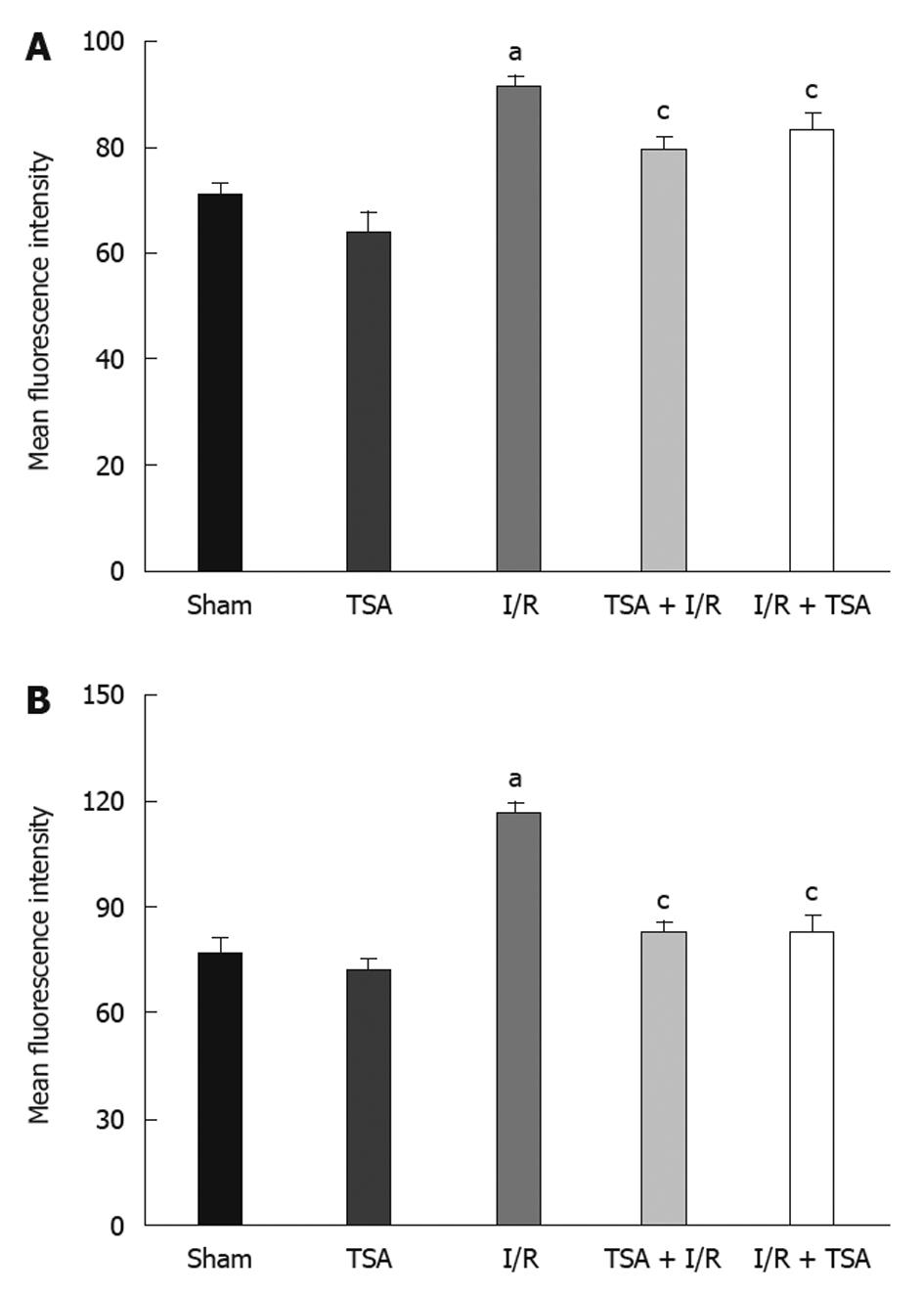
The images of fluorescence intensity of DHR in the venular walls in each group at baseline, 10 and 60 min after the reperfusion are illustrated in Figure 3A. No DHR fluorescence was detected on rat mesenteric venular walls before I/R (a1-e1). I/R induced a pronounced DHR fluorescence on mesenteric venular walls (c3). Pre-treatment with TSA significantly attenuated the I/R-induced DHR fluorescence enhancement (d3). Post-treatment with TSA inhibited the I/R-induced increase in DHR fluorescence ratio in a similar fashion (e3).
The time course of changes in DHR fluorescence ratio on the venular walls is presented in Figure 3B. In the Sham group and TSA group, there was no significant change in the DHR fluorescence ratio on the venular wall throughout the observation. In the I/R group, the intensity of DHR fluorescence on the venular wall increased apparently and lineally until the end of reperfusion. Pre-treatment with TSA apparently attenuated the I/R-induced DHR fluorescence intensity on the venular walls at 10 min after reperfusion. Post-treatment with TSA attenuated the further increase of DHR fluorescence intensity on the venular walls at 20 min after reperfusion, with the fluorescence intensity of DHR on the venular wall keeping nearly constant over the remaining time.
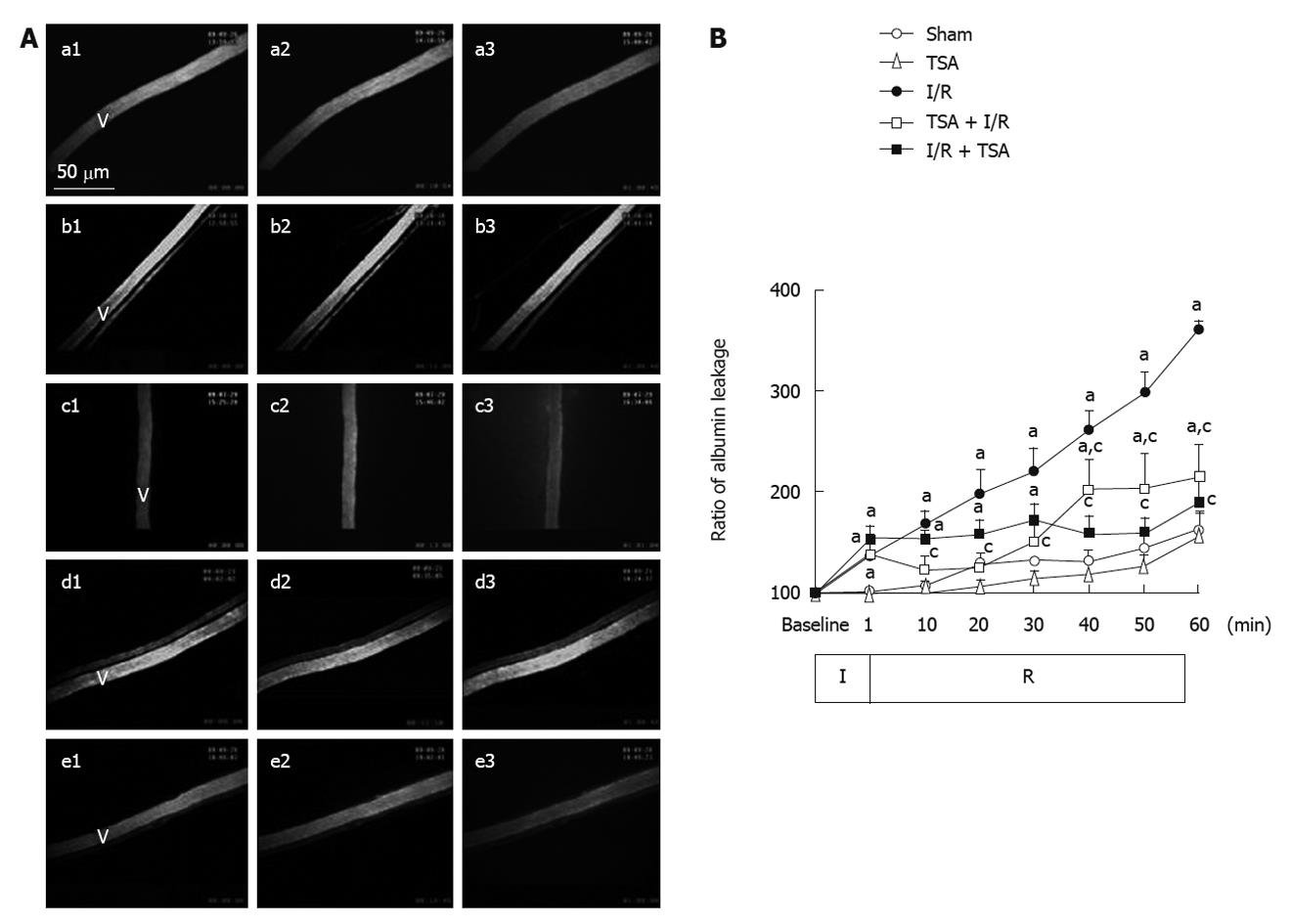
The images of albumin leakage in each group at baseline, 10 and 60 min after the reperfusion are illustrated in Figure 4A. No albumin leakage was observed before ischemia in all conditions (a1-e1). In the I/R group, apparent leakage was observed at 60 min after reperfusion (c3). Pre-treatment with TSA significantly prevented FITC-labeled albumin leakage from rat mesentery venules challenged by I/R (d3). Post-treatment with TSA prevented further increase in the albumin leakage (e3).
The changes in albumin leakage from the rat mesenteric venules were quantitated and the result is shown in Figure 4B. In the Sham group and TSA group, there was no significant change in the albumin leakage ratio from venular walls throughout the observation. In the I/R group, the albumin leakage from venules increased immediately after the initiation of reperfusion in a time-dependent manner. Pre-treatment with TSA significantly attenuated the albumin leakage from venular walls at 10 min after reperfusion. Post-treatment with TSA attenuated the further increase in the albumin leakage from venular walls from 20 min after reperfusion.
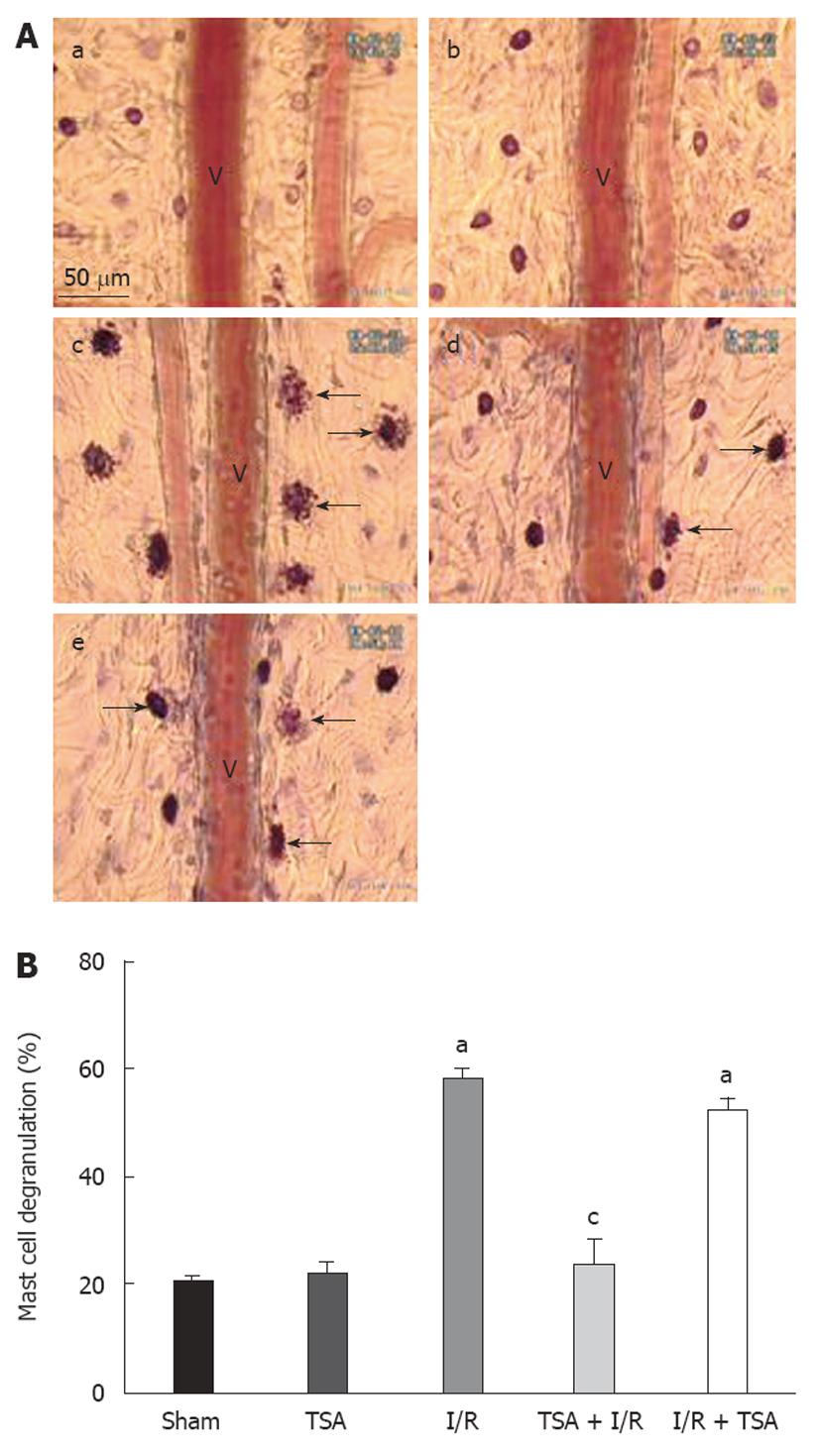
Mast cell degranulation was examined after 60 min of reperfusion in various conditions (Figure 5A). I/R evoked an apparent increase in mast cell degranulation (c), while pre-treatment with TSA significantly inhibited the mast cell degranulation (d). The results were quantified as the percent of the number of degranulated mast cells to the total number of mast cells examined (Figure 5B). The percentages of degranulated mast cells in the Sham group and TSA group were 20.7% ± 0.7% and 21.7% ± 2.5%, respectively, representing the spontaneous occurrence of the mast cell degranulation in the present case. I/R resulted in an apparent increase in the mast cell degranulation (62.2% ± 2.5%), and this increase was suppressed significantly by pre-treatment with TSA (23.7% ± 4.7%). Post-treatment with TSA almost had no effect on mast cell degranulation compared to I/R group (52.4% ± 2.2% vs 62.2% ± 2.5%).
An in vitro study was performed to determine the fluorescence intensity of adhesion molecules CD11b and CD18 in neutrophils (Figure 6). The fluorescence intensity of CD11b (Figure 6B) and CD18 (Figure 6A) was significantly increased by I/R compared to the Sham group. Pre-treatment with TSA significantly inhibited the increase in fluorescence intensity of CD11b and CD18 in neutrophils induced by I/R. A similar effect was observed for post-treatment with TSA.
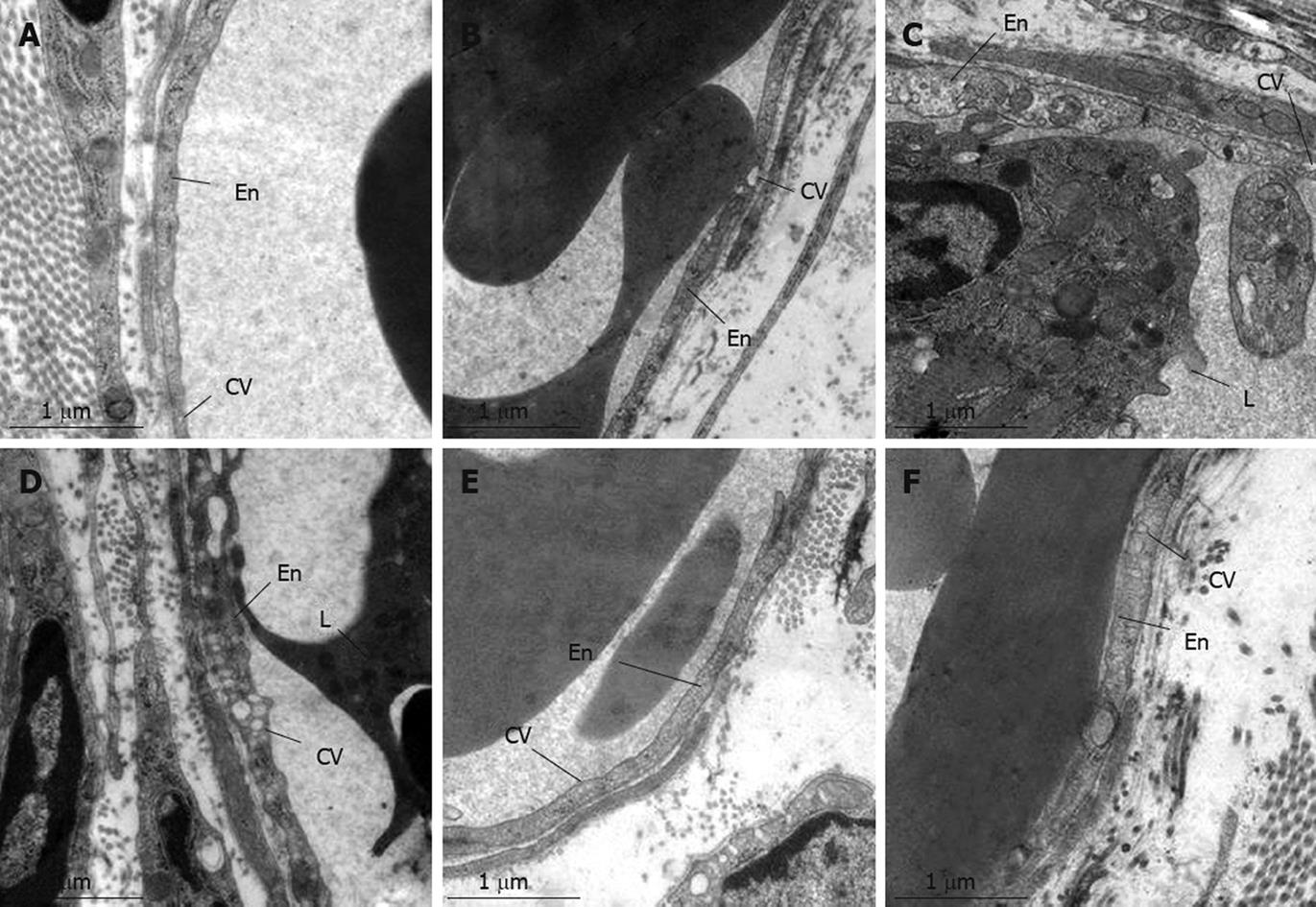
Figure 7 presents the electron micrographs of rat mesentery post-capillary venules in each group. In the Sham group (A) and TSA group (B) venules were lined by a layer of endothelial cells, which exhibited a rather smooth inner face with occasionally occurring vesicles in the cytoplasm. At 10 min after reperfusion (C) in I/R group, the adherent leukocyte and platelet were observed within the lumen of venules in rat mesentery, and numerous caveolae emerged in the endothelial cells of post-capillary venules. At 60 min after reperfusion, an even more apparent alteration in the ultrastructure of the endothelial cell occurred, characterized by the increase in the number and size of caveolae in the cytoplasm (D). I/R-induced alterations in the ultrastructures of endothelial cell were abated by pre-and post-treatment with TSA (E and F).
With the animal model of rat mesenteric microcirculation disturbance induced by I/R, this research demonstrated that pre-treatment with TSA has an improvement effect on the adhesion of leukocytes to venules, production of peroxides in the venular wall, albumin leakage and mast cell degranulation induced by I/R. Post-treatment with TSA displays an improvement action on the I/R-induced insults likewise, except for the mast cell degranulation.
The diameters of the mesenteric venules were not changed after I/R, and TSA had no effect on venular diameter during I/R, suggesting that TSA does not affect vascular tone. Therefore, TSA exerts protective effects on I/R-induced microcirculatory disturbance through a way other than vasodilation. In addition, both pre- and post- treatment with TSA had no apparent influence on the decrease of RBCs velocity induced by I/R, indicating that TSA does not affect the hemodynamics of the rats.
One of the interesting results in this study is that pre- and post-treatment with TSA inhibited the DHR fluorescence intensity in the venular walls following I/R. It has been reported that I/R challenge leads to production of oxygen-free radicals by xanthine oxidase[25], and leukocyte adhered to vascular walls generates a peroxide burst through the NADPH oxidase[26-29] is one of the reasons of injury in vascular endothelium and basement membrane[30,31]. So inhibition of the production of oxygen-free radical induced by I/R can help protect the blood vessels. Previous studies have shown that DLA can clear the negative oxygen anions produced through the xanthine oxidase system[32-34]. Sal A was reported to inhibit cerebral lipid peroxidation after I/R, and scavenge oxygen-free radical in vitro[18]. Sal B could eliminate 1,1-diphenyl-2-picrylhydrazyl (DPPH), inhibit lipid peroxidation[35] and production of reactive oxygen species[36], remove hydrogen peroxide in a dose-dependent way and inhibit the activity of NADPH and peroxide generation induced by TNF-α[37]. However, the effect of TSA on the production of oxygen free radicals on venular walls induced by I/R in vivo has not been reported. In the present study, we assessed the production of hydrogen peroxide (H2O2) in the venular wall with the aid of DHR, a fluorescence probe that transforms to rhodamine when reacting with hydrogen peroxide, and proved that TSA can inhibit the DHR fluorescence intensity on the venular walls following I/R. The mechanism for TSA to inhibit the hydrogen peroxide remains to be elucidated, and it is most likely that its ingredients DLA, Sal A and Sal B work in coordination through their own pathways to produce a collective outcome.
Another important observation in this study was that pre- or post-treatment with TSA can inhibit I/R induced adhesion of leukocytes to venular walls. It was reported that I/R promotes the expression of ICAM-1 on endothelium[38] and CD11b/CD18 in neutrophils, leading to the adhesion of leukocyte to vascular wall[39,40]. Inhibiting the adhesion of leukocytes to the vascular wall is another important part of improving the microcirculatory disturbance induced by I/R. Our previous study also demonstrated that DLA can inhibit I/R-induced adhesion of leukocyte to rat mesenteric venule and expression of adhesion molecule CD11 b/CD18 in vitro[20]. No report has been published so far concerning inhibition of TSA on leukocyte adhesion to venular walls induced by I/R. This study proved that pre- or post-treatment with TSA can inhibit I/R induced adhesion of leukocytes to venular walls, which was associated with the inhibition of TSA on the expression of CD11b/CD18 in neutrophils.
I/R induced mast cell degranulation, resulting in release of pro-inflammatory factors[41,42] and vasoactive substances that attack the vessels from outside, increasing the vascular damage. Inhibiting the degranulation of mast cells after I/R can reduce the attacks on vessels from outside. This study proved that pre-treatment with TSA can inhibit I/R induced mast cell degranulation. However, based on the result that post-treatment with TSA could not inhibit mast cells degranulation, the inhibitory effect of TSA upon mast cells degranulation is considered to be an indirect result.
In the present study, we demonstrated that pre- or post-treatment with TSA could inhibit FITC-albumin leakage from venules. It was documented in the lung[43] and myocardium[44] that the increased vascular permeability induced by inflammatory stimulations is due to the enlarged population of caveolae, which play a significant role in microvascular permeability[44]. In line with these findings, the result of electron microscopy in the present study showed that the number of caveolae in the endothelial cells of venules was apparently increased at 60 min after reperfusion, whereas the endothelial cells themselves and the intercellular junctions remained intact, implying that I/R-induced albumin leakage observed in the present situation was mainly accomplished by the caveola. The present study demonstrated that pre- or post-treatment with TSA could reduce the number of endothelial caveola as well as FITC-labeled albumin leakage from venular wall induced by I/R, suggesting that it inhibited albumin leakage from venular wall by modulating the number of endothelial caveola. In addition, the inhibition of TSA on FITC-albumin leakage may be also related to inhibition of peroxide generation and the adhesion of leukocytes to venules, thus protecting the venule from inside.
In summary, the present study demonstrated that pre-treatment with TSA significantly improved the microcirculatory dysfunction in rat mesentery induced by I/R. TSA post-treatment starting from 20-min reperfusion ameliorated I/R-induced microcirculatory disorders in rat mesentery alike but in a distinct manner. The in vitro experiment revealed that H2O2-elicited enhancement in the expression of CD11b and CD18 on neutrophils was significantly attenuated by treatment with TSA, raising the possibility that the ability of TSA to inhibit the leukocyte adhesion may be correlated with its potential to suppress the expression of adhesion molecules. It is likely that the antioxidant activity of TSA and its inhibition on the expression of adhesion molecules CD11b/CD18 on leukocyte are underlying its ameliorating action on I/R-induced microcirculatory disturbance in rat mesentery.
Ischemia-reperfusion (I/R) injury occurs in a wide range of situations, including trauma, vascular reflow after contraction, percutaneous transluminal coronary angioplasty, thrombolysis treatment, organ transplantation, and hypovolemic shock with resuscitation. I/R-induced microcirculatory disturbance with diverse manifestations is considered to be the pathological basis of I/R injury. An agent that targets the multiple insults simultaneously would be appealing for management of I/R-induced microcirculatory dysfunction. However, the study in this regard remains limited. Total salvianolic acid (TSA) is the major water-soluble ingredient of Salvia miltiorrhiza (SM), SM and SM-containing preparations have been clinically used in the prevention and treatment of various vascular diseases. However, whether the TSA can improve microcirculatory disturbance induced by I/R are not clear. The results of the present study provide evidence for the beneficial role of TSA in attenuating microcirculatory disorders, using I/R-induced rat mesenteric microcirculatory dysfunction as a model.
3,4-dihydroxy-phenyl lactic acid, salvianolic acid A and salvianolic acid B are the main ingredients of TSA. However, the extraction process of 3,4-dihydroxy-phenyl lactic acid, salvianolic acid A and salvianolic acid B is complex and the extraction conditions are hard to control. On the other hand, TSA, if taking as a whole, has the advantage of high output and low cost in preparation. The present work shows the potential of TSA for management of I/R-induced microcirculatory dysfunctions. Innovations and breakthroughs: this study proves, for the first time, the ability of TSA to inhibit I/R-induced dihydrorhodamine 123 fluorescence intensity, and adhesion of leukocytes to venular walls, the latter of which was possibly associated with the inhibition of TSA on the expression of CD11b/CD18 in neutrophils.
These results provide a theoretical support for TSA containing Chinese medicine intervention in microcirculatory disturbance induced by thrombolytic therapy or other I/R-related clinical events.
TSA is the water-soluble extract of SM, consisting of 3,4-dihydroxy-phenyl lactic acid, salvianolic acid A and salvianolic acid B as major ingredients.
The authors demonstrated the inhibitory effect of total salvianolic acid inhibiting several markers associated with ischemia-reperfusion injury. This paper was well-organized and well-investigated, and has a novel finding in this manuscript.
Peer reviewer: Yuji Naito, Professor, Kyoto Prefectural University of Medicine, Kamigyo-ku, Kyoto 602-8566, Japan
S- Editor Tian L L- Editor Rutherford A E- Editor Ma WH
| 1. | Han JY, Fan JY, Horie Y, Miura S, Cui DH, Ishii H, Hibi T, Tsuneki H, Kimura I. Ameliorating effects of compounds derived from Salvia miltiorrhiza root extract on microcirculatory disturbance and target organ injury by ischemia and reperfusion. Pharmacol Ther. 2008;117:280-295. |
| 2. | Han JY, Miura S, Akiba Y, Higuchi H, Kato S, Suzuki H, Yokoyama H, Ishii H. Chronic ethanol consumption exacerbates microcirculatory damage in rat mesentery after reperfusion. Am J Physiol Gastrointest Liver Physiol. 2001;280:G939-G948. |
| 3. | Jones SP, Trocha SD, Strange MB, Granger DN, Kevil CG, Bullard DC, Lefer DJ. Leukocyte and endothelial cell adhesion molecules in a chronic murine model of myocardial reperfusion injury. Am J Physiol Heart Circ Physiol. 2000;279:H2196-H2201. |
| 4. | Lan W, Harmon D, Wang JH, Ghori K, Shorten G, Redmond P. The effect of lidocaine on in vitro neutrophil and endothelial adhesion molecule expression induced by plasma obtained during tourniquet-induced ischaemia and reperfusion. Eur J Anaesthesiol. 2004;21:892-897. |
| 5. | Kurose I, Wolf R, Cerwinka W, Granger DN. Microvascular responses to ischemia/reperfusion in normotensive and hypertensive rats. Hypertension. 1999;34:212-216. |
| 6. | Panés J, Granger DN. Neutrophils generate oxygen free radicals in rat mesenteric microcirculation after abdominal irradiation. Gastroenterology. 1996;111:981-989. |
| 7. | Carden D, Xiao F, Moak C, Willis BH, Robinson-Jackson S, Alexander S. Neutrophil elastase promotes lung microvascular injury and proteolysis of endothelial cadherins. Am J Physiol. 1998;275:H385-H392. |
| 8. | Kubo Y, Fukuishi N, Yoshioka M, Kawasoe Y, Iriguchi S, Imajo N, Yasui Y, Matsui N, Akagi M. Bacterial components regulate the expression of Toll-like receptor 4 on human mast cells. Inflamm Res. 2007;56:70-75. |
| 9. | Patella V, Marinò I, Arbustini E, Lamparter-Schummert B, Verga L, Adt M, Marone G. Stem cell factor in mast cells and increased mast cell density in idiopathic and ischemic cardiomyopathy. Circulation. 1998;97:971-978. |
| 10. | Singh M, Saini HK. Resident cardiac mast cells and ischemia-reperfusion injury. J Cardiovasc Pharmacol Ther. 2003;8:135-148. |
| 11. | Horie Y, Han JY, Mori S, Konishi M, Kajihara M, Kaneko T, Yamagishi Y, Kato S, Ishii H, Hibi T. Herbal cardiotonic pills prevent gut ischemia/reperfusion-induced hepatic microvascular dysfunction in rats fed ethanol chronically. World J Gastroenterol. 2005;11:511-515. |
| 12. | Wang F, Liu YY, Liu LY, Guo J, Sun K, Wang CS, Fan JY, Han JY. Inhibition effect of cardiotonic pills on venous thrombosis induced in rat mesentery by photochemical reaction. Clin Hemorheol Microcirc. 2006;34:131-138. |
| 13. | Zhao N, Liu YY, Wang F, Hu BH, Sun K, Chang X, Pan CS, Fan JY, Wei XH, Li X. Cardiotonic pills, a compound Chinese medicine, protects ischemia-reperfusion-induced microcirculatory disturbance and myocardial damage in rats. Am J Physiol Heart Circ Physiol. 2010;298:H1166-H1176. |
| 14. | Han JY, Horie Y, Fan JY, Sun K, Guo J, Miura S, Hibi T. Potential of 3,4-dihydroxy-phenyl lactic acid for ameliorating ischemia-reperfusion-induced microvascular disturbance in rat mesentery. Am J Physiol Gastrointest Liver Physiol. 2009;296:G36-G44. |
| 15. | Wang SB, Yang XY, Tian S, Yang HG, Du GH. Effect of salvianolic acid A on vascular reactivity of streptozotocin-induced diabetic rats. Life Sci. 2009;85:499-504. |
| 16. | Lin TJ, Zhang KJ, Liu GT. Effects of salvianolic acid A on oxygen radicals released by rat neutrophils and on neutrophil function. Biochem Pharmacol. 1996;51:1237-1241. |
| 17. | Wang XJ, Wang ZB, Xu JX. Effect of salvianic acid A on lipid peroxidation and membrane permeability in mitochondria. J Ethnopharmacol. 2005;97:441-445. |
| 18. | Du G, Zhang J. Protective effects of salvianolic acid A against impairment of memory induced by cerebral ischemia-reperfusion in mice. Chin Med J (Engl). 1997;110:65-68. |
| 19. | Li L. [Protective effects of schisanhenol, salvianolic acid A and SY-L on oxidative stress induced injuries of cerebral cells and their mechanisms]. Shengli Kexue Jinzhan. 1998;29:35-38. |
| 20. | Guo J, Sun K, Wang CS, Fang SP, Horie Y, Yang JY, Liu YY, Wang F, Liu LY, Fan JY. Protective effects of dihydroxylphenyl lactic acid and salvianolic acid B on LPS-induced mesenteric microcirculatory disturbance in rats. Shock. 2008;29:205-211. |
| 21. | Du GH, Qiu Y, Zhang JT. Salvianolic acid B protects the memory functions against transient cerebral ischemia in mice. J Asian Nat Prod Res. 2000;2:145-152. |
| 22. | Chen YH, Du GH, Zhang JT. Salvianolic acid B protects brain against injuries caused by ischemia-reperfusion in rats. Acta Pharmacol Sin. 2000;21:463-466. |
| 23. | Yuan Q, Liu YY, Sun K, Chen CH, Zhou CM, Wang CS, Li A, Zhang SW, Ye ZL, Fan JY. Improving effect of pretreatment with yiqifumai on LPS-induced microcirculatory disturbance in rat mesentery. Shock. 2009;32:310-316. |
| 24. | Han JY, Horie Y, Miura S, Akiba Y, Guo J, Li D, Fan JY, Liu YY, Hu BH, An LH. Compound Danshen injection improves endotoxin-induced microcirculatory disturbance in rat mesentery. World J Gastroenterol. 2007;13:3581-3591. |
| 25. | Parks DA, Granger DN. Ischemia-induced vascular changes: role of xanthine oxidase and hydroxyl radicals. Am J Physiol. 1983;245:G285-G289. |
| 26. | Chen H, Song YS, Chan PH. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J Cereb Blood Flow Metab. 2009;29:1262-1272. |
| 27. | Hu Q, Yu ZX, Ferrans VJ, Takeda K, Irani K, Ziegelstein RC. Critical role of NADPH oxidase-derived reactive oxygen species in generating Ca2+ oscillations in human aortic endothelial cells stimulated by histamine. J Biol Chem. 2002;277:32546-32551. |
| 28. | Inauen W, Suzuki M, Granger DN. Mechanisms of cellular injury: potential sources of oxygen free radicals in ischemia/reperfusion. Microcirc Endothelium Lymphatics. 1989;5:143-155. |
| 29. | Wolin MS, Ahmad M, Gupte SA. Oxidant and redox signaling in vascular oxygen sensing mechanisms: basic concepts, current controversies, and potential importance of cytosolic NADPH. Am J Physiol Lung Cell Mol Physiol. 2005;289:L159-L173. |
| 30. | Zimmerman BJ, Grisham MB, Granger DN. Mechanisms of oxidant-mediated microvascular injury following reperfusion of the ischemic intestine. Basic Life Sci. 1988;49:881-886. |
| 31. | Moore TM, Khimenko PL, Taylor AE. Endothelial damage caused by ischemia and reperfusion and different ventilatory strategies in the lung. Chin J Physiol. 1996;39:65-81. |
| 32. | Cao EH, Liu XQ, Wang JJ, Xu NF. Effect of natural antioxidant tanshinone II-A on DNA damage by lipid peroxidation in liver cells. Free Radic Biol Med. 1996;20:801-806. |
| 33. | Yang R, Liu A, Ma X, Li L, Su D, Liu J. Sodium tanshinone IIA sulfonate protects cardiomyocytes against oxidative stress-mediated apoptosis through inhibiting JNK activation. J Cardiovasc Pharmacol. 2008;51:396-401. |
| 34. | Fu J, Huang H, Liu J, Pi R, Chen J, Liu P. Tanshinone IIA protects cardiac myocytes against oxidative stress-triggered damage and apoptosis. Eur J Pharmacol. 2007;568:213-221. |
| 35. | Lin YL, Wu CH, Luo MH, Huang YJ, Wang CN, Shiao MS, Huang YT. In vitro protective effects of salvianolic acid B on primary hepatocytes and hepatic stellate cells. J Ethnopharmacol. 2006;105:215-222. |
| 36. | Lin YH, Liu AH, Wu HL, Westenbroek C, Song QL, Yu HM, Ter Horst GJ, Li XJ. Salvianolic acid B, an antioxidant from Salvia miltiorrhiza, prevents Abeta(25-35)-induced reduction in BPRP in PC12 cells. Biochem Biophys Res Commun. 2006;348:593-599. |
| 37. | Zhang HS, Wang SQ. Salvianolic acid B from Salvia miltiorrhiza inhibits tumor necrosis factor-alpha (TNF-alpha)-induced MMP-2 upregulation in human aortic smooth muscle cells via suppression of NAD(P)H oxidase-derived reactive oxygen species. J Mol Cell Cardiol. 2006;41:138-148. |
| 38. | Han MK, Kim JS, Park BH, Kim JR, Hwang BY, Lee HY, Song EK, Yoo WH. NF-kappaB-dependent lymphocyte hyperadhesiveness to synovial fibroblasts by hypoxia and reoxygenation: potential role in rheumatoid arthritis. J Leukoc Biol. 2003;73:525-529. |
| 39. | Banda MA, Lefer DJ, Granger DN. Postischemic endothelium-dependent vascular reactivity is preserved in adhesion molecule-deficient mice. Am J Physiol. 1997;273:H2721-H2725. |
| 40. | Ren DC, Du GH, Zhang JT. Inhibitory effect of the water-soluble extract of Salvia miltiorrhiza on neutrophil-endothelial adhesion. Jpn J Pharmacol. 2002;90:276-280. |
| 41. | Rocha MF, Aguiar JE, Sidrim JJ, Costa RB, Feitosa RF, Ribeiro RA, Lima AA. Role of mast cells and pro-inflammatory mediators on the intestinal secretion induced by cholera toxin. Toxicon. 2003;42:183-189. |
| 42. | Wierzbicki M, Brzezińska-Błaszczyk E. [The role of mast cells in the development of inflammatory bowel diseases]. Postepy Hig Med Dosw (Online). 2008;62:642-650. |
| 43. | Heckel K, Kiefmann R, Dörger M, Stoeckelhuber M, Goetz AE. Colloidal gold particles as a new in vivo marker of early acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2004;287:L867-L878. |