Published online Jan 28, 2010. doi: 10.3748/wjg.v16.i4.508
Revised: November 19, 2009
Accepted: November 26, 2009
Published online: January 28, 2010
AIM: To evaluate the detection and differentiation ability of contrast-enhanced intraoperative ultrasonography (CE-IOUS) in hepatocellular carcinoma (HCC) operations.
METHODS: Clinical data of 50 HCC patients were retrospective analyzed. The sensitivity, specificity, false negative and false positive rates of contrast enhanced magnetic resonance imaging (CE-MRI), IOUS and CE-IOUS were calculated and compared. Surgical strategy changes due to CE-IOUS were analyzed.
RESULTS: Lesions detected by CE-MRI, IOUS and CE-IOUS were 60, 97 and 85 respectively. The sensitivity, specificity, false negative rate, false positive rate of CE-MRI were 98.2%, 98.6%, 98.6%, 60.0%, respectively; for IOUS were 50.0%, 90.9%, 1.8%, 1.4%, respectively; and for CE-IOUS were 1.4%, 40.0%, 50.0%, 9.1%, respectively. The operation strategy of 9 (9/50, 18.0%) cases was changed according to the results of CE-IOUS.
CONCLUSION: Compared with CE-MRI, CE-IOUS performs better in detection and differentiation of small metastasis and regenerative nodules. It plays an important role in the decision-making of HCC operation.
- Citation: Wu H, Lu Q, Luo Y, He XL, Zeng Y. Application of contrast-enhanced intraoperative ultrasonography in the decision-making about hepatocellular carcinoma operation. World J Gastroenterol 2010; 16(4): 508-512
- URL: https://www.wjgnet.com/1007-9327/full/v16/i4/508.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i4.508
The incidence rate of hepatocellular carcinoma (HCC) and cirrhosis in the hepatic B infection population is high in China, about 53.8%-85.9%, and more than 95% in some reports. There are several stages of hepatocarcinogenesis, from regenerative nodule, to degenerative nodule and to HCC. While comparing preoperative imaging results and pathological results after operation, the sensitivity of contrast enhanced magnetic resonance imaging (CE-MRI) is not satisfactory and can hardly detect some lesions[1,2]. It has been shown that intraoperative ultrasound (IOUS) is the most accurate diagnostic technique for detecting focal liver lesions (FLL) and has a great impact on the surgical approach to liver tumors[3,4]. However, in cirrhotic patients with HCC, not all nodules detected by IOUS are neoplastic[5]. How to differentiate small HCC from the nodules detected by IOUS poses a big challenge for surgeons. The application of intravenous ultrasound contrast agents during transcutaneous ultrasonography of the liver has been shown to improve nodule characterization in comparison with unenhanced ultrasound[6-10]. Therefore, we investigated whether the application of contrast-enhanced ultrasound examination intraoperatively could solve the aforementioned deficiencies of IOUS during liver exploration.
The data from 50 HCC patients, including 38 males, 12 females, mean age 45 years (range, 19-67 years) was retrospectively analyzed. Thirty nine cases had a history of hepatitis B infection and 2 of hepatitis C infection; nine had no hepatitis history. Three cases had undergone surgical resection for HCC before. Preoperative MRI, IOUS and contrast-enhanced intraoperative ultrasonography (CE-IOUS) were performed in 395 liver segments of 50 patients.
CE-MRI examinations were performed with a 1.5 T imaging system (Gyroscan Intera, Philips Medical Systems Best, Netherlands), using a breathhold 3D gradient echo sequence with fat saturation sequence, following an iv bolus of 0.1 mmol gadobenate dimeglumine (MultiHance, Bracco SpA, Milan, Italy) per kg of body weight at a rate of 2 mL/s. Data was acquired in the hepatic arterial, portal venous, and equilibrium phases.
IOUS scans were done by a VIVID4 (GE, US) ultrasound system with I-shaped 10-4 MHz intraoperative probe. After mobilization of the liver, IOUS was performed to search for nodules. Suspected lesions were counted and mapped.
CE-IOUS scans were carried out both for lesion characterization and new nodule detection. Since no specific intraoperative probe is available for contrast study, we used the IU22 unit (Philips, USA) equipped with a 5-2 MHz convex transducer and a 9-3 MHz linear transducer instead. Both of the probes have the capacity for contrast enhanced ultrasound studies. The contrast agent was SonoVue (Bracco Imaging, Milan, Italy) which consists of sulphur hexafluoride microbubbles stabilized by a phospholipid shell; 4.8 mL of SonoVue per exploration was injected intravenously through a peripheral vein. A low mechanical index (MI) < 0.1 mode was used. All phases of contrast enhancement, including arterial (10-20 s to 25-35 s after injection), portal (30-45 s to 120 s) and late parenchymal (> 120 s) phases were recorded and analyzed.
In the CE-IOUS study, HCC is characterized by arterial phase hyper-enhancing and wash out of microbubbles during the portal and late phase, while benign solid lesions are characterized by persistence of contrast enhancement during the portal and late phase.
Lesions considered malignant were removed surgically. Ultrasound-guided biopsy and ethanol ablation would be an alternative if the lesion can not be removed surgically. Nodules regarded as benign were removed only in cases located close to the main lesion and others were followed by examinations of α-fetoprotein (AFP) level and ultrasound and/or CE-MRI every 3 mo for more than 6 mo.
Pathologic examination was taken as the golden standard. Those unresected lesions with negative findings during 3 mo follow-up were regarded benign.
χ2 tests were used to analyze the data, P < 0.05 was considered to be statistically significant.
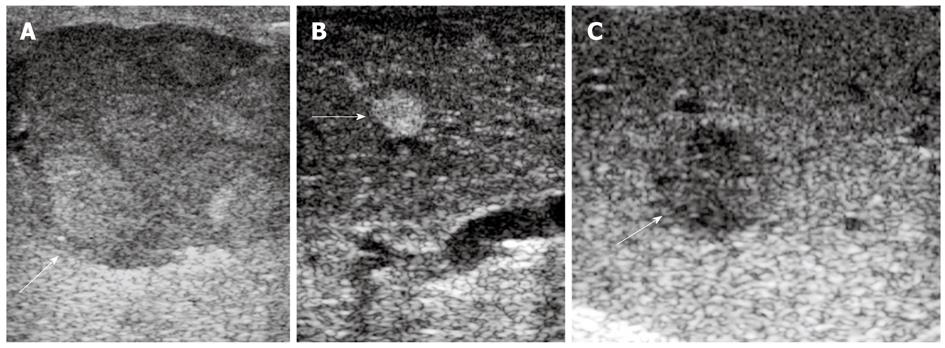
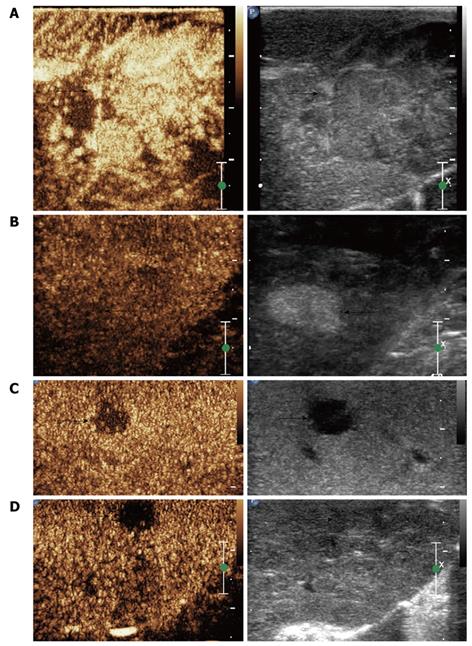
Preoperative MRI detected 60 lesions in total and IOUS found 96 nodules in 50 patients (Figure 1A-C). A total of 85 lesions were detected by CE-IOUS, among them, 73 HCC (Figure 2A and C) and 7 benign nodules (Figure 2B) were proved by pathology; another 5 lesions which were considered benign were not removed. Follow-up ultrasound showed no sign of malignancy with normal AFP levels after 6-15 mo in 4 patients and the size of the lesion in the other patient increased during 3 mo follow up. Further surgery was performed in this patient and proved to be HCC at histology.
Malignant and benign lesions detected by CE-MRI, IOUS and CE-IOUS were listed in Table 1.
| Malignant lesions | Pathology | Benign lesions | Pathology + follow up | |
| CE-MRI | 56 | 54 | 4 | 3 |
| IOUS | 89 | 72 | 8 | 7 |
| CE-IOUS | 74 | 73 | 11 | 10 |
The sensitivity, specificity, false negative ratio and false positive ratio of CE-MRI, IOUS and CE-IOUS, respectively, were shown in Table 2.
Particularly, one isoechoic HCC nodule was missed by IOUS, but showed a typical contrast agent wash-out pattern on CE-IOUS late parenchymal phase (Figure 2D). Another hypo-enhanced nodule was diagnosed as malignant by CE-IOUS but proved to be a necrotic nodule at histology.
Among 18 additional malignant lesions detected by CE-IOUS, 1 patient had 3 lesions, 4 patients had 2 lesions, and 7 patients had 1 lesion. The size of lesions was 5-20 mm (mean 12 mm). Surgical strategies of 9 patients (18.0%, 9/50) were changed because newly detected lesions were not in the same segment as the old ones (Table 3).
Recently, the incidence of HCC has had a tendency to increase and radical resection is considered to be the most effective therapy[11,12]. The rate of HCC with cirrhosis is high in China; therefore, differentiation of regenerative nodules from malignant ones plays an important role in the decision-making about surgery. However, the performance of preoperative CE-MRI is not satisfactory in detection of small lesions and unenhanced IOUS can hardly make the differential diagnosis of regenerative nodules from malignant ones in cirrhotic patients. The purpose of our research is to find a better imaging method with high sensitivity and specificity.
CE-IOUS is a real time gray scale imaging with low mechanical index (MI), which can clearly show microcirculation and perfusion of a tumor. HCC is a typical hyper-vascular tumor with the majority of blood contained in microvessels which can be demonstrated by CE-IOUS. The contrast agent we used in our research is SonoVue which consists of sulphur hexafluoride microbubbles stabilized by a phospholipid shell. Microbubbles of SonoVue can stay in blood for about 8 min which makes it possible for us to observe dynamic changes of liver enhancement. Pulse inversion harmonic technology can use non-linear signals in low acoustic pressure while restraining the linear signals from liver parenchyma[13], so it has the high sensitivity of harmonic signal detection.
Sensitivity of CE-MRI (98.2%), IOUS (98.6%) and CE-IOUS (98.6%) were high, but compared with CE-IOUS, the specificity of CE-MRI and IOUS were fairly poor. In our study, 85 lesions were diagnosed in 50 patients finally, preoperative CE-MRI only detected 60 lesions, indicating the diagnosis rate of CE-MRI for micro lesions is poor. In the 97 lesions that were detected by IOUS, only 74 malignant lesions were finally diagnosed, and the false positive rate of IOUS is too high compared with CE-IOUS. Consequently, we believe that CE-IOUS is an ideal diagnostic method in the decision-making about hepatocellular carcinoma surgery. Among 85 lesions detected by CE-IOUS, we had one false negative and one false positive case. The reason for the false negative case is that the minor lesion was located under the right diaphragm, and the probe we used for CE-IOUS was too big to thoroughly scan that area. The reason for the false positive case is that the minor nodule was a necrosis nodule. Parenchymal phase was used to search for malignant nodules during the CE-IOUS study and both necrotic nodules and small HCC shows hypoenhancement in parenchymal phase.
CE-MRI had 19 false negative cases (27%), with size 5-20 mm (mean 12 mm). Among these, 3 HCCs and 5 metastatic nodules had typical hyper-enhancement in artery phase and hypo-enhancement in portal phase in CE-IOUS. All patients accepted surgery and malignancy was proved by pathological results. Research found that CE-IOUS with low MI is more sensitive than CE-MRI in revealing artery perfusion of liver tumors. Time resolution of CE-MRI is relatively low, so it can not observe the dynamic enhancement of lesions, which is very important in differential diagnosis of HCC. Therefore, CE-IOUS is proved to be another sensitive imaging method with high diagnostic value[14].
CE-IOUS is of vital importance in detection and differentiation of lesions which were not detected pre-operatively with other imaging methods. Newly found tumors in pre-considered normal liver segments may lead to expanded resection, or combination treatment with radiofrequency ablation and ethanol injection. Sometimes, surgeons were obliged to give up or change their operation strategy. It had been reported that IOUS changed 18%-51% of operation strategies in liver metastasis patients with rectal cancer[15-19]. Eighteen newly detected malignant liver nodules by CE-IOUS in all 50 patients were proved by histology. The primary operation strategy was changed in 9 cases but not in another 3 cases. Two cases proved to have micro-metastatic lesions in another lobe of the liver during operation which can not be radical cured. One had half liver resection and ethanol injection of another nodule. Another patient had right part of liver resection and ethanol injection of another nodule. After operation, the patient’s AFP level decreased to normal and gradually increased over 2 and 4 mo later, he received cadaveric liver transplantation.
In our preliminary study, CE-IOUS is proved to be better than CE-MRI and IOUS in detecting and differentiating micro-metastatic liver lesions and hyperplastic nodules, which helped decision-making about surgical strategy. However its impact on increasing the long-term survival rate need further follow-up.
In cirrhotic patients with hepatocellular carcinoma (HCC), preoperative imaging was unsatisfactory in detection of some lesions. Intraoperative ultrasonography (IOUS) was sensitive in finding small lesions but not all nodules detected by IOUS are neoplastic. How to differentiate small HCC from the nodules detected by IOUS poses a big challenge for surgeons. Contrast enhanced ultrasound of the liver has been shown to improve nodule characterization. Therefore, the authors investigated if the application of contrast-enhanced ultrasound examination intraoperatively could solve the aforementioned problems during liver exploration.
Radical resection is the goal of surgery for HCC and the key is to find all the malignant lesions.
CE-IOUS proved to be better than CE-MRI and IOUS in detecting and differentiating micro-metastatic liver lesions and hyperplastic nodules, which helped decision-making about operation strategy.
The application of CE-IOUS in surgery for HCC has a positive impact on the decision making about surgical strategy.
CE-IOUS is an ultrasound exam performed by applying contrast agent during surgery. Besides the high sensitivity of detecting focal liver lesion, it shows tumor vascularity and tissue microcirculation, thus helping differentiate malignant nodules from benign ones.
This article aims to determine the role of CE-IOUS vs IOUS vs CE-MRI in the detection and characterization of suspicious nodules in cirrhotic patients.
Peer reviewer: Brian Kim Poh Goh, MBBS, MMed, MSc, FRCS, FAMS, Department of Surgery, Singapore General Hospital, Outram Road, Singapore 169608, Singapore
S- Editor Wang YR L- Editor O’Neill M E- Editor Ma WH
| 1. | Shirabe K, Shimada M, Gion T, Hasegawa H, Takenaka K, Utsunomiya T, Sugimachi K. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg. 1999;188:304-309. |
| 2. | Krinsky GA, Lee VS, Theise ND, Weinreb JC, Morgan GR, Diflo T, John D, Teperman LW, Goldenberg AS. Transplantation for hepatocellular carcinoma and cirrhosis: sensitivity of magnetic resonance imaging. Liver Transpl. 2002;8:1156-1164. |
| 3. | Cerwenka H. Intraoperative ultrasonography during planned liver resections remains an important surgical tool. Surg Endosc. 2008;22:1137-1138. |
| 4. | Kane RA. Intraoperative ultrasonography: history, current state of the art, and future directions. J Ultrasound Med. 2004;23:1407-1420. |
| 5. | Kruskal JB, Kane RA. Intraoperative US of the liver: techniques and clinical applications. Radiographics. 2006;26:1067-1084. |
| 6. | Takigawa Y, Sugawara Y, Yamamoto J, Shimada K, Yamasaki S, Kosuge T, Makuuchi M. New lesions detected by intraoperative ultrasound during liver resection for hepatocellular carcinoma. Ultrasound Med Biol. 2001;27:151-156. |
| 7. | Bartolotta TV, Taibbi A, Galia M, Runza G, Matranga D, Midiri M, Lagalla R. Characterization of hypoechoic focal hepatic lesions in patients with fatty liver: diagnostic performance and confidence of contrast-enhanced ultrasound. Eur Radiol. 2007;17:650-661. |
| 8. | Brannigan M, Burns PN, Wilson SR. Blood flow patterns in focal liver lesions at microbubble-enhanced US. Radiographics. 2004;24:921-935. |
| 9. | Cosgrove D, Blomley M. Liver tumors: evaluation with contrast-enhanced ultrasound. Abdom Imaging. 2004;29:446-454. |
| 10. | Morin SH, Lim AK, Cobbold JF, Taylor-Robinson SD. Use of second generation contrast-enhanced ultrasound in the assessment of focal liver lesions. World J Gastroenterol. 2007;13:5963-5970. |
| 11. | Jievaltas M, Stoskuviene L, Petrenkiene V, Barauskas G, Pundzius J. Results of treatment of primary liver cancer at Kaunas University of Medicine Hospital. Medicina (Kaunas). 2004;40:127-134. |
| 12. | Capussotti L, Muratore A, Massucco P, Ferrero A, Polastri R, Bouzari H. Major liver resections for hepatocellular carcinoma on cirrhosis: early and long-term outcomes. Liver Transpl. 2004;10:S64-S68. |
| 13. | von Herbay A, Vogt C, Häussinger D. Late-phase pulse-inversion sonography using the contrast agent levovist: differentiation between benign and malignant focal lesions of the liver. AJR Am J Roentgenol. 2002;179:1273-1279. |
| 14. | Lu Q, Luo Y, Yuan CX, Zeng Y, Wu H, Lei Z, Zhong Y, Fan YT, Wang HH, Luo Y. Value of contrast-enhanced intraoperative ultrasound for cirrhotic patients with hepatocellular carcinoma: a report of 20 cases. World J Gastroenterol. 2008;14:4005-4010. |
| 15. | Krix M, Kiessling F, Essig M, Herth F, Karcher A, Le-Huu M, Kauczor HU, Delorme S. Low mechanical index contrast-enhanced ultrasound better reflects high arterial perfusion of liver metastases than arterial phase computed tomography. Invest Radiol. 2004;39:216-222. |
| 16. | Zacherl J, Scheuba C, Imhof M, Zacherl M, Längle F, Pokieser P, Wrba F, Wenzl E, Mühlbacher F, Jakesz R. Current value of intraoperative sonography during surgery for hepatic neoplasms. World J Surg. 2002;26:550-554. |
| 17. | Cervone A, Sardi A, Conaway GL. Intraoperative ultrasound (IOUS) is essential in the management of metastatic colorectal liver lesions. Am Surg. 2000;66:611-615. |
| 18. | Conlon R, Jacobs M, Dasgupta D, Lodge JP. The value of intraoperative ultrasound during hepatic resection compared with improved preoperative magnetic resonance imaging. Eur J Ultrasound. 2003;16:211-216. |