Published online Oct 21, 2010. doi: 10.3748/wjg.v16.i39.4932
Revised: June 4, 2010
Accepted: June 11, 2010
Published online: October 21, 2010
AIM: To investigate the role of hepatitis B virus X-protein (HBx)-induced reactive oxygen species (ROS) on liver carcinogenesis in HBx transgenic mice and HepG2-HBx cells.
METHODS: Cell growth rate was analyzed, and through western blotting, mitogenic signaling was observed. Endogenous ROS from wild and HBx transgenic mice and HepG2-Mock and HBx cells were assayed by FACScalibur. Identification of oxidized and reduced phosphatase and tensin homolog (PTEN) was analyzed through N-ethylmaleimide alkylation, nonreducing electrophoresis.
RESULTS: We observed that the cell-proliferation-related phosphoinositide 3-kinase/Akt pathway is activated by HBx in vivo and in vitro. Increased ROS were detected by HBx. Tumor suppressor PTEN, via dephosphorylation of Akt, was oxidized and inactivated by increased ROS. Increased oxidized PTEN activated the mitogenic pathway through over-activated Akt. However, treatment with ROS scavenger N-acetyl cysteine can reverse PTEN to a reduced form. Endogenously produced ROS also stimulated HBx expression.
CONCLUSION: HBx induced ROS promoted Akt pathways via oxidized inactive PTEN. HBx and ROS maintained a positive regulatory loop, which aggravated carcinogenesis.
-
Citation: Ha HL, Yu DY. HBx-induced reactive oxygen species activates hepatocellular carcinogenesis
via dysregulation of PTEN/Akt pathway. World J Gastroenterol 2010; 16(39): 4932-4937 - URL: https://www.wjgnet.com/1007-9327/full/v16/i39/4932.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i39.4932
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide and the third most common cause of cancer mortality. Among other risk factors (including alcohol abuse, cirrhosis, and aflatoxin B1), chronic hepatitis B virus (HBV) infection plays a central role in the etiology of HCC[1]. About 53% of HCC cases are related to HBV, and the risk of HCC in chronic HBV carriers is approximately 100 times greater than in uninfected individuals[2]. Among the four proteins encoded by the HBV genome, X protein (HBx) is a multifunctional regulatory protein that is closely linked to HCC, but its role in tumor growth has not been fully clarified. Prior work from this laboratory has shown that HBx induces liver cancer in transgenic mice[3]. HBx does not bind directly to DNA, but affects transcriptional activation through interaction with nuclear transcription factors and by cytoplasmic modulation of signal transduction pathways[4]. HBx also mediates the activation of the Ras/Raf/extracellular signal-regulated kinase and mitogen-activated protein kinase kinase kinase-1/c-Jun NH2-terminal kinase cascades, which leads to the induction of activator protein-1 and nuclear factor κB[5,6]. One of the most well-known pathways activated by HBx is phosphoinositide 3-kinase (PI3K)/Akt, which is associated with anti-apoptotic activity and cell proliferation[7-9]. Therefore, HBx is thought to be associated with the development of human HCC, but the precise function of HBx in the tumorigenic transformation of liver cells remains unclear.
Previous studies have indicated that HBx protein directly interacts with the membrane proteins of mitochondria, the major site of reactive oxygen species (ROS) production, and alters the mitochondrial membrane potential in a hepatoma cell line. HBx also increases the level of mitochondrial ROS and lipid peroxide production[10]. The results of many previous studies have shown that normal cells exposed to low levels of H2O2 can increase their proliferation[11]. In this context, many types of cancer cells manifest increased production of H2O2[12].
Protein tyrosine phosphatases (PTPs) are a group of enzymes that remove phosphate groups from phosphorylated tyrosine residues on proteins. Together with tyrosine kinases, PTPs regulate the phosphorylation state of many important signaling molecules. They have been suggested to be direct targets of H2O2[13,14]. In general, PTPs exert an inhibitory effect on cancer signaling by opposing the tyrosine phosphorylation initiated by activated receptor kinases. Cell stimulation induces the transient activation of class-I PI3K, and the subsequent production of PI 3,4,5-trisphosphate (PIP3) which is important for the activation of a variety of downstream signaling molecules, including the protein kinase Akt, that mediate promotion of cell proliferation and survival[15]. The reaction catalyzed by PI3K is reversed by phosphatase and tensin homolog (PTEN), which functions as a PIP3 3-phosphatase. Indeed, by negatively modulating the PI3K signaling pathway, PTEN acts as a tumor suppressor. PTEN is also a member of the PTP family. It has been previously demonstrated that Cys-124 in the catalytic site of human PTEN is readily oxidized by exogenous H2O2 to form a disulfide with Cys-71[16].
In the present study, we attempted to determine the effect of HBx on the activated Akt pathways. We showed that HBx-produced H2O2 induces reversible inactivation of PTEN and activation of Akt. We suggest that scavenging H2O2 could be a therapeutic target for abnormal cell signaling to reactivate PTEN.
The production of HBx transgenic mice used in this study has been reported previously[3]. HBx homozygous (+/+) transgenic mice were produced by mating HBx heterozygous transgenic mice with each other. To generate HBx homozygous transgenic mice on a mixed background of C57BL/6 and CBA strains, HBx homozygous mice with C57BL/6 backgrounds were crossed with CBA wild-type mice. The heterozygous transgenic offspring with a mixed background of C57BL/6 and CBA strains were cross mated. Among their offspring, HBx homozygous transgenic mice were selected by genotyping the next generation. Selected mice were then crossed up to F12, which is applicable for the study as an inbred strain with a mixed genetic background (C57BL/6 and CBA). In the current study, these F12 mice were used for in vivo analyses. HBx (+/+) transgenic mice were verified by polymerase chain reaction (PCR) analysis. The PCR primers used were as follows: one set was sense primer 5'-TTCTCATCTGCCGGTCCGTG-3' and antisense primer 5'-GGGTCAATGTCCATGCCCCA-3', and another set was sense primer 5'-GAAAACACACTCACTGTTCAGAG-3' and antisense primer 5'-GTAAGCCGCTTTCTCTTATGCAG-3'. The wild-type mice were derived from littermates between HBx heterozygous transgenic male and female mice, with a mixed genetic background (C57BL/6 and CBA). Mice were housed in a specific pathogen-free environment. Mice were maintained in accordance with the guidelines of the Institutional Animal Care and Use Committee at the Korea Research Institute of Bioscience and Biotechnology (Daejeon, Korea).
HepG2-HBx cells derived from HepG2 cells were stably transfected and expressed HBx. HepG2 cells were grown in an atmosphere that contained 5% CO2 at 37°C in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin and 100 U/mL streptomycin.
Cell proliferation was determined by the crystal violet staining method, as described previously[17].
Proteins (20 μg/sample) were separated on 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (Millipore, Bedford, MA, USA). The membranes were blotted at 4°C overnight with primary antibodies. The membranes were washed five times with 10 mmol/L Tris-HCl (pH 7.5) plus 150 mmol/L NaCl (Tris-buffered saline; TBS) that contained 0.2% Tween-20, and incubated with horseradish peroxidase (HRP)-conjugated IgG. After the removal of excess antibodies by washing with TBS, specific binding was detected using a chemiluminescence detection system (Amersham, Berks, UK) according to the manufacturer’s instructions. Mouse monoclonal antibody to PTEN was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit polyclonal antibodies to phospho-Akt (Ser-473), Akt, and monoclonal antibodies to cyclin D1, were purchased from Cell Signaling Technology (Beverly, MA, USA). Rabbit polyclonal antibodies to GAPDH were from Lab Frontier (Seoul, Korea), and HRP-conjugated goat antibodies to mouse or rabbit IgG were from Amersham and Sigma.
Total RNA was isolated from the HepG2-HBx cells, or liver tissues from HBx transgenic mice, using TRIzol reagent (Invitrogen, Seoul, Korea) according to the manufacturer’s specifications. The concentration of total RNA in the final elutes was determined by nano-drop. Total RNA was converted into single-strand cDNA using a cDNA synthesis kit (Fermentas, Glen Burnie, MD, USA). Amplification of the target genes by real-time reverse transcriptase (RT)-PCR was conducted using SYBR Green (Takara, Otsu, Shiga, Japan) followed by analysis using the ExicyclerTM 96 Real-Time Quantitative Thermal block (Bioneer, Daejeon, Korea). Relative gene expression was calculated using the comparative Ct (2-ΔΔCt) method.
Cells were harvested, washed once with PBS, and resuspended in 0.2 mL 100 mmol/L Tris-HCl (pH 6.8) that contained 2% SDS and 40 mmol/L N-ethylmaleimide (Sigma). Protein (20 μg/sample) was loaded and subjected to SDS-PAGE under nonreducing conditions. The separated proteins were then transferred to nitrocellulose membranes and immunoblotted with a mouse anti-PTEN antibody. Binding was detected by an HRP-conjugated anti-mouse Ig (1:10 000, Sigma) and enhanced chemiluminescence reagents (Pierce, Rockford, IL, USA).
Hepatocytes were isolated using the same methods as previously reported[18].
Cells treated with 500 μmol/L H2O2 and 10 mmol/L N-acetylcysteine (NAC) were stained for 15 min with 5 μmol/L H2O2-sensitive fluorescent dye dichlorofluorescein diacetate (DCFDA, FL-1; Molecular Probes, Eugene, OR, USA) at 37°C in the dark, washed three times with PBS, and subsequently assayed by FACSCalibur (BD Biosciences, San Jose, CA, USA).
Comparisons were analyzed for statistical significance by unpaired or paired Student’s t test using Microsoft Excel software. P < 0.001 was considered as significant. All data are reported as mean ± SD.
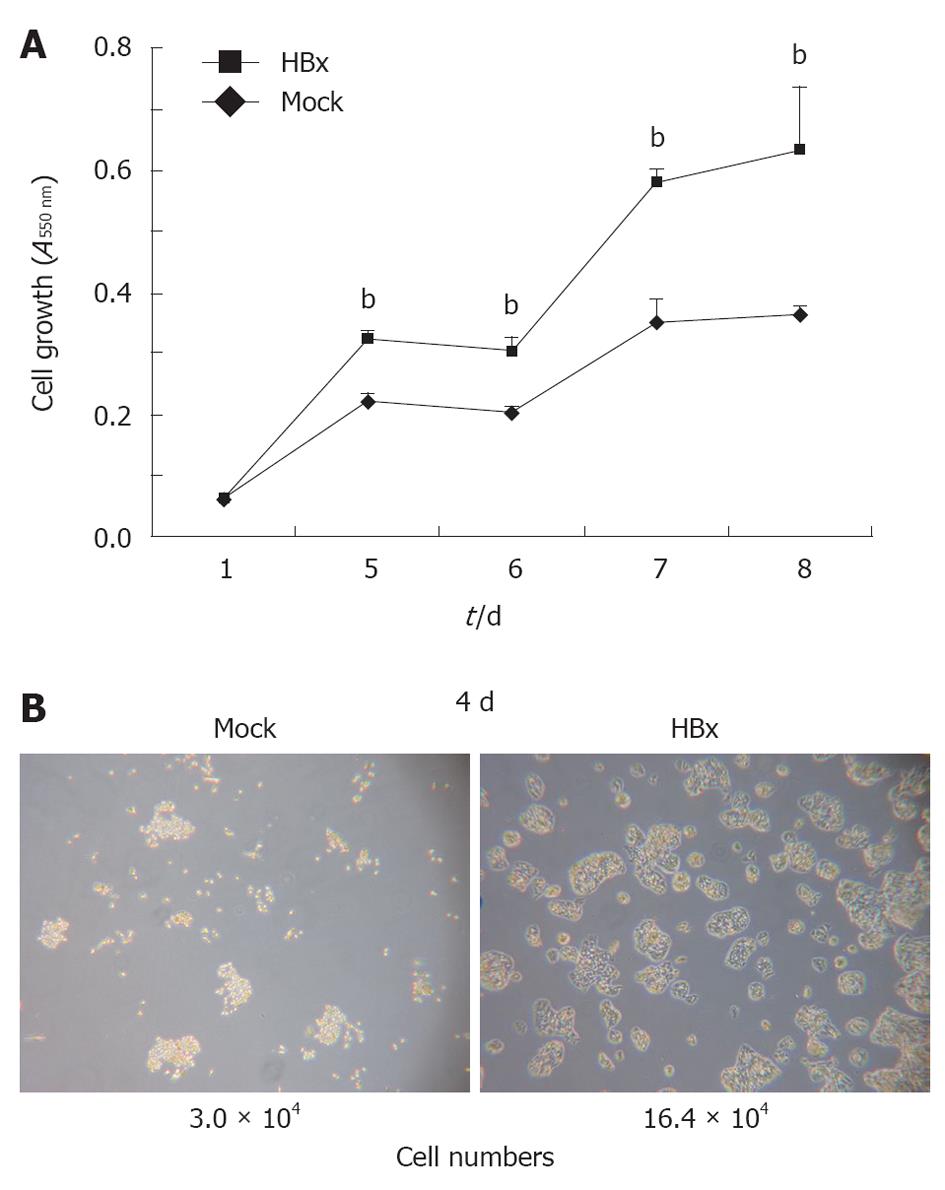
The HBx protein is considered to be closely associated with the development of HCC. HBx transgenic mice, previously developed in this laboratory[3], developed dysplasia around 4 wk of age, and hepatic tumors developed from 6 mo of age[19]. Several studies have shown that HBx stimulates cell proliferation and growth through the activation of signal transduction pathways such as Akt. To study the role of the HBx protein in cancer generation at the cellular level, HepG2-HBx cells were obtained by stably transfecting HepG2 cells with an HBx expression plasmid. The growth rate of the HepG2-HBx cells was approximately double that of the HepG2 control cells (Figure 1A and B). There were differences not only in cell growth, but also in morphology. HepG2-HBx cells showed aberrant actin bundling. Taken together, these results show that HBx has a role in the development of the liver tumor by activating proliferation and changing cell characteristics.
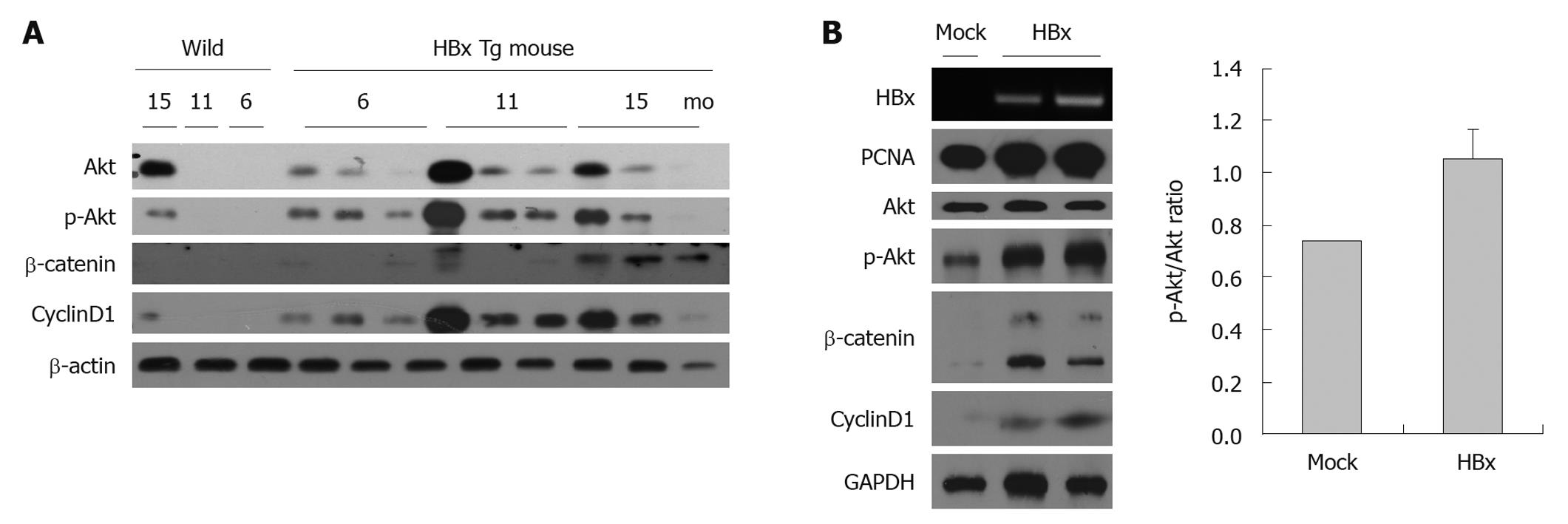
The PI3K/Akt signaling pathway is crucial to many aspects of cell growth and survival. To determine whether HBx-associated HCC is also accompanied by activation of the Akt pathway, lysates from the mouse liver tissue and cells transfected with HBx or an empty vector were used. As expected, the livers of HBx transgenic mice and HepG2-HBx cells displayed an activated Akt pathway. Accumulated β-catenin, phosphorylated Akt, and increased cyclin D1 were detected (Figure 2A). Even though cancer cell lines might have activated Akt, total Akt per p-Akt of HepG2 HBx cells was increased 1.4-fold compared with the HepG2 control cells (Figure 2B).
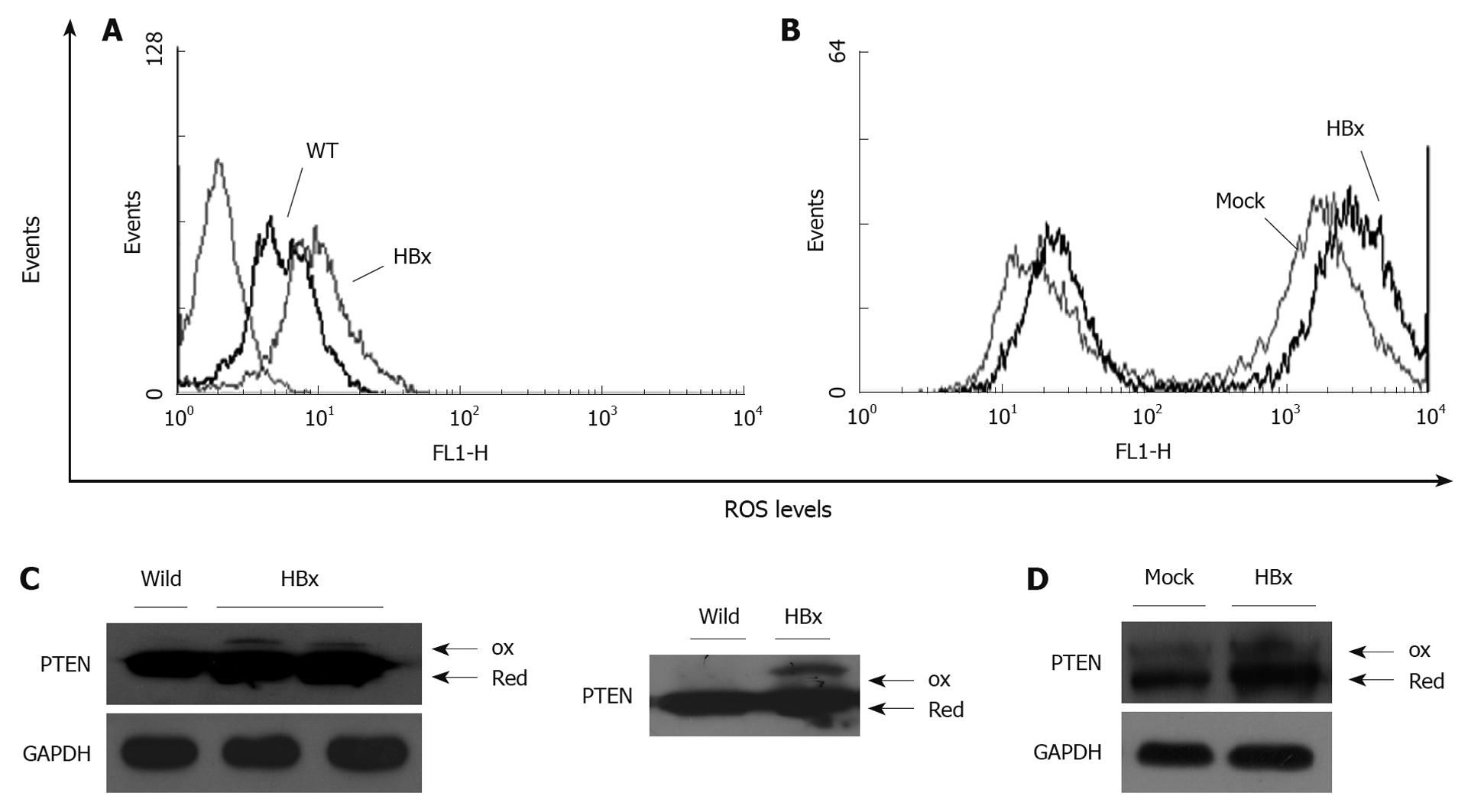
Peroxides are known to modify PTPs by oxidation. PTEN is also known to be inactivated through H2O2-mediated oxidation[20]. FACS analysis was used to verify HBx-induced ROS in mice and HepG2 cells. Primary hepatocytes were isolated from HBx transgenic and wild-type mice at the same age. ROS levels were significantly increased in HBx transgenic hepatocytes and HepG2-HBx cells compared to controls (Figure 3A and B). HBx expression was also associated with decreased mitochondrial membrane potential (data not shown). To examine the effect of HBx-induced ROS on PTEN inactivation, a PTEN oxidation assay was performed. HBx-expressing cells had higher ROS levels, and showed higher levels of oxidized PTEN when evaluated in primary hepatocytes and in HepG2 cells. HBx-induced ROS inactivated PTEN by promoting oxidation of cysteine residues within PTEN, thereby inactivating PTEN and promoting the function of Akt.
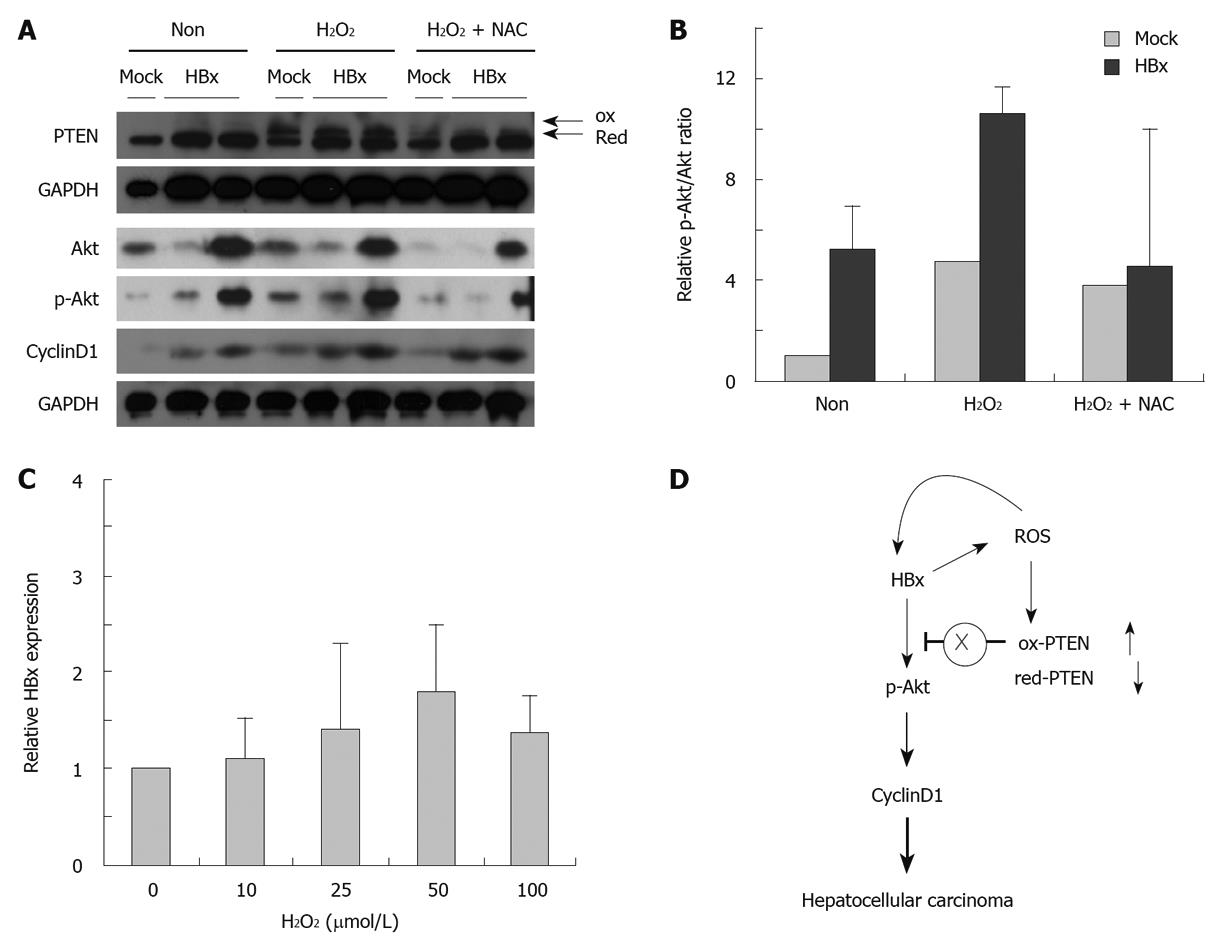
To investigate the activation of Akt in the presence of ROS-inactivated PTEN, we examined the Akt pathway activity, which was detected in 0 and 500 μmol/L H2O2. Increases in oxidized PTEN were associated with a higher p-Akt/total Akt ratio and increased cyclin D1 expression. To investigate further whether induced ROS is required for activation of the Akt pathway, HepG2 cells were treated with H2O2 in the presence or absence of NAC, a ROS quencher. Scavenging ROS through NAC were able to block the Akt pathway (Figure 4A and B). These observations are consistent with the hypothesis that HBx-mediated generation of ROS inactivates PTEN, thereby activating the Akt pathway in carcinogenesis. In addition, elevated ROS was also associated with elevated levels of HBx (Figure 4C).
One of the HBV-encoded proteins, HBx, is considered to be a major risk factor for HCC. It is well known that HBx activates cell signal transduction pathways, such as PI3K. Mutations or inactivation of the tumor suppressor, PTEN, regulates Akt activation[21]. This is considered one of the reasons for activation of Akt signaling in cancer. For example, endogenously produced H2O2 has been shown to inactivate PTEN in a macrophage cell line and cancer cell lines[16,22]. In this study, HBx-triggered ROS were associated with the oxidation and functional inactivation of PTEN. Although quantification of the extent of PTEN oxidation in the cells was not possible, the level of oxidized, inactivated PTEN was associated with several factors, such as Akt activation and accelerated HepG2 cell growth, and thus might be associated with hepatocarcinogenesis in HBx transgenic mice. Both cell growth and abnormal actin filaments were observed in HepG2-HBx cells. It has been reported that reorganization of actin filaments can cause loss of focal adhesions and cell-cell contact, which leads to an epithelial-mesenchymal transition that consequently disrupts monolayer integrity[23]. The HBx-induced ROS appear to stimulate HBx expression further, which suggests the existence of a positive feedback loop. Such feedback would be expected to cause a rapid increase in the abundance of H2O2. This localized H2O2 accumulation would be expected to result in the oxidation of only those PTEN molecules located nearby, possibly explaining the small proportion of PTEN molecules that undergo oxidative inactivation in HepG2-HBx cells and mouse livers.
The scheme presented in Figure 4D represents the HBx-induced generation of H2O2. H2O2 participates in intracellular signaling by targeting PTEN, and regulation of HBx gene expression, depending on the concentration. The results of the present study suggest that the HBx-mediated activation of Akt is regulated, at least in part, by the effects of HBx-induced ROS upon PTEN.
In summary, these studies further strengthen the case for a close relationship between oxidative stress and tumorigenesis. The studies reported herein have shown that HBx-induced generation of ROS can promote cellular transformation signaling by altering the function of PTEN. H2O2-oxidized PTEN leads to the activation of Akt. This is significant from a mechanistic as well as therapeutic point of view. Hence, drugs that scavenge endogenous ROS might slow down progression to HBx-induced liver cancer.
Hepatocellular carcinoma (HCC) is one of the most common cancers in the world. HCC is closely associated with hepatitis B virus (HBV) infection, especially in Asia. Among the HBV-encoding proteins, X protein (HBx) is a potential candidate for involvement in HBV-related HCC. One of the best-known pathways activated by HBx is phosphoinositide 3-kinase (PI3K)/Akt, which is associated with anti-apoptotic activity and cell proliferation. The reaction catalyzed by PI3K is reversed by phosphatase and tensin homolog (PTEN), which functions as a PI 3,4,5-trisphosphate 3-phosphatase. Indeed, by negatively modulating the PI3K signaling pathway, PTEN acts as a tumor suppressor.
HCC is one of the cancers with poor prognosis. HBV carriers are approximately 100 times greater than in uninfected individuals. Finding a diagnostic marker and preventing severe liver damage are important areas in liver cancer research.
There have been several studies about HBx-induced reactive oxygen species (ROS). However, most of the studies have used in vitro models. This is believed to be the first study of HBx-induced ROS in mice and HepG2 cells, and the increased ROS promoted Akt pathways via oxidized inactive PTEN.
The suggestions in this study are significant not only from a mechanistic point of view - HBx-induced ROS activate the Akt pathway - but also from a therapeutic point of view - prevention of overactivation of the Akt pathway by scavenging ROS.
In this experimental study, the molecular pathway of HBx-associated HCC tumorigenesis via PI3K/Akt was addressed. The authors demonstrated an important role for ROS as HBx-dependent tumorigenesis mediators. This paper is well written and concise.
Peer reviewers: Nikolaus Gassler, Professor, Institute of Pathology, University Hospital RWTH Aachen, Pauwelsstrasse 30, 52074 Aachen, Germany; Takashi Kojima, DVM, PhD, Department of Pathology, Sapporo Medical University School of Medicine, S.1, W.17, Chuo-ku, Sapporo 060-8556, Japan
S- Editor Tian L L- Editor Kerr C E- Editor Lin YP
| 1. | Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674-687. |
| 3. | Yu DY, Moon HB, Son JK, Jeong S, Yu SL, Yoon H, Han YM, Lee CS, Park JS, Lee CH. Incidence of hepatocellular carcinoma in transgenic mice expressing the hepatitis B virus X-protein. J Hepatol. 1999;31:123-132. |
| 4. | Haviv I, Shamay M, Doitsh G, Shaul Y. Hepatitis B virus pX targets TFIIB in transcription coactivation. Mol Cell Biol. 1998;18:1562-1569. |
| 5. | Benn J, Su F, Doria M, Schneider RJ. Hepatitis B virus HBx protein induces transcription factor AP-1 by activation of extracellular signal-regulated and c-Jun N-terminal mitogen-activated protein kinases. J Virol. 1996;70:4978-4985. |
| 6. | Chirillo P, Falco M, Puri PL, Artini M, Balsano C, Levrero M, Natoli G. Hepatitis B virus pX activates NF-kappa B-dependent transcription through a Raf-independent pathway. J Virol. 1996;70:641-646. |
| 7. | Lee YH, Yun Y. HBx protein of hepatitis B virus activates Jak1-STAT signaling. J Biol Chem. 1998;273:25510-25515. |
| 8. | Lee YI, Kang-Park S, Do SI. The hepatitis B virus-X protein activates a phosphatidylinositol 3-kinase-dependent survival signaling cascade. J Biol Chem. 2001;276:16969-16977. |
| 9. | Suzuki A, Hayashida M, Kawano H, Sugimoto K, Nakano T, Shiraki K. Hepatocyte growth factor promotes cell survival from fas-mediated cell death in hepatocellular carcinoma cells via Akt activation and Fas-death-inducing signaling complex suppression. Hepatology. 2000;32:796-802. |
| 10. | Lee YI, Hwang JM, Im JH, Kim NS, Kim DG, Yu DY, Moon HB, Park SK. Human hepatitis B virus-X protein alters mitochondrial function and physiology in human liver cells. J Biol Chem. 2004;279:15460-15471. |
| 11. | Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med. 1995;18:775-794. |
| 12. | Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794-798. |
| 13. | Lee SR, Kwon KS, Kim SR, Rhee SG. Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem. 1998;273:15366-15372. |
| 14. | Ushio-Fukai M, Alexander RW, Akers M, Yin Q, Fujio Y, Walsh K, Griendling KK. Reactive oxygen species mediate the activation of Akt/protein kinase B by angiotensin II in vascular smooth muscle cells. J Biol Chem. 1999;274:22699-22704. |
| 15. | Maehama T, Taylor GS, Dixon JE. PTEN and myotubularin: novel phosphoinositide phosphatases. Annu Rev Biochem. 2001;70:247-279. |
| 16. | Lee SR, Yang KS, Kwon J, Lee C, Jeong W, Rhee SG. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336-20342. |
| 17. | Kim YM, Chung HT, Simmons RL, Billiar TR. Cellular non-heme iron content is a determinant of nitric oxide-mediated apoptosis, necrosis, and caspase inhibition. J Biol Chem. 2000;275:10954-10961. |
| 18. | Wang AG, Moon HB, Lee MR, Hwang CY, Kwon KS, Yu SL, Kim YS, Kim M, Kim JM, Kim SK. Gender-dependent hepatic alterations in H-ras12V transgenic mice. J Hepatol. 2005;43:836-844. |
| 19. | Kim SY, Lee PY, Shin HJ, Kim do H, Kang S, Moon HB, Kang SW, Kim JM, Park SG, Park BC. Proteomic analysis of liver tissue from HBx-transgenic mice at early stages of hepatocarcinogenesis. Proteomics. 2009;9:5056-5066. |
| 20. | Leslie NR, Bennett D, Lindsay YE, Stewart H, Gray A, Downes CP. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003;22:5501-5510. |
| 21. | Keniry M, Parsons R. The role of PTEN signaling perturbations in cancer and in targeted therapy. Oncogene. 2008;27:5477-5485. |
| 22. | Kwon J, Lee SR, Yang KS, Ahn Y, Kim YJ, Stadtman ER, Rhee SG. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc Natl Acad Sci USA. 2004;101:16419-16424. |
| 23. | Hirohashi S, Kanai Y. Cell adhesion system and human cancer morphogenesis. Cancer Sci. 2003;94:575-581. |