Published online Oct 21, 2010. doi: 10.3748/wjg.v16.i39.4913
Revised: June 10, 2010
Accepted: June 17, 2010
Published online: October 21, 2010
Abdominal involvement in angioedema is often a challenge to diagnose. Acute onset abdominal pain is its most common presenting symptom, and misdiagnosis may lead to unnecessary surgical intervention. Familiarity with the types and presentations of angioedema can be invaluable to clinicians as they consider the differential diagnoses of a patient presenting with abdominal pain. Detailed personal and family histories, careful physical examination of the patient, combined with knowledge of angioedema types, can help clinicians perform their diagnostic evaluation. An accurate diagnosis is essential in order to provide appropriate treatment to patients with angioedema. Depending upon the diagnosis, treatment may be the avoidance of provoking factors (such as allergens or medications), inhibiting histamine-provoked reactions, or treating C1 esterase inhibitor deficiency.
- Citation: Nzeako UC. Diagnosis and management of angioedema with abdominal involvement: A gastroenterology perspective. World J Gastroenterol 2010; 16(39): 4913-4921
- URL: https://www.wjgnet.com/1007-9327/full/v16/i39/4913.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i39.4913
About 10% to 20% of people worldwide will develop an episode of angioedema or urticaria at some point in their lifetime, women being more prone than men[1]. Angioedema is characterized by localized temporary swelling, which can affect all layers of the skin or of the walls of hollow viscera, such as the oropharynx, respiratory system, or the gastrointestinal (GI) tract. Peripheral non-pitting edema is typical of cutaneous manifestations. However, the effects of visceral angioedema are variable, ranging from life-threatening episodes when the respiratory system (larynx) is involved, to pain of varied severity associated with nausea or vomiting, when abdominal viscera such as the intestines are involved[2].
Abdominal pain associated with angioedema may manifest as severe acute onset abdominal pain, or as chronic recurrent abdominal pain of moderate severity. The abdominal pain is described as cramping or colicky and is rated as severe to excruciating in 87% of patients[3]. Vomiting and diarrhea occur in 78% and 65%, respectively, of patients with abdominal symptoms[3].
Patients with these symptoms typically present to one of four medical specialists, namely emergency room physicians, primary care physicians (internists, pediatricians, family medicine), general surgeons, and gastroenterologists. Physicians in each of these specialties should be familiar with the signs and symptoms of cutaneous and visceral angioedema, and be able to order appropriate tests to investigate this group of differential diagnoses. This brief review provides a perspective of angioedema diagnoses and management, including new therapeutic options, for practicing gastroenterologists.
Classification of angioedema types is based on their etiology or pathophysiology. Broadly, these include allergic angioedema, angiotensin converting enzyme inhibitor (ACE-I)-mediated angioedema, non-steroidal anti-inflammatory drug (NSAID)-mediated angioedema, hereditary angioedema (HAE), inherited angioedema with normal C1 esterase inhibitor (formerly called HAE type 3), and acquired C1 esterase inhibitor deficiency angioedema [acquired angioedema (AA)]. Angioedema can result from mast cell degranulation with massive histamine release or from increased accumulation of bradykinin either via increased production or decreased inactivation (Table 1).
| Type of AE | Mediator | Mechanism |
| Allergic AE | Histamine (mast cells) | Allergens react with IgE antibodies on the surface of mast cells, causing degranulation and release of histamine |
| ACE-I-induced | Bradykinin | ACE-Is prevent the conversion of bradykinin to inactive metabolites, leading to bradykinin accumulation |
| NSAID-induced AE | Leukotrienes (mast cells) | Inhibition of COX-1 leads to overproduction of vasoactive substances by shunting arachidonic acid metabolism through the lipoxygenase pathway, creating leukotrienes. Vasoactive leukotrienes act on cell-surface receptors to increase vascular permeability and promote inflammation |
| HAE type 1 | Bradykinin | Genetic mutations in the C1 INH gene result in low levels of C1 INH. Major roles of C1 INH include inactivating coagulation factors XIIa, XIIf and XIa; blocking C1 complement autoactivation; and inhibiting activated kallikrein. Removal of these inhibitory actions results in complement activation and elevated bradykinin levels |
| HAE type 2 | Bradykinin | Genetic mutations in the C1 INH gene result in normal levels of C1 INH, but the C1 INH is dysfunctional. Plasma cascades are unregulated in the presence of dysfunctional C1 INH, leading to bradykinin accumulation as in HAE type 1 |
| Inherited AE with normal C1 INH | Bradykinin | Missense mutation in factor XII gene confers a significant increase in the protease activity of each activated factor XII molecule, which increases bradykinin generation. Decreased activity of enzymes such as ACE and aminopeptidase P have also been noted |
| Acquired AE | Bradykinin | Type 1: Immune complex formation associated with rheumatologic, lymphoproliferative, and neoplastic disorders continuously activate C1, causing C1 INH depletion and bradykinin accumulation |
| Type 2: Autoantibodies inactivate C1 INH, leading to bradykinin accumulation | ||
| Idiopathic recurrent AE | Unknown | Unknown |
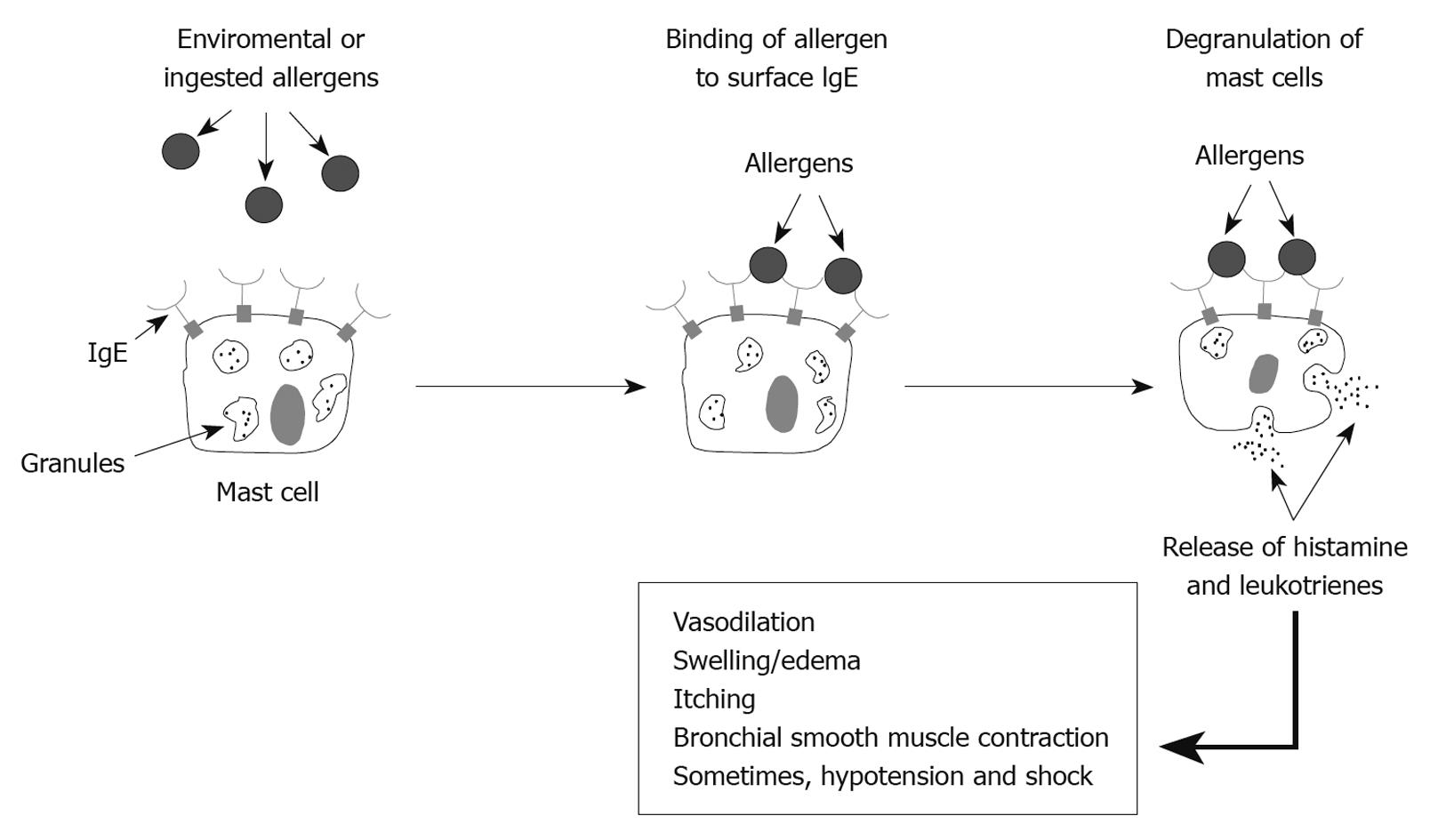
Allergic angioedema is caused by reaction to foods (such as shellfish, nuts, some fruits), medications, insect bites, latex, or other environmental allergens, and results from IgE-mediated mast cell degranulation, with resultant histamine release that causes local tissue swelling[2]. Sensitization, through prior exposure to the allergen, is usual. Swelling in allergic angioedema can occur throughout the body and is typically associated with urticaria and pruritus. Ingested allergens may cause angioedema symptoms that include abdominal pain and vomiting. Most episodes of allergic angioedema resolve 1 to 3 d after ceasing contact with the allergen[2].
A variety of medications can induce a non-IgE-mediated form of angioedema, including ACE-Is, NSAIDs, and rarely, angiotensin-2-receptor blockers (ARBs).
ACE-I-induced angioedema occurs in 0.1% to 2.2% of patients receiving these drugs[6,7]. It manifests within the first month of treatment in one quarter of patients taking ACE-Is, but delay of onset as long as 10 years has been reported[8]. Bradykinin is converted by ACE into inactive metabolites. Thus, ACE-Is inhibit the degradation of bradykinin, causing it to accumulate[9]. This accumulation causes angioedema via bradykinin-induced vasodilation, increased capillary permeability, and plasma extravasation[8,10]. ACE-I-induced angioedema primarily affects the head and neck (especially the lips and tongue), and is more common in women and people of African descent[8]. However, there have been case reports of abdominal visceral involvement with ACE-I-induced angioedema presenting with abdominal pain as the only symptom[11]. ACE-Is should always be considered in the differential diagnosis of unexplained abdominal pain. Although switching patients to ARBs is safe in most patients with ACE-I-induced angioedema, continued bouts of angioedema have been reported in some patients after switching to ARBs[12]. Observational data suggest that the combined use of ACE-I and ARBs may be more likely to result in angioedema[13].
NSAID-induced angioedema is present in 0.1% to 0.3% of patients receiving NSAIDs[14,15]. This is a class-specific reaction mediated by inhibition of cyclooxygenase (COX)-1, which results in the over-production of a variety of vasoactive substances, including cysteinyl leukotrienes. It is often characterized by periorbital swelling and occurs in combination with respiratory symptoms in one third of patients[14]. Observational data suggest that the combined use of ACE-Is and NSAIDs may also be more likely to result in angioedema adverse effects[13].
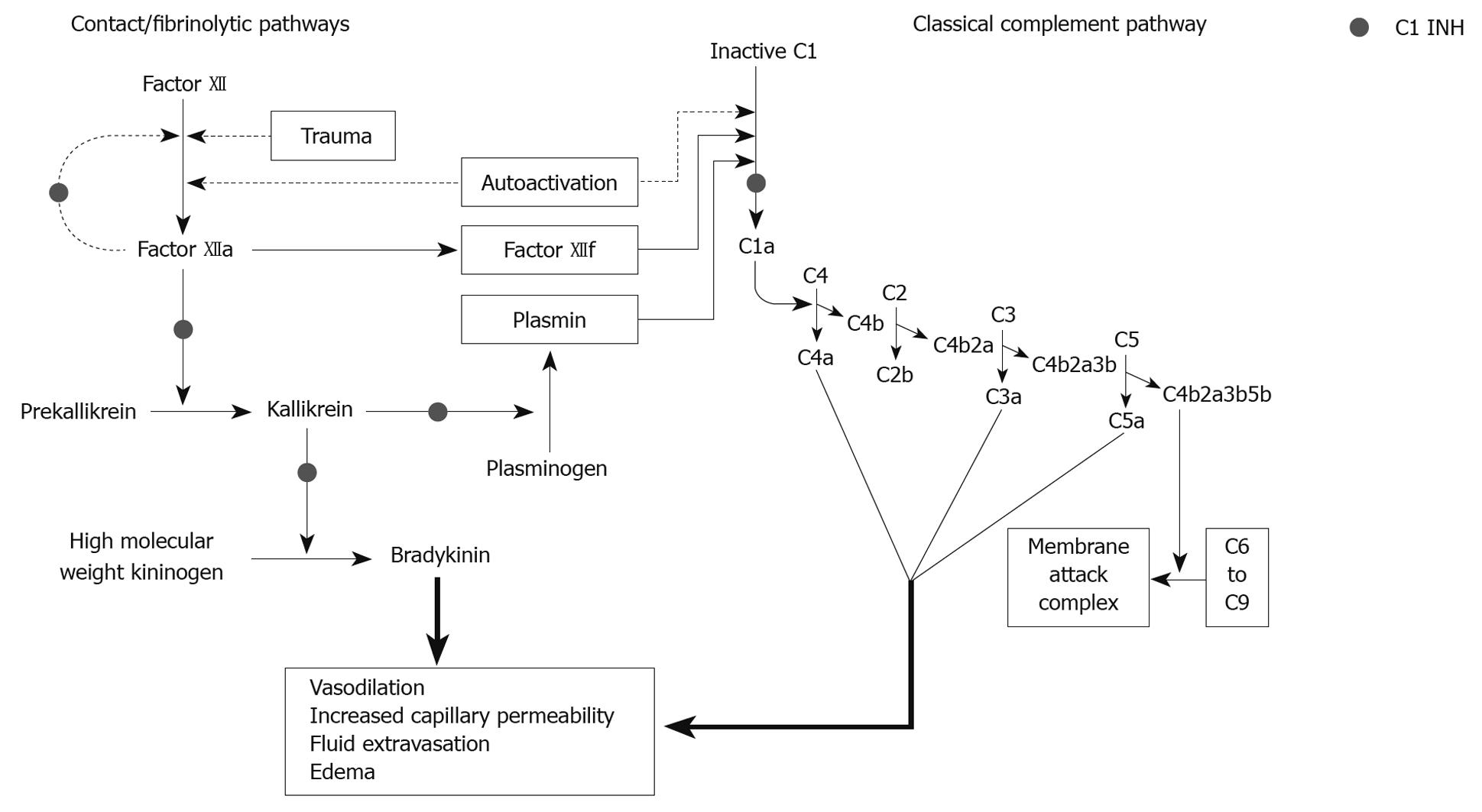
HAE occurs in 1:10 000 to 1:50 000 people and results from mutations in the C1 esterase inhibitor (C1 INH) gene[16]. Type 1 HAE is caused by a deficiency in the amount of functional C1 INH produced, while type 2 HAE is characterized by dysfunctional C1 INH. Although primarily inherited in an autosomal dominant manner, HAE appears de novo in one quarter of patients due to new mutations. C1 INH plays an important role in complement, contact, and fibrinolytic pathways, which have been described in other literature[17]. The end result of quantitative or functional C1 INH deficiency is massive bradykinin release, which is thought to mediate many symptoms of HAE and AA[17]. Bradykinin causes edema, ascites, and swelling via increasing vascular permeability; congestion, hypotension, and erythema due to vasodilation; and cramps, spasms, and pain due to contraction of nonvascular smooth muscle[4].
HAE can manifest anywhere in the body, including the head and neck, extremities, GI tract, genitals, trunk, and larynx, and shows wide variability in presentation within patients and families[18,19]. Up to 80% of patients with HAE have recurrent abdominal pain, while half will have a potentially life-threatening laryngeal attack[19-22]. Such abdominal pain symptoms may occur for many years without concomitant cutaneous or respiratory symptoms[23]. HAE-mediated abdominal pain can be mistaken for other causes of abdominal pain, such as acute appendicitis[16]. Attacks are frequently accompanied by a prodromal phase. In the case of GI manifestations, nonspecific complaints of irritability, aggressiveness, fatigue, or hunger may precede an attack[3]. Intestinal wall swelling (thickening on imaging studies), ascites, and rarely, hypovolemic shock, occur due to massive fluid accumulation in the intestinal wall and lumen, and in the peritoneal cavity[23]. Attacks typically resolve over 2 to 5 d, and may be triggered by trauma, stress, medical procedures (e.g. instrumentation or surgery), estrogens, and certain medications (e.g. ACE-Is)[4].
Inherited angioedema with normal C1 INH is a rare disorder caused by a mutation in the coagulation factor XII gene, which in turn leads to increased gene expression, and consequently increased levels of bradykinin[24]. It is clinically indistinguishable from HAE, is thought to be autosomal dominant, and although occurring predominantly in women, has also been reported in men[25]. Similar to HAE, estrogen-containing birth control pills, estrogen-replacement therapy, and pregnancy may precipitate or worsen symptoms[25,26].
AA is a rare syndrome that occurs as a result of increased catabolism of C1 INH and overactivation of the classical complement pathway[27]. Although the clinical picture is identical to HAE, the underlying immunologic disturbance is not hereditary in nature, and precise mechanisms remain unclear. AA has been associated with lymphoproliferative/neoplastic disorders (type 1 AA) or autoimmunity (type 2 AA). Symptoms often localize to the abdomen and upper respiratory tract, as well as skin. Abdominal symptoms of angioedema have been described previously. Symptoms typically resolve over 2 d.
Idiopathic recurrent angioedema refers to swelling episodes that occur at least three times within a 6 to 12 mo period, have no identifiable cause, and are typically recalcitrant to treatment[1]. The mechanism is unknown, but autoimmune processes have been implicated. In most cases, swelling is accompanied by urticaria. When urticaria is present, it is accompanied by pruritus. Swelling can last from hours to days. Abdominal symptoms include pain, nausea, and vomiting.
Different cells, and different cell components, play roles in the prevention or causation of various types of angioedema.
Mast cells, with cell surface IgE receptors, mediate allergic angioedema when an allergen interacts with the Fab fragment of the IgE molecule (Figure 1). Such allergen-Fab interaction results in intracellular signaling, which causes degranulation of the mast cells with release of histamine and leukotrienes, thereby resulting in angioedema symptoms.
HAE and AA occur due to the loss of inhibitory control of the contact/fibrinolytic pathway and the classical complement pathway caused by low levels or subnormal activity of C1 INH (Figure 2). Unlike allergic angioedema, these events occur in the extracellular milieu. C1 esterase inhibitor is synthesized mainly by hepatocytes and, to a lesser extent, by circulating blood monocytes. In AA, C1 INH is catabolized at a rate which is faster than the rate of synthesis, thus resulting in attacks of angioedema. HAE is caused by genetic mutations which result either in significantly low production of normal C1 INH (type 1), or production of normal or elevated quantities of a dysfunctional C1 INH which is unable to bind the usual substrates and thus is unable to inhibit activation of the contact/fibrinolytic and classical pathways (type 2)[4].
Angiotensin converting enzyme (ACE) is also known as kininase II, because one of its main roles is to metabolize bradykinin into inactive metabolites. When ACE activity is inhibited, another enzyme - aminopeptidase P - metabolizes bradykinin, thus preventing excess accumulation. ACE-I-induced angioedema results from accumulation of bradykinin after inhibition of ACE by ACE-I drugs, and appears to occur more often among individuals who have subnormal activity of aminopeptidase P. Such subnormal aminopeptidase P activity may be genetic (caused by a mutation), or acquired (caused by another drug the patient is taking)[28].
Inherited angioedema with normal C1 INH results from missense mutations in the factor XII gene, which confer a significant increase in the protease activity of transcribed factor XII, resulting in increased bradykinin production[29]. Some of these patients also appear to have concomitant mutations in the ACE gene, which result in production of ACE with reduced ability to degrade bradykinin[30]. Angioedema attacks are therefore more common in patients with these mutations due to increased production, and decreased metabolism, of bradykinin.
NSAID-induced angioedema results from the inhibition of COX-1, which results in the channeling of larger quantities of arachidonic acid into the lipoxygenase pathway, resulting in the increased production of vasoactive leukotrienes (Figure 3).
For the practicing gastroenterologist, several patients with abdominal involvement by angioedema will present as referrals for evaluation at a time when the acute episode has subsided, or during a subacute episode of abdominal pain. In such cases, identification of angioedema triggers (e.g. medications, allergens, trauma, or disease) and careful consideration of patient and family history may provide clues. The absence of a family or prior personal history of angioedema should not preclude consideration of this diagnosis.
A thorough history of patient illness, family history, and review of current and recent medications will raise suspicion of the diagnosis in appropriate circumstances. Physical examination, cross-sectional imaging studies of the abdomen, and relevant laboratory tests will often confirm the diagnosis during the acute episode. The physical examination may reveal characteristics of cutaneous angioedema (urticaria, pruritis, cutaneous swelling). For episodes with abdominal involvement, palpation may reveal diffuse abdominal tenderness, with or without rebound[23]. Bowel sounds may be hypoactive or hyperactive[20]. Shifting dullness may be present.
Contrast-enhanced abdominal computed tomography (CT) scan may show intestinal wall and mucosal thickening consistent with edema, fluid accumulation in dilated small or large bowel loops, and ascites[23]. Plain abdominal X-ray may show various degrees of obstruction with or without air-fluid levels, thumb printing, and dilated intestinal loops. Abdominal ultrasonography may detect ascites and edematous viscera. These findings are visible only during acute angioedema attacks and are fully reversible.
Endoscopy of the GI tract or oropharynx is not recommended for patients with suspected HAE due to the risk of inducing a potentially life-threatening laryngeal attack. In cases where endoscopy must be used for additional clinical reasons, prophylactic measures to protect against the possibility of laryngeal swelling should be initiated. Diffuse mucosal edema and erythema, and bulging masses of gastric mucosa (resembling a submucosal tumor), have been reported upon upper GI endoscopy in patients with HAE and abdominal attacks[31].
Laboratory testing should be conducted for patients suspected of angioedema to aid in differential diagnosis. Elevated serum tryptase and urine histamine levels can detect IgE-mediated angioedema, and allergy testing can be used to help identify the source of the offending allergen[32]. Assessment of complement markers provides useful information for helping delineate between acquired and hereditary forms of angioedema. Patient characteristics and test results can help differentiate between types of angioedema, as shown in Table 2.
| HAE | Inherited | AA | Idiopathic | ACE-I | NSAID | Allergic | |
| Location | Anywhere | Anywhere | Anywhere | Especially lips and face | Especially lips, tongue, intestines | Especially face | Anywhere |
| Urticaria | No | No | No | Usually | Rare | Usually | Usually |
| Family history | Yes | Yes | No | No | No | No | No |
| Age of onset | 6-20 yr | 6-20 yr | > 40 yr | Any age | Any age | Any age | Any age |
| Trauma as trigger | Yes | Yes | Yes | No | No | No | No |
| C1q | Normal | Normal | Low (type 1) | Normal | Normal | Normal | Normal |
| Low/normal (type 2) | |||||||
| C1 INH levels | Low (type 1) | Normal | Low (type 1) | Normal | Normal | Normal | Normal |
| Normal (type 2) | Low/normal (type 2) | ||||||
| C1 INH function | Low (type 1) | Normal | Low | Normal | Normal | Normal | Normal |
| Low (type 2) | |||||||
| C4 | Low | Normal | Low | Normal | Normal | Normal | Normal |
| C3 | Normal | Normal | Low/normal | Normal | Normal | Normal | Normal |
The various types of angioedema have symptoms that overlap with several other acute and subacute conditions, and as noted above, can occasionally present atypically. As such, the diagnosis may be obscured. Isolated abdominal pain from angioedema, without associated skin or respiratory system symptoms, may lead to wrong diagnoses or unnecessary interventions. In one study, misdiagnosis of abdominal symptoms led to unnecessary appendectomy, laparotomy, or both, in 35% of patients[20]. Such inappropriate treatment may be attributed to the multiple interpretations that can be made of GI findings. For example, abdominal pain with intestinal wall edema on CT scan can be misinterpreted as ischemia and result in unnecessary laparotomy. Abdominal pain with intestinal obstruction in cases of severe edema may be interpreted as an “acute abdomen”, resulting in unnecessary laparotomy. Abdominal pain with gallbladder distension may be interpreted as biliary colic, and result in cholecystectomy. Abdominal pain with transient ascites on CT scan or ultrasonography may misdirect the focus to the liver as the cause of abdominal symptoms. Surgical exploration of abdominal symptoms should be avoided in the absence of signs of acute abdomen (i.e. absence of fever, leukocytosis, or peritoneal signs; presence of bowel sounds)[23]. Alternate diagnoses for recurrent abdominal symptoms should be considered to avoid unnecessary medical procedures.
Without intervention, angioedema attacks typically last from 1 to 5 d, depending on the underlying cause. However, acute treatment is often necessary to prevent a fatal outcome when the respiratory system is involved, or to alleviate pain when the abdominal viscera are involved.
Acute treatment of angioedema varies by type. Airway integrity is the first priority. Establishing intravenous access early and initiating appropriate intravenous hydration is also essential. For acute allergic angioedema, adrenaline by the intramuscular or subcutaneous route, and intravenous diphenhydramine, will help reduce edema. Hydrocortisone or methylprednisolone can reduce the risk of relapse[32]. Allergen avoidance is the best prophylaxis for allergic angioedema[32]. The same principles are true for acute treatment of medication-induced angioedema. Clinicians need to consider the short-term risk of relapse despite the discontinuation of the culprit medication. Hospital admission may be necessary to ensure close observation following a medication-induced attack.
Table 3 reviews clinical trial data of agents for the treatment of patients with HAE. For HAE, C1 INH concentrate has shown efficacy in aborting acute attacks of angioedema and has been used in this capacity in Europe for over 20 years. In the emergency room or critical care setting, administration of C1 INH may be a necessary urgent intervention in a patient with severe oropharyngeal, respiratory or abdominal symptoms who has a known diagnosis of HAE. Antihistamines, glucocorticoids or epinephrine typically do not improve acute HAE exacerbations[5]. Fresh frozen plasma (FFP) has also shown efficacy in aborting acute attacks of HAE, with only mild and transient adverse events[41]. However, because FFP contains contact-system proteins that could contribute to increased bradykinin production, there is a small risk of exacerbating an attack[18]. Although long available in Europe, the plasma-derived C1 INH, BERINERT® P [C1 esterase inhibitor (human); CSL Behring GmbH, Marburg, Germany] was approved by the United States Food and Drug Administration (FDA) in October 2009 [under the name BERINERT®, C1 esterase inhibitor (human); CSL Behring LLC, Kankakee, IL] for the treatment of acute abdominal and facial attacks of HAE in adolescents and adults in the United States[37]. Patients who received the recommended dose experienced a significantly shorter time to symptom relief compared with patients given placebo. Common potential side effects of BERINERT include subsequent HAE attack, headache, abdominal pain, nausea, muscle spasms, pain, diarrhea, and vomiting[37]. Rarely, a paradoxical increase in severity of pain associated with HAE may occur with BERINERT treatment[37].
| Trial design | Primary efficacy outcome result | AE | Other safety notes | |
| Routine prophylaxis | ||||
| CINRYZE[34] C1 inhibitor (human) (1000 units every 3-4 d for 12 wk) IV | Randomized, double-blind, placebo-controlled, cross-over study (n = 24) | Decreased the number of attacks (mean 12.7 for placebo vs 6.1, P < 0.0001) | Sinusitis, rash, headache, upper respiratory tract infection | Precautions: hypersensitivity reactions, thrombotic events, and risk of transmission of infectious agents |
| DANOCRINE[35,36] Danazol (range, 40-1000 mg, mean: 171.2 mg/d) oral route | Retrospective (n = 118) | Decreased the number of attacks (33.3 per year when untreated vs 9.7 per year when treated) | Clinical: weight gain, menstrual irregularities, virilization in women, headache | 8 patients with long-term therapy had serious adverse events (i.e. myocardial infarction, stroke, deep vein thrombosis, acute pancreatitis, hepatocellular adenoma) |
| Laboratory: elevated liver enzymes, elevated cholesterol | Warnings: use in pregnancy is contraindicated. Thrombotic events, peliosis hepatis, benign hepatic adenoma, and intracranial hypertension have been reported | |||
| Acute attacks | ||||
| BERINERT[37] C1 esterase inhibitor (human) (10 or 20 units/kg body weight) IV | Randomized, double-blind, placebo-controlled study (n = 124) | 20 mg/kg dose: reduction in time to onset of symptom relief (> 4 h for placebo vs 50 min, P = 0.0016) | Headache, nausea, abdominal pain, dysgeusia, vomiting | Treatment-emergent AEs: laryngeal edema, facial attack with laryngeal edema, swelling (shoulder and chest) exacerbation of HAE, and laryngospasm |
| Precautions: hypersensitivity reactions, thrombotic events, and risk of transmission of infectious agents | ||||
| FIRAZYR[38,39] Icatibant (30 mg) SQ | Randomized, double-blind, comparator-group study [n = 56 (placebo comparator study)] [n = 74 (tranexamic acid comparator study)] | Decreased time to onset of symptom relief (4.6 h placebo vs 2.5 h, P = 0.142; 12 h tranexamic acid vs 2 h, P < 0.001 ) | Injection site reactions; common events include recurrent attacks, nausea, abdominal pain, headache, asthenia, rash | Precautions: caution should be used in patients with acute ischemic heart disease or unstable angina pectoris, and in patients in the weeks following a stroke |
| KALBITOR[40] Ecallantide (30 mg) SQ | Randomized, double-blind, placebo-controlled trial (n = 96) | Decreased symptom severity measured by Mean Symptom Complex Severity scores (-0.4 placebo vs -0.8, P = 0.010) | Headache, nausea, diarrhea, pyrexia, injection site reactions, nasopharyngitis | Warnings: hypersensitivity reactions, including anaphylaxis |
| KALBITOR[40] Ecallantide (30 mg) SQ | Randomized, double-blind, placebo-controlled trial (n = 72) | Improved symptom response to treatment measured by Treatment Outcome Scores (36 placebo vs 63, P = 0.045) |
Two agents that work using different mechanisms have recently become available for treating acute attacks of HAE. Icatibant (FIRAZYR®; Jerini AG, Berlin, Germany) is a bradykinin-2 receptor antagonist that was approved by the European Commission in July 2008 for the treatment of acute attacks of HAE in adults[38]. In December 2009, the kallikrein inhibitor, ecallantide (KALBITOR®; Dyax Corporation, Cambridge, MA), was approved by the FDA for the treatment of acute attacks of HAE in patients aged 16 and older[40]. A black box warning notes that ecallantide should only be administered by a healthcare professional with appropriate medical support due to a risk of anaphylaxis after administration of ecallantide.
In Europe, the C1 esterase inhibitor, CETOR® (Sanquin; Amsterdam, Netherlands) is approved for the treatment of acute attacks of AA[42]. No treatments are approved for acute attacks of AA in the United States. Acute treatment has typically paralleled treatment of acute attacks of HAE, with the use of FFP or C1 INH concentrate. Although patients with AA generally require higher doses of C1 INH, treatment of the underlying malignancy or lymphoproliferative disorder will best prevent recurrent symptoms and laboratory abnormalities. Additionally, plasmapheresis is sometimes necessary to decrease autoantibody levels in AA type 2.
Although patient safety and symptom resolution are the primary goals of acute angioedema treatment, routine and pre-procedure prophylaxis focus on preventing acute events and are particularly appropriate in HAE, AA, and idiopathic recurrent angioedema. For patients with idiopathic recurrent angioedema, routine prophylaxis includes avoidance of provoking factors and low sedation antihistamines, supplemented as necessary with sedating antihistamines at night[1].
In HAE and AA, pre-procedure prophylaxis increases the quantity of circulating C1 INH prior to invasive medical or surgical procedures, so as to reduce the risk of developing life-threatening acute angioedema during the peri-operative period. Thus, an accurate diagnosis of angioedema type is central to developing an appropriate long-term management strategy. Once a gastroenterologist has reached a diagnosis of HAE or AA, a physician specializing in allergy and immunology should be consulted to guide further management.
Attenuated androgens (e.g. danazol and stanozolol), C1 INH, and the antifibrinolytic, tranexamic acid, are used in Europe for prophylaxis[43]. In the United States, the nanofiltered C1 INH concentrate, CINRYZE™ [C1 esterase inhibitor (human); ViroPharma Incorporated, Exton, PA; approved by the FDA in October 2008 for intravenous administration] and oral attenuated androgens (e.g. danazol), are the only FDA-approved treatments for routine prophylaxis of HAE attacks[34,35]. Antifibrinolytics (e.g. tranexamic acid) have also been shown to be effective. However, the nanofiltered C1 INH concentrate has become the preferred therapy for attack prophylaxis due to the significant risk for adverse events associated with attenuated androgens and antifibrinolytics[5]. Biweekly administration of CINRYZE (every 3-4 d) has been shown to reduce angioedema attack rate, attack severity, and time to symptom resolution in patients with HAE[34]. Common potential side effects include upper respiratory infections, sinusitis, skin rash, and headache[34]. CINRYZE is approved for patient self-administration.
Prior to any surgical or dental procedure, or any invasive procedure associated with tissue trauma, patients with HAE should receive prophylaxis with either C1 INH concentrate 1 h prior to surgery with subsequent doses available, or oral attenuated androgens starting 5 d before and 2 d after the procedure, or FFP at least 1 h before surgery[16,28]. These measures will help prevent an acute HAE attack during the peri-operative period.
Since C1 INH is purified from human plasma, there exists a theoretical risk of transmission of viral infections or Creutzfeldt-Jakob agent[34,37]. To reduce the risk of viral transmission, both C1 INH products referenced in this paper start with plasma donor screening for human immunodeficiency virus, hepatitis B, and hepatitis C viruses. Further steps within the manufacturing process are designed to reduce the risk of viral transmission, with both products ultimately using different processes to this end. For CINRYZE, no transmission of disease has been reported[34]. For BERINERT, a few suspected cases of viral transmission have been reported[37]. C1 INH has been associated with a risk of thrombosis if used off-label at high doses[34,37]. Severe hypersensitivity reactions may rarely occur, and since such reactions are clinically indistinguishable from an acute HAE attack, epinephrine injection should be available at all times during administration of C1 INH, for use if necessary[34,37]. Table 4 summarizes the FDA approved drugs for prophylaxis and treatment of HAE.
| Drug | FDA approved indication | Usual adult dose | Range |
| Cinryze | Prophylaxis | 1000 units IV | Every 3rd or 4th day |
| Danazol | Prophylaxis | 200 mg/d | 100 mg every 3rd day to 600 mg/d |
| Berinert | Acute attacks | 20 units/kg body weight IV | Per attack |
| Icatibant | Acute attacks | 30 mg SQ | Per attack |
| Ecallantide | Acute attacks | 30 mg SQ | Per attack |
Patients experiencing angioedema with abdominal involvement often present to the gastroenterologist with a history of recurrent episodes of abdominal pain. A careful history and physical examination, imaging studies, laboratory assessments, and awareness of angioedema types can help the clinician order appropriate tests to explore the differential diagnosis. Suspicion may be raised from the patient or family history, but the physical examination, imaging studies, and laboratory tests may confirm a diagnosis. Several reports have shown that abdominal pain can sometimes be the only manifestation of HAE or drug-induced angioedema. Therefore, these differential diagnoses need to be considered even in the absence of a history of prior cutaneous, oropharyngeal or respiratory symptoms, because recurrent abdominal symptoms may predate these other presentations by several years. Once a correct diagnosis has been made, appropriate treatments can be considered.
It is this author’s opinion that patients receiving ACE-Is who present with unexplained recurrent or chronic abdominal pain should be tested for HAE. However, since these drugs can have abdominal pain adverse effects independent of a diagnosis of HAE, via a pathway leading to excessive bradykinin concentration, negative tests for HAE should not completely remove these drugs from consideration as a cause of the patient’s symptoms. Furthermore, the absence of classical intestinal thickening on imaging studies such as CT scan should not automatically absolve this drug class of blame in cases of unexplained abdominal pain, without a brief trial of drug withdrawal to see if symptoms improve.
If a diagnosis of HAE is made, appropriate immediate and short-term treatment with one of the approved agents should be undertaken. In addition, a plan for long-term management should be discussed with the patient and their family. Appropriate testing of family members, to identify those at risk, should be offered.
A multi-disciplinary approach to management of angioedema is necessary. A specialist in allergy and immunology should be engaged to guide additional evaluation and to provide the patient with long-term management.
Peer reviewer: Fabrizio Montecucco, MD, Assistant Professor, Division of Cardiology, Department of Internal Medicine, University of Geneva, Avenue de la Roseraie 64, 1211 Geneva, Switzerland
S- Editor Tian L L- Editor Logan S E- Editor Lin YP
| 1. | Frigas E, Park M. Idiopathic recurrent angioedema. Immunol Allergy Clin North Am. 2006;26:739-751. |
| 2. | Kaplan AP, Greaves MW. Angioedema. J Am Acad Dermatol. 2005;53:373-388; quiz 389-392. |
| 3. | Bork K, Staubach P, Eckardt AJ, Hardt J. Symptoms, course, and complications of abdominal attacks in hereditary angioedema due to C1 inhibitor deficiency. Am J Gastroenterol. 2006;101:619-627. |
| 4. | Nzeako UC, Frigas E, Tremaine WJ. Hereditary angioedema: a broad review for clinicians. Arch Intern Med. 2001;161:2417-2429. |
| 5. | Nzeako UC, Frigas E, Tremaine WJ. Hereditary angioedema as a cause of transient abdominal pain. J Clin Gastroenterol. 2002;34:57-61. |
| 6. | Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens. 2004;17:103-111. |
| 7. | Vleeming W, van Amsterdam JG, Stricker BH, de Wildt DJ. ACE inhibitor-induced angioedema. Incidence, prevention and management. Drug Saf. 1998;18:171-188. |
| 8. | Malde B, Regalado J, Greenberger PA. Investigation of angioedema associated with the use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Ann Allergy Asthma Immunol. 2007;98:57-63. |
| 9. | Gainer JV, Morrow JD, Loveland A, King DJ, Brown NJ. Effect of bradykinin-receptor blockade on the response to angiotensin-converting-enzyme inhibitor in normotensive and hypertensive subjects. N Engl J Med. 1998;339:1285-1292. |
| 10. | Molinaro G, Cugno M, Perez M, Lepage Y, Gervais N, Agostoni A, Adam A. Angiotensin-converting enzyme inhibitor-associated angioedema is characterized by a slower degradation of des-arginine(9)-bradykinin. J Pharmacol Exp Ther. 2002;303:232-237. |
| 11. | Khan MU, Baig MA, Javed RA, Ali S, Qamar UR, Vasavada BC, Khan IA. Benazepril induced isolated visceral angioedema: a rare and under diagnosed adverse effect of angiotensin converting enzyme inhibitors. Int J Cardiol. 2007;118:e68-e69. |
| 12. | Cicardi M, Zingale LC, Bergamaschini L, Agostoni A. Angioedema associated with angiotensin-converting enzyme inhibitor use: outcome after switching to a different treatment. Arch Intern Med. 2004;164:910-913. |
| 13. | Wong JT, Hsu Y, Chen HJL, Bloch KJ. Severe angioedema: Interaction between ACEI, ASA/NSAID, narcotics, and other contributing factors. J Allergy Clin Immunol. 2002;109:S128. |
| 14. | Sánchez-Borges M, Capriles-Hulett A, Caballero-Fonseca F. NSAID-induced urticaria and angioedema: a reappraisal of its clinical management. Am J Clin Dermatol. 2002;3:599-607. |
| 15. | Strom BL, Carson JL, Morse ML, West SL, Soper KA. The effect of indication on hypersensitivity reactions associated with zomepirac sodium and other nonsteroidal antiinflammatory drugs. Arthritis Rheum. 1987;30:1142-1148. |
| 16. | Bowen T, Cicardi M, Bork K, Zuraw B, Frank M, Ritchie B, Farkas H, Varga L, Zingale LC, Binkley K. Hereditary angiodema: a current state-of-the-art review, VII: Canadian Hungarian 2007 International Consensus Algorithm for the Diagnosis, Therapy, and Management of Hereditary Angioedema. Ann Allergy Asthma Immunol. 2008;100:S30-S40. |
| 17. | Cugno M, Zanichelli A, Foieni F, Caccia S, Cicardi M. C1-inhibitor deficiency and angioedema: molecular mechanisms and clinical progress. Trends Mol Med. 2009;15:69-78. |
| 18. | Zuraw BL. Clinical practice. Hereditary angioedema. N Engl J Med. 2008;359:1027-1036. |
| 19. | Winnewisser J, Rossi M, Späth P, Bürgi H. Type I hereditary angio-oedema. Variability of clinical presentation and course within two large kindreds. J Intern Med. 1997;241:39-46. |
| 20. | Agostoni A, Cicardi M. Hereditary and acquired C1-inhibitor deficiency: biological and clinical characteristics in 235 patients. Medicine (Baltimore). 1992;71:206-215. |
| 21. | Frank MM, Gelfand JA, Atkinson JP. Hereditary angioedema: the clinical syndrome and its management. Ann Intern Med. 1976;84:580-593. |
| 22. | Bork K, Siedlecki K, Bosch S, Schopf RE, Kreuz W. Asphyxiation by laryngeal edema in patients with hereditary angioedema. Mayo Clin Proc. 2000;75:349-354. |
| 23. | De Backer AI, De Schepper AM, Vandevenne JE, Schoeters P, Michielsen P, Stevens WJ. CT of angioedema of the small bowel. AJR Am J Roentgenol. 2001;176:649-652. |
| 24. | Bork K, Gül D, Hardt J, Dewald G. Hereditary angioedema with normal C1 inhibitor: clinical symptoms and course. Am J Med. 2007;120:987-992. |
| 25. | Bork K. Hereditary angioedema with normal C1 inhibitor activity including hereditary angioedema with coagulation factor XII gene mutations. Immunol Allergy Clin North Am. 2006;26:709-724. |
| 26. | Bouillet L, Longhurst H, Boccon-Gibod I, Bork K, Bucher C, Bygum A, Caballero T, Drouet C, Farkas H, Massot C. Disease expression in women with hereditary angioedema. Am J Obstet Gynecol. 2008;199:484.e1-484.e4. |
| 27. | Zingale LC, Castelli R, Zanichelli A, Cicardi M. Acquired deficiency of the inhibitor of the first complement component: presentation, diagnosis, course, and conventional management. Immunol Allergy Clin North Am. 2006;26:669-690. |
| 28. | Agostoni A, Aygören-Pürsün E, Binkley KE, Blanch A, Bork K, Bouillet L, Bucher C, Castaldo AJ, Cicardi M, Davis AE. Hereditary and acquired angioedema: problems and progress: proceedings of the third C1 esterase inhibitor deficiency workshop and beyond. J Allergy Clin Immunol. 2004;114:S51-S131. |
| 29. | Cichon S, Martin L, Hennies HC, Müller F, Van Driessche K, Karpushova A, Stevens W, Colombo R, Renné T, Drouet C. Increased activity of coagulation factor XII (Hageman factor) causes hereditary angioedema type III. Am J Hum Genet. 2006;79:1098-1104. |
| 30. | Duan QL, Binkley K, Rouleau GA. Genetic analysis of Factor XII and bradykinin catabolic enzymes in a family with estrogen-dependent inherited angioedema. J Allergy Clin Immunol. 2009;123:906-910. |
| 31. | Hara T, Shiotani A, Matsunaka H, Yamanishi T, Oka H, Ishiguchi T, Saika A, Itoh H, Nishioka S. Hereditary angioedema with gastrointestinal involvement: endoscopic appearance. Endoscopy. 1999;31:322-324. |
| 33. | Zuraw BL. Hereditary angiodema: a current state-of-the-art review, IV: short- and long-term treatment of hereditary angioedema: out with the old and in with the new? Ann Allergy Asthma Immunol. 2008;100:S13-S18. |
| 34. | CINRYZE [package insert]. New York: Lev Pharmaceuticals 2009; . |
| 35. | DANOCRINE [package insert]. New York: Sanofi-Synthelabo 2003; . |
| 36. | Bork K, Bygum A, Hardt J. Benefits and risks of danazol in hereditary angioedema: a long-term survey of 118 patients. Ann Allergy Asthma Immunol. 2008;100:153-161. |
| 37. | BERINERT [package insert]. Kankakee: CSL Behring 2009; . |
| 38. | FIRAZYR [package insert]. Berlin: Jerini AG 2009; . |
| 39. | CHMP assessment report for Firazyr. Accessed 4 June 2010. Available from: http://www.ema.europa.eu/humandocs/PDFs/EPAR/firazyr/H-899-en6.pdf. |
| 40. | KALBITOR [package insert]. Cambridge: Dyax Corp 2009; . |
| 41. | Prematta M, Gibbs JG, Pratt EL, Stoughton TR, Craig TJ. Fresh frozen plasma for the treatment of hereditary angioedema. Ann Allergy Asthma Immunol. 2007;98:383-388. |
| 42. | CETOR [patient information leaflet]. Amsterdam: Sanquin 2003; . |
| 43. | Gompels MM, Lock RJ, Abinun M, Bethune CA, Davies G, Grattan C, Fay AC, Longhurst HJ, Morrison L, Price A. C1 inhibitor deficiency: consensus document. Clin Exp Immunol. 2005;139:379-394. |