Published online Oct 14, 2010. doi: 10.3748/wjg.v16.i38.4846
Revised: June 12, 2010
Accepted: June 19, 2010
Published online: October 14, 2010
AIM: To investigate whether microsomal prostaglandin E synthase-1 (mPGES-1) expression in hepatocellular carcinoma (HCC) and in non-cancerous liver affects HCC prognosis after hepatectomy.
METHODS: The relationship between patient clinical profiles, tumor factors, surgical determinants, and mPGES-1 expression and the recurrence-free survival rate were examined in 64 patients who underwent curative hepatectomy between March 2003 and December 2006.
RESULTS: The scores for mPGES-1 expression were higher in well differentiated and moderately differentiated HCC tissues than in poorly differentiated HCC tissues (well differentiated, 5.1 ± 2.7; moderately differentiated, 5.1 ± 1.7; poorly differentiated, 3.0 ± 1.8). In non-cancerous liver tissues, the mPGES-1 levels were higher in injured liver tissues than in normal tissues. Cirrhotic livers had higher mPGES-1 levels than livers with chronic hepatitis (normal livers, 3.3 ± 0.7; chronic hepatitic livers, 5.4 ± 1.9; cirrhotic livers, 6.4 ± 1.6). A univariate analysis revealed that the recurrence-free survival rate was significantly lower in patients with vascular invasion, a higher mPGES-1 level in non-cancerous liver tissue, a larger tumor diameter (≥ 5 cm), and a lower serum albumin level (≤ 3.7 g/dL). The mPGES-1 expression in HCC tissues did not correlate well with postoperative recurrence. A multivariate analysis demonstrated that the presence of vascular invasion and higher mPGES-1 levels were statistically significant independent predictors for early postoperative recurrence of HCC.
CONCLUSION: Increased mPGES-1 expression in non-cancerous liver tissues is closely associated with the early recurrence of HCC after curative resection.
- Citation: Nonaka K, Fujioka H, Takii Y, Abiru S, Migita K, Ito M, Kanematsu T, Ishibashi H. mPGES-1 expression in non-cancerous liver tissue impacts on postoperative recurrence of HCC. World J Gastroenterol 2010; 16(38): 4846-4853
- URL: https://www.wjgnet.com/1007-9327/full/v16/i38/4846.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i38.4846
Hepatocellular carcinoma (HCC) is a common cause of cancer death worldwide[1,2]. Hepatectomy is one of the best treatment modalities for HCC. Recent advances in surgical techniques and perioperative management have led to improved survival after curative resection. However, the rates of postoperative recurrence remain high (60%-80%)[3], and such recurrences can originate from intrahepatic metastases of the primary HCC and from the multicentric occurrence of new tumors[4]. With regard to the latter, many studies have reported a significant association between HCC development and underlying liver disease[5,6]. Therefore, HCC tumor factors as well as the underlying hepatic status should be carefully examined to predict tumor recurrence after curative resection and to choose optimal treatments.
A variety of malignant tumors in many visceral sites have appeared after chronic inflammation[7]. Clinical and biochemical evidence suggests that prostaglandin E2 (PGE2) produced at inflammation sites and its receptors play an important role in the development of malignant tumors, including HCC and other cancers[8,9]. The biosynthesis of PGE2 from arachidonic acid requires two enzymatic activities that include cyclooxygenase (COX) and prostaglandin E synthase (PGES), which is the terminal enzyme for PGE2 biosynthesis. Three PGES isoforms have been identified, including microsomal PGES-1 (mPGES-1), mPGES-2, and cytosolic PGES[10,11]. In particular, mPGES-1, an enzyme induced by pro-inflammatory stimuli, has received much attention[8,11]. Previous studies have indicated that mPGES-1 overexpression was associated with various types of cancer, including HCC[12,13]. Therefore, mPGES-1 may play an important role in HCC recurrence in the remnant liver tissue after curative resection for HCC.
The aim of the present study was to clarify whether mPGES-1 expression in HCC and non-cancerous liver tissues affects the clinical course of HCC patients undergoing curative resection.
Sixty-four consecutive patients (42 males and 22 females) underwent curative liver resection for HCC at the Division of Surgery, National Hospital Organization, Nagasaki Medical Center, between March 2003 and December 2006. In all cases, the diagnosis of HCC was confirmed by pathological examination of the resected specimens.
The inclusion criteria for the study were as follows: (1) the absence of extrahepatic metastasis; (2) curative resection defined as histological evidence of the complete removal of HCC tumors; and (3) no additional therapies or multi-modality treatment for HCC until the development of recurrence. Written informed consent was obtained from all the patients. They were regularly followed up at our outpatient clinic and were prospectively monitored for disease recurrence by serum levels of α-fetoprotein (AFP) and des-γ-carboxy prothrombin (DCP), and ultrasonography or computed tomography every 3 mo. Suspected intra-hepatic recurrence was confirmed by hepatic angiography, and if necessary, by percutaneous needle biopsy. The follow-up period was at least 12 mo or until death in patients who died within 12 mo of their operation. The study was conducted in accordance with the Helsinki Declaration and the guidelines issued by the Ministry of Education, Culture, Sports, Science and Technology of Japan, and the Ethics Committee at National Hospital Organization, Nagasaki Medical Center.
HCC tissues and non-cancerous liver tissues from the opposite liver lobe in which HCC developed were obtained. The tissues were frozen in liquid nitrogen and stored at -80°C until use. For immunohistochemical analysis, the tissues were formalin-fixed and paraffin-embedded.
Histologically “normal” livers (free of hepatitis B or C viral infections and without any significant pathological abnormalities) were obtained from 7 patients with liver metastases from colorectal cancer.
For immunohistochemical analysis of the mPGES-1 protein, formalin-fixed and paraffin-embedded tissue blocks were cut into 4 μm-thick sections. The sections were deparaffinized in xylene and subsequently rehydrated in sequential ethanol (100%-70%). After washing 3 times with 10 mmol/L phosphate-buffered saline (PBS) (pH 7.4), antigen retrieval was performed by first heating in a microwave at 95°C for 20 min, then by washing twice in PBS for 10 min. The sections were treated with peroxidase-blocking solution (DAKO Japan, Kyoto, Japan) for 5 min, and incubated with the primary antibody for 60 min at room temperature. The primary antibody used was a 1:100 dilution of a mPGES-1 polyclonal antibody (Cayman Chemical, Ann Arbor, MI, USA). A standardized two-step method with ENVISION plus (DAKO) was used for detection. The reaction products were visualized using diaminobenzidine as a chromogen (DAKO), and counterstained with Mayer’s hematoxylin (DAKO). The specificity of the antibody was checked by the adsorption with corresponding blocking peptides (Cayman Chemical) using a 1:1 ratio of primary antibody to blocking peptide.
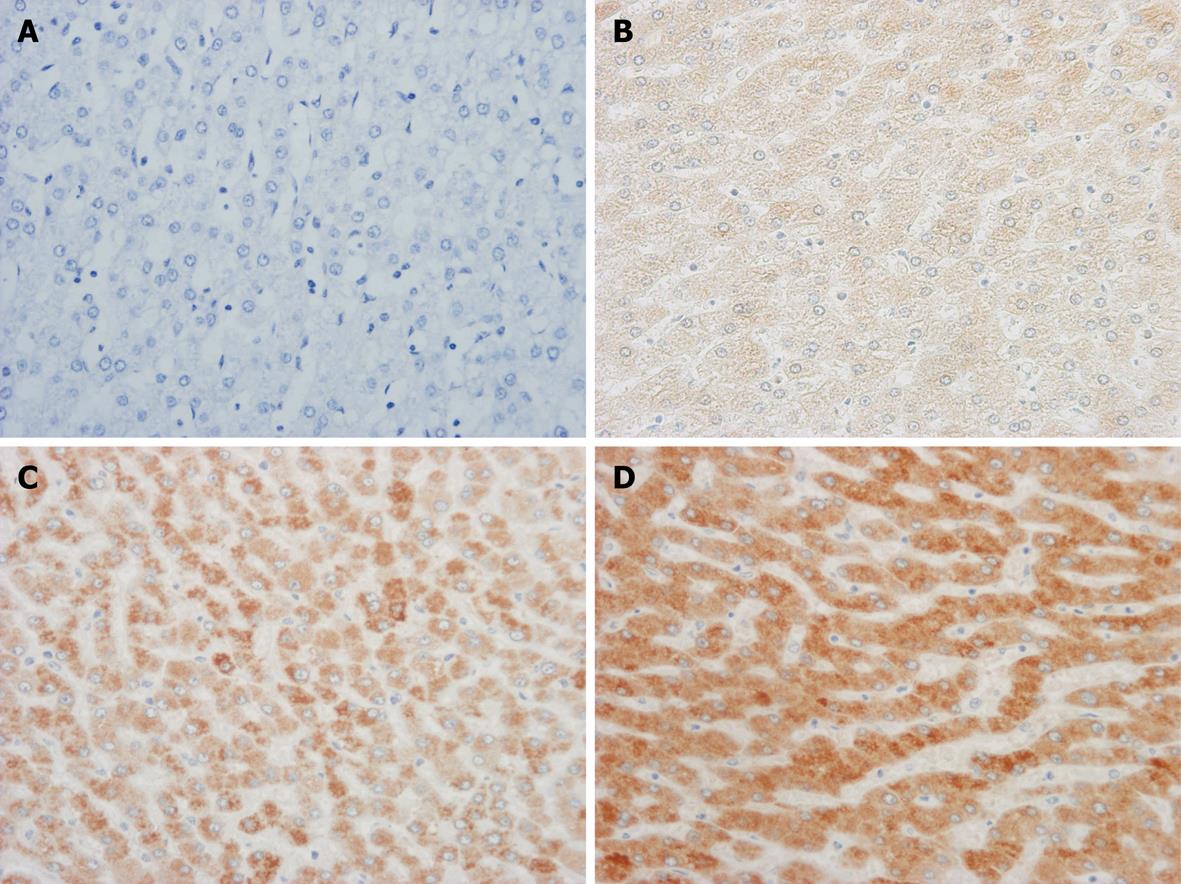
Two blinded investigators (MI and KN) evaluated the immunostained sections. To assess the mPGES-1 protein staining results, the cytoplasmic immunoreactive intensity was scored as previously described[14]. In summary, the staining intensity for mPGES-1 was scored in each specimen on a scale of 0-3, with 0 = negative staining, 1 = weakly positive staining, 2 = moderately positive staining, and 3 = strongly positive staining (Figure 1). The staining intensity was evaluated for the maximum intensity among positive cells (‘‘maximum intensity of staining’’, I) and the intensity level observed in the largest number of positive cells (‘‘most extensive intensity level’’, II). The extent to which positive cells were observed in each specimen (‘‘extent of distribution of positive cells”, III) was estimated and scored on a scale of 0-4, with 0 = negative, 1 = positive in 1%-25% of cells, 2 = positive in 26%-50% of cells, 3 = positive in 51%-75% of cells, and 4 = positive in 76%-100% of cells. Each section was evaluated for the sum of these three parameters (I + II + III). Immunoreactivity for mPGES-1 protein was compared statistically using the average of the sum in each histological category. The patients in the present study were divided into two groups, including the higher expression (the sum of the categorical score, 6 to 10) and the lower expression groups (the sum of the categorical score; less than 6).
We performed a Western blotting analysis on representative samples of HCC and non-cancerous liver tissues. The tissues were homogenized on ice in RIPA buffer [PBS, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate (SDS)] containing 100 ng/mL phenylmethylsulphonyl fluoride, 4 mg/mL aprotinin, 2 mg/mL leupeptin, 1 mg/mL pepstatin, 10 mg/mL antipain, 10 mg/mL soybean trypsin inhibitor, and 2 mmol/L ethylenediamine-tetraacetic acid. The homogenates were clarified by centrifugation. Protein concentrations were measured using the Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). After boiling for 5 min in the presence of 2-mercaptoethanol, samples containing 50 mg of tissue lysates were separated on 12.5% SDS-polyacrylamide gels and then transferred onto equilibrated Hybond PVDF membranes (Amersham International, Buckinghamshire, UK). After skim milk blocking, the membranes were then incubated with the mPGES-1 polyclonal antibody (at a dilution of 1:500). Bound antibodies were detected with horseradish peroxidase-labeled rabbit anti-goat IgG (Southern Biotechnology Associates, Birmingham, AL, USA) using an enhanced chemiluminescence detection system (ECL kit; Amersham International, Buckinghamshire, UK).
The following clinicopathological factors were evaluated for their association with HCC recurrence: age, gender, presence of hepatitis B surface antigen (HBsAg) or anti-hepatitis C virus antibody (anti-HCV Ab), platelet count, preoperative blood chemistry (serum levels of total bilirubin, alanine aminotransferase and albumin), presence of liver cirrhosis, and mPGES-1 expression. The evaluated operative factors included the intraoperative blood loss and the hepatectomy method. The tumor factors were the greatest tumor diameter, the number of tumor nodules, the presence of vascular invasion, the presence of capsular formation, the histological grade, and the serum levels of AFP and DCP. The hepatectomy method was classified as anatomical or non-anatomical resection according to the methods described by Makuuchi et al[15] and Takayama et al[16]. The anatomic resection consisted of the systematic removal of the hepatic segment which is confined by the tumor-bearing portal tributaries. In the non-anatomic resection, the liver was divided along a line so as to secure a surgical margin of at least 5 mm, if possible.
Statistical analyses were performed using either Student’s t-test or the Mann-Whitney U test to compare variables between the groups. A recurrence-free survival curve was plotted using the Kaplan-Meier method. A statistical comparison of the recurrence-free survival was performed using the log-rank test. A multivariate analysis by the Cox proportional hazard model was used to identify the independent risk factors for tumor recurrence. A P value < 0.05 was considered statistically significant. Statistical analyses were performed using the StatView for Windows software program (version 5.0, SAS Institute Inc., Cary, NC, USA).
There were 42 male (65.6%) and 22 female (34.4%) patients. The mean age was 64 years (range, 38-86 years). Twenty-one patients were positive for HBsAg, 32 were positive for anti-HCV Ab, and 11 were negative for both. Thirty-one patients had a cirrhotic liver, while 33 did not. The maximum tumor size was 12 cm, and 57 patients (89.1%) had a solitary tumor. More than 90% of the patients enrolled in the study had a Child-Pugh classification of A for liver function. Fifty percent of the patients had a tumor size > 3 cm. In the pathological differentiation, HCC was well differentiated in 18 patients, moderately differentiated in 40, and poorly differentiated in 6. The median observation period was 49 mo (range, 3-74 mo).
The expression of mPGES-1 protein in the HCC and non-cancerous liver tissues was examined immunohistochemically. Various degrees of staining for mPGES-1 protein were observed. The scores for mPGES-1 expression in the HCC and non-cancerous liver tissues are summarized in Table 1. The marked expression of mPGES-1 was demonstrated in well differentiated as well as in moderately differentiated HCC tissues (scores; 5.1 ± 2.7 and 5.1 ± 1.7, respectively). Conversely, mPGES-1 expression was significantly weaker in poorly differentiated HCC tissues (score; 3.0 ± 1.8, P < 0.05). Seven of 18 cases (38.9%) with well differentiated HCC and 14 of 40 cases (35.0%) with moderately differentiated HCC had high expression scores, whereas none of the patients with poorly differentiated HCC had high expression scores.
| No. of cases | Patients with higher scores | Patients with lower scores | Scores (mean ± SD) | P | |
| Hepatocellular carcinoma tissues | |||||
| Well differentiated | 18 | 7/18 (38.9) | 11/18 (61.1) | 5.1 ± 2.7 | - |
| Moderately differentiated | 40 | 14/40 (35.0) | 26/40 (65.0) | 5.1 ± 1.7 | 0.959a |
| Poorly differentiated | 6 | 0 | 6/6 (100) | 3.0 ± 1.8 | 0.009a |
| Non-cancerous liver tissues | |||||
| Normal | 2 | 1/2 (50.0) | 1/2 (50.0) | 3.3 ± 0.7 | - |
| Chronic hepatitis | 31 | 19/31 (61.3) | 12/31 (38.7) | 5.4 ± 1.9 | 0.006b |
| Cirrhosis | 31 | 15/31 (48.4) | 16/31 (51.6) | 6.4 ± 1.6 | 0.002b, 0.039c |
| Normal livers from colorectal cancer | 7 | 0 | 7/7 (100) | 3.5 ± 0.5 | - |
The mPGES-1 levels increased significantly with fibrotic stage of the liver tissues (scores; normal liver 3.3 ± 0.7, chronic hepatitic livers 5.4 ± 1.9, cirrhotic livers 6.4 ± 1.6). High expression scores were observed in 1 of 2 normal livers (50%), 19 of 31 chronic hepatitic livers (61.3%), and 15 of 31 cirrhotic livers (48.4%). There was no significant correlation between tumor differentiation and non-cancerous liver tissue in the expression of mPGES-1 (data not shown). Additionally, mPGES-1 expression in normal livers obtained from 7 patients with liver metastasis was lower than that in damaged livers (P < 0.05).
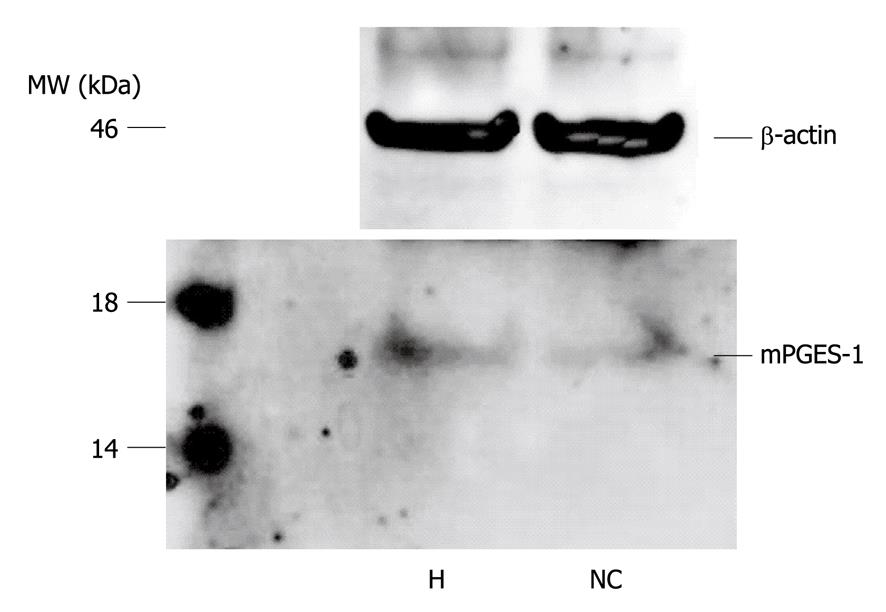
To confirm the specificity of the mPGES-1 antibody and the presence of mPGES-1 protein in the specimen, Western blotting analysis was performed on representative samples of HCC and non-cancerous liver tissues. Both tissue types yielded a single band with a molecular weight of 16 kDa, indicating the presence of mPGES-1 protein (Figure 2).
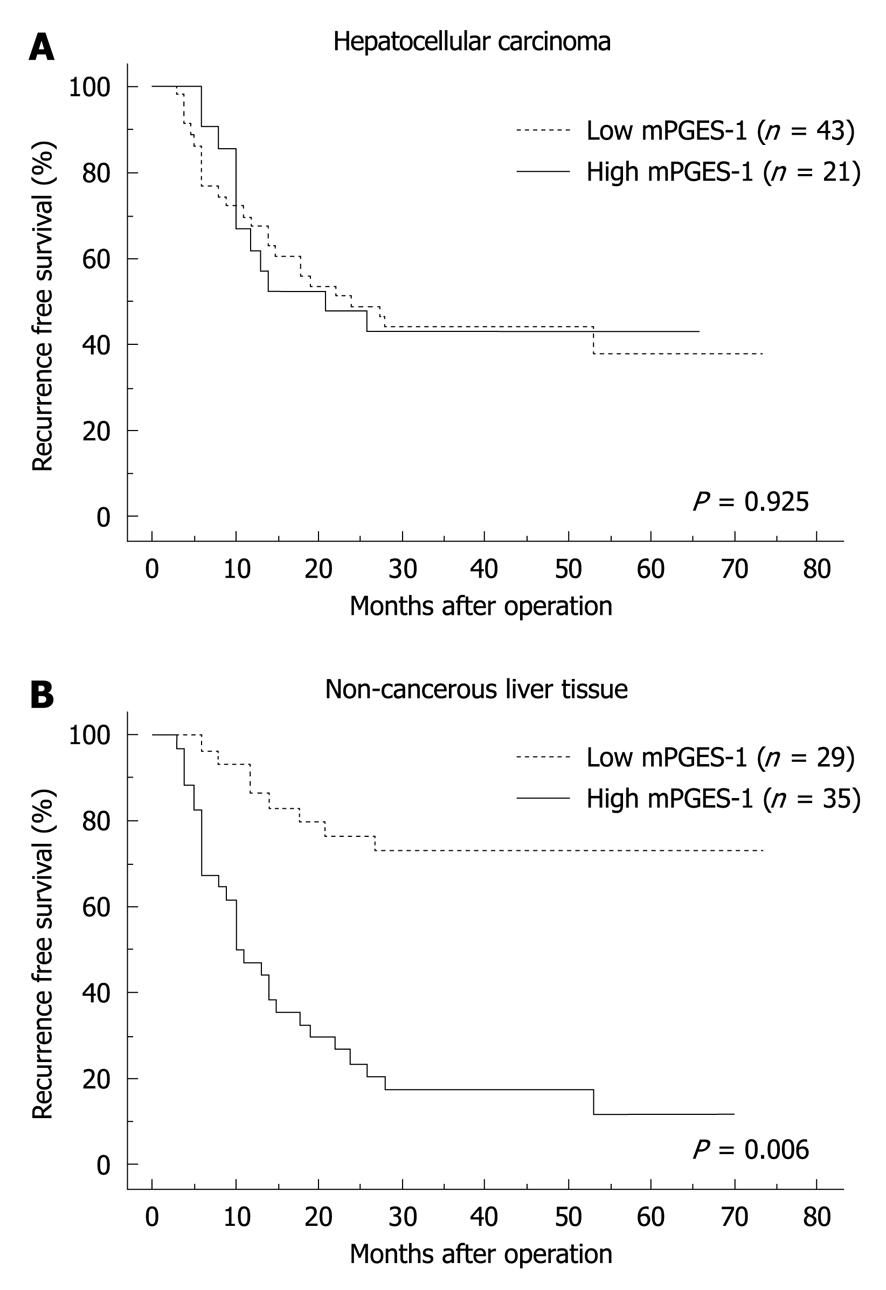
We evaluated the correlation between the levels of mPGES-1 expression in HCC and non-cancerous liver tissues and recurrence-free survival time. No statistically significant difference was observed in the recurrence-free survival time between the higher and lower expression groups in HCC tissues (Figure 3A). In contrast, a statistically significant difference in the recurrence-free survival time was observed between the higher and lower expression groups in non-cancerous liver tissues (P = 0.006, Figure 3B).
Various clinicopathological parameters were evaluated for their association with HCC recurrence (Table 2). A univariate analysis revealed that recurrence-free survival time was shorter in cases with vascular invasion, higher mPGES-1 levels in non-cancerous liver tissue, a larger tumor diameter (≥ 5 cm), and lower levels of serum albumin (< 37 g/L). The operative factors were not significantly correlated with recurrence-free survival time. A multivariate analysis demonstrated that the presence of vascular invasion and higher mPGES-1 levels in the non-cancerous liver tissue were significant independent predictors for the early recurrence of HCC after curative hepatectomy (Table 3).
| No. of patients | Postoperative recurrence | P | ||
| Yes | No | |||
| Age (yr) | ||||
| ≥ 60 | 45 | 23 | 22 | 0.733 |
| < 60 | 19 | 9 | 10 | |
| Gender | ||||
| Male | 43 | 20 | 23 | 0.481 |
| Female | 21 | 12 | 9 | |
| Hepatitis B surface antigen | ||||
| Positive | 21 | 13 | 8 | 0.147 |
| Negative | 43 | 19 | 24 | |
| Hepatitis C virus antibody | ||||
| Positive | 32 | 12 | 20 | 0.151 |
| Negative | 32 | 20 | 12 | |
| Total bilirubin (mg/dL) | ||||
| ≥ 1.0 | 29 | 7 | 22 | 0.987 |
| < 1.0 | 35 | 13 | 22 | |
| Alanine aminotransferase (IU/L) | ||||
| ≥ 50 | 26 | 11 | 15 | 0.635 |
| < 50 | 38 | 21 | 17 | |
| Albumin (g/dL) | ||||
| ≥ 3.7 | 49 | 24 | 25 | 0.022 |
| < 3.7 | 15 | 13 | 2 | |
| Platelets (104/μL) | ||||
| ≥ 10 | 46 | 21 | 25 | 0.465 |
| < 10 | 18 | 11 | 7 | |
| Liver cirrhosis | ||||
| Present | 31 | 16 | 15 | 0.772 |
| Absent | 33 | 16 | 17 | |
| T mPGES-1 | ||||
| High | 21 | 12 | 9 | 0.925 |
| Low | 43 | 25 | 18 | |
| NC mPGES-1 | ||||
| High | 35 | 23 | 11 | 0.006 |
| Low | 29 | 9 | 21 | |
| Hepatectomy | ||||
| Anatomic | 31 | 21 | 10 | 0.149 |
| Non-anatomic | 33 | 16 | 17 | |
| Operative blood loss (mL) | ||||
| ≥ 500 | 15 | 12 | 3 | 0.091 |
| < 500 | 49 | 25 | 24 | |
| α-fetoprotein (ng/mL) | ||||
| ≥ 100 | 15 | 10 | 5 | 0.347 |
| < 100 | 49 | 27 | 22 | |
| DCP (mAU/mL) | ||||
| ≥ 400 | 22 | 15 | 7 | 0.081 |
| < 400 | 42 | 19 | 23 | |
| Tumor diameter (cm) | ||||
| ≥ 5 | 16 | 12 | 3 | 0.018 |
| < 5 | 48 | 20 | 29 | |
| Tumor number | ||||
| Multiple | 7 | 3 | 4 | 0.789 |
| Solitary | 57 | 29 | 28 | |
| Histological grade | ||||
| Well | 18 | 8 | 10 | 0.495 |
| Moderate | 40 | 21 | 19 | |
| Poor | 6 | 3 | 3 | |
| Capsular formation | ||||
| Present | 49 | 25 | 24 | 0.320 |
| Absent | 5 | 7 | 8 | |
| Vascular invasion | ||||
| Present | 16 | 11 | 5 | < 0.001 |
| Absent | 48 | 9 | 39 | |
| Variables | Hazard ratio | 95% CI | P |
| Vascular invasion (present) | 4.116 | 1.813-9.344 | < 0.001 |
| NC mPGES-1 expression (high) | 4.074 | 1.760-9.428 | 0.001 |
| Tumor diameter (≥ 5 cm) | 2.060 | 0.860-4.935 | 0.105 |
| Albumin (< 3.7 g/dL) | 1.745 | 0.589-3.165 | 0.315 |
The present study demonstrated that the rate of HCC recurrence was high after curative resection. This finding was consistent with those described in other recent reports[1-6]. Tumor recurrence is caused by metastatic lesions, residual microscopic lesions that remain after curative resection, or multicentric occurrence in the setting of hepatitis or cirrhosis[17,18]. The prevention of tumor recurrence is key to the improvement of prognosis for HCC patients after a hepatectomy[19]. In the present study, a multivariate analysis indicated that the two independent predictors for HCC recurrence after curative resection were the presence of vascular invasion and increased mPGES-1 expression in the non-cancerous liver tissue.
Vascular invasion is a well-known risk factor for a poor prognosis after curative resection. The presence of vascular invasion is considered one of the strongest predictors of intrahepatic metastasis caused by the spread of cancer cells via the portal venous system[17-19]. Although several reports have demonstrated that postoperative adjuvant therapy prevented postoperative HCC recurrence[20,21], its efficacy has yet to be determined. Other therapeutic modalities for treating postoperative recurrence are urgently needed.
The most interesting finding in the present study was that increased mPGES-1 expression in the non-cancerous liver tissue was an independent predictor for early HCC recurrence after curative resection. Increased mPGES-1 levels induce PGE2 synthesis, which may create a suitable environment for occult intrahepatic metastases to survive and spread after hepatectomy. This hypothesis is supported by several studies showing that PGE2 was implicated in migration, secretion of various types of matrix metallo-proteinases, and cell adhesion in HCC cells[22-24]. Additionally, increased PGE2 levels in the non-cancerous liver tissue leads to prolonged acceleration of necroinflammation and regeneration in the remnant liver[25]. The inflamed liver may also provide a good environment for occult intrahepatic metastases to grow in response to different growth factors[26]. In the present study, active hepatitic and/or cirrhotic livers had increased mPGES-1 expression compared to normal livers. The repeated cycles of necroinflammation, degeneration, and regeneration increase hepatocyte turnover, which facilitates spontaneous mutation and may hinder DNA repair[26]. The release of reactive oxygen species including superoxide and H2O2 in this situation may also contribute to uncontrolled cell growth, apoptosis, and senescence[27]. Another possible mechanism is that mPGES-1 itself may act as a landscaping tumor promoter. mPGES-1 lies downstream of the PGE2-biosynthetic pathway of COX-2. Recent studies reported that mPGES-1 was expressed in several cancers and was linked to carcinogenesis[12,28]. mPGES-1 derived from the stromal component may promote tumor growth by producing bioactive PGE2, which acts angiogenetically or immunosuppressively, and affects carcinoma cells in a paracrine fashion[12,28]. Therefore, the increased expression of mPGES-1 in the non-cancerous liver tissue may create conditions suitable for HCC recurrence from metastasis or multicentric occurrence. However, the precise mechanisms remain to be elucidated.
The mPGES-1 expression in HCC tissues did not correlate well with postoperative recurrence. This finding suggested that the mPGES-1 in HCC tissues per se did not determine the malignant potential of HCC tissues, although overexpression of mPGES-1 was associated with various types of cancer[12,13].
The data indicate that COX-2 inhibitors are chemopreventive for several kinds of cancers[29], however, there have been no reports on HCC patients. Although the COX-2 inhibitors have a reduced gastrointestinal toxicity in comparison to traditional non-steroidal anti-inflammatory drugs, some adverse effects have been reported[30]. From this standpoint, more selective inhibition of the prostanoid pathway to PGE2 is thus highly desirable. mPGES-1 is the terminal enzyme for PGE2 biosynthesis, and thus it is considered the most selective agent for that pathway. Although there have been several reports concerning the selective inhibitors of mPGES-1[31,32], further studies are still needed in clinical settings.
In conclusion, increased mPGES-1 expression in non-cancerous liver tissue is closely associated with the early recurrence of HCC after curative resection. The present study also indicates that an inhibitor of mPGES-1 may be a new therapeutic option to improve the survival rate of HCC patients after curative resection.
Microsomal prostaglandin E synthase-1 (mPGES-1) is the terminal enzyme in the formation of prostaglandin E2 from prostaglandin H2. Data indicate that increased expression of mPGES-1 is associated with various types of cancers. However, the impact of mPGES-1 expression on the clinical course of hepatocellular carcinoma (HCC) has not yet been elucidated.
In HCC, tumor recurrence is caused by metastatic lesions, residual microscopic lesions that remain even after curative resection, and multicentric occurrence in the setting of hepatitis or cirrhosis. The research was performed to clarify the risk factors for the recurrence of HCC after curative resection in Nagasaki Medical Center.
The present study demonstrates that various degrees of mPGES-1 expression occur in HCC and non-cancerous liver tissues. This is the first report to demonstrate that increased expression of mPGES-1 in non-cancerous liver tissue is an independent predictor for HCC recurrence after curative resection.
mPGES-1 expression in non-cancerous liver tissue could be a useful biomarker for screening high risk groups of patients with HCC after curative resection. In the near future, a selective mPGES-1 inhibitor may prevent postoperative recurrence of HCC and improve the prognosis of HCC patients.
mPGES-1 is a protein belonging to the membrane-associated proteins involved in eicosanoid and glutathione metabolism super family. mPGES-1 is induced by pro-inflammatory stimuli, down-regulated by anti-inflammatory glucocorticoids, and functionally coupled with cyclooxygenase-2. Thus, mPGES-1 plays a central role in the biosynthesis of prostaglandin E2.
The paper reported that increase in mPGES-1 in non-cancerous liver was an independent prognostic factor in patients received surgical therapy to HCC. Although the results might be of importance, several questions are addressed, and several points to be improved are suggested.
Peer reviewer: Hitoshi Tsuda, MD, PhD, Diagnostic Pathology Section, Clinical Laboratory Division, National Cancer Center Hospital, 5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan
S- Editor Wang YR L- Editor Webster JR E- Editor Zheng XM
| 1. | Ryu SH, Chung YH, Lee H, Kim JA, Shin HD, Min HJ, Seo DD, Jang MK, Yu E, Kim KW. Metastatic tumor antigen 1 is closely associated with frequent postoperative recurrence and poor survival in patients with hepatocellular carcinoma. Hepatology. 2008;47:929-936. |
| 2. | Sumie S, Kuromatsu R, Okuda K, Ando E, Takata A, Fukushima N, Watanabe Y, Kojiro M, Sata M. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol. 2008;15:1375-1382. |
| 3. | Shah SA, Cleary SP, Wei AC, Yang I, Taylor BR, Hemming AW, Langer B, Grant DR, Greig PD, Gallinger S. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery. 2007;141:330-339. |
| 4. | Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2000;89:500-507. |
| 5. | Park JH, Koh KC, Choi MS, Lee JH, Yoo BC, Paik SW, Rhee JC, Joh JW. Analysis of risk factors associated with early multinodular recurrences after hepatic resection for hepatocellular carcinoma. Am J Surg. 2006;192:29-33. |
| 6. | Koike Y, Shiratori Y, Sato S, Obi S, Teratani T, Imamura M, Hamamura K, Imai Y, Yoshida H, Shiina S. Risk factors for recurring hepatocellular carcinoma differ according to infected hepatitis virus-an analysis of 236 consecutive patients with a single lesion. Hepatology. 2000;32:1216-1223. |
| 7. | Schottenfeld D, Beebe-Dimmer J. Chronic inflammation: a common and important factor in the pathogenesis of neoplasia. CA Cancer J Clin. 2006;56:69-83. |
| 8. | Kamei D, Murakami M, Nakatani Y, Ishikawa Y, Ishii T, Kudo I. Potential role of microsomal prostaglandin E synthase-1 in tumorigenesis. J Biol Chem. 2003;278:19396-19405. |
| 9. | Morinaga S, Tarao K, Yamamoto Y, Nakamura Y, Rino Y, Miyakawa K, Ohkawa S, Akaike M, Sugimasa Y, Takemiya S. Overexpressed cyclo-oxygenase-2 in the background liver is associated with the clinical course of hepatitis C virus-related cirrhosis patients after curative surgery for hepatocellular carcinoma. J Gastroenterol Hepatol. 2007;22:1249-1255. |
| 10. | Tanioka T, Nakatani Y, Semmyo N, Murakami M, Kudo I. Molecular identification of cytosolic prostaglandin E2 synthase that is functionally coupled with cyclooxygenase-1 in immediate prostaglandin E2 biosynthesis. J Biol Chem. 2000;275:32775-32782. |
| 11. | Jakobsson PJ, Thorén S, Morgenstern R, Samuelsson B. Identification of human prostaglandin E synthase: a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc Natl Acad Sci USA. 1999;96:7220-7225. |
| 12. | Yoshimatsu K, Golijanin D, Paty PB, Soslow RA, Jakobsson PJ, DeLellis RA, Subbaramaiah K, Dannenberg AJ. Inducible microsomal prostaglandin E synthase is overexpressed in colorectal adenomas and cancer. Clin Cancer Res. 2001;7:3971-3976. |
| 13. | Breinig M, Rieker R, Eiteneuer E, Wertenbruch T, Haugg AM, Helmke BM, Schirmacher P, Kern MA. Differential expression of E-prostanoid receptors in human hepatocellular carcinoma. Int J Cancer. 2008;122:547-557. |
| 14. | Koga H, Sakisaka S, Ohishi M, Kawaguchi T, Taniguchi E, Sasatomi K, Harada M, Kusaba T, Tanaka M, Kimura R. Expression of cyclooxygenase-2 in human hepatocellular carcinoma: relevance to tumor dedifferentiation. Hepatology. 1999;29:688-696. |
| 15. | Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet. 1985;161:346-350. |
| 16. | Takayama T, Makuuchi M, Kubota K, Harihara Y, Hui AM, Sano K, Ijichi M, Hasegawa K. Randomized comparison of ultrasonic vs clamp transection of the liver. Arch Surg. 2001;136:922-928. |
| 17. | Cha C, Fong Y, Jarnagin WR, Blumgart LH, DeMatteo RP. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg. 2003;197:753-758. |
| 18. | Kaibori M, Ishizaki M, Saito T, Matsui K, Kwon AH, Kamiyama Y. Risk factors and outcome of early recurrence after resection of small hepatocellular carcinomas. Am J Surg. 2009;198:39-45. |
| 19. | Shirabe K, Wakiyama S, Gion T, Motomura K, Koyanagi T, Sakamoto S, Nagaie T. Clinicopathological risk factors linked to recurrence pattern after curative hepatic resection for hepatocellular carcinoma--results of 152 resected cases. Hepatogastroenterology. 2007;54:2084-2087. |
| 20. | Zhong C, Guo RP, Li JQ, Shi M, Wei W, Chen MS, Zhang YQ. A randomized controlled trial of hepatectomy with adjuvant transcatheter arterial chemoembolization versus hepatectomy alone for Stage III A hepatocellular carcinoma. J Cancer Res Clin Oncol. 2009;135:1437-1445. |
| 21. | Zhou WP, Lai EC, Li AJ, Fu SY, Zhou JP, Pan ZY, Lau WY, Wu MC. A prospective, randomized, controlled trial of preoperative transarterial chemoembolization for resectable large hepatocellular carcinoma. Ann Surg. 2009;249:195-202. |
| 22. | Adachi E, Maeda T, Matsumata T, Shirabe K, Kinukawa N, Sugimachi K, Tsuneyoshi M. Risk factors for intrahepatic recurrence in human small hepatocellular carcinoma. Gastroenterology. 1995;108:768-775. |
| 23. | Mayoral R, Fernández-Martínez A, Boscá L, Martín-Sanz P. Prostaglandin E2 promotes migration and adhesion in hepatocellular carcinoma cells. Carcinogenesis. 2005;26:753-761. |
| 24. | Han C, Michalopoulos GK, Wu T. Prostaglandin E2 receptor EP1 transactivates EGFR/MET receptor tyrosine kinases and enhances invasiveness in human hepatocellular carcinoma cells. J Cell Physiol. 2006;207:261-270. |
| 25. | Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18:7908-7916. |
| 26. | Chung YH, Kim JA, Song BC, Lee GC, Koh MS, Lee YS, Lee SG, Suh DJ. Expression of transforming growth factor-alpha mRNA in livers of patients with chronic viral hepatitis and hepatocellular carcinoma. Cancer. 2000;89:977-982. |
| 27. | Cheung YS, Chan HL, Wong J, Lee KF, Poon TC, Wong N, Lai PB. Elevated perioperative transaminase level predicts intrahepatic recurrence in hepatitis B-related hepatocellular carcinoma after curative hepatectomy. Asian J Surg. 2008;31:41-49. |
| 28. | Mehrotra S, Morimiya A, Agarwal B, Konger R, Badve S. Microsomal prostaglandin E2 synthase-1 in breast cancer: a potential target for therapy. J Pathol. 2006;208:356-363. |
| 29. | Abiru S, Nakao K, Ichikawa T, Migita K, Shigeno M, Sakamoto M, Ishikawa H, Hamasaki K, Nakata K, Eguchi K. Aspirin and NS-398 inhibit hepatocyte growth factor-induced invasiveness of human hepatoma cells. Hepatology. 2002;35:1117-1124. |
| 30. | Crofford LJ, Lipsky PE, Brooks P, Abramson SB, Simon LS, van de Putte LB. Basic biology and clinical application of specific cyclooxygenase-2 inhibitors. Arthritis Rheum. 2000;43:4-13. |
| 31. | AbdulHameed MD, Hamza A, Liu J, Huang X, Zhan CG. Human microsomal prostaglandin E synthase-1 (mPGES-1) binding with inhibitors and the quantitative structure-activity correlation. J Chem Inf Model. 2008;48:179-185. |
| 32. | Côté B, Boulet L, Brideau C, Claveau D, Ethier D, Frenette R, Gagnon M, Giroux A, Guay J, Guiral S. Substituted phenanthrene imidazoles as potent, selective, and orally active mPGES-1 inhibitors. Bioorg Med Chem Lett. 2007;17:6816-6820. |