INTRODUCTION
Angiogenesis can be envisaged as a dynamic, hypoxia-stimulated and growth factor-dependent ubiquitous process leading to the formation of new vessels from pre-existing blood vessels[1-5], and should be distinguished from other terms that, although related to vessel formation and growth, define significantly different processes such as vasculogenesis, arteriogenesis and collateral vessel growth. Angiogenesis occurs virtually in almost all organs and tissues and is considered a critical step in either physiological conditions or in tissue repair and growth in several pathophysiological conditions[1-5], including chronic liver diseases (CLDs)[6-8].
Where the liver is concerned, physiological and pathological angiogenesis can occur during liver regeneration (after acute liver injury or after partial hepatectomy), in ischemic conditions, during chronic inflammatory and fibrogenic liver diseases as well as in hepatocellular carcinoma and in metastatic liver cancers[6-8]. The steps and mechanisms of hepatic angiogenesis mostly overlap with those described in other organs or tissues but a number of liver parenchyma peculiarities are likely to make the overall scenario more complex[6]. These include the existence of two different kinds of microvascular structures (portal vessels and liver sinusoids, lined by continuous or fenestrated and discontinuous endothelium, respectively), the expression of a putative liver specific angiopoietin-like peptide defined as ANGPTL3[9] and, most relevant, the unique and heterogenous phenotypic profile and functional role of hepatic stellate cells (HSCs) that, although regarded as liver specific pericytes in normal liver, also represent the most relevant pro-fibrogenic cell lineage[10-13] in CLDs. HSCs, particularly in their activated and myofibroblast-like phenotype (HSC/MFs), are indeed emerging as cells that may have an active role in modulating angiogenesis that differs from the one attributed to microcapillary pericytes[14]. The overall scenario is even more complex if one considers that in CLDs hepatic myofibroblast-like cells (MFs) constitute a heterogenous population of pro-fibrogenic cells. These highly proliferative and contractile cells may also originate from portal (myo) fibroblasts, bone marrow-derived stem cells and, as recently suggested, also from hepatocytes or cholangiocytes through a process of epithelial to mesenchymal transition[10-13]. In the following section of this editorial we will try to focus on those relevant features that link angiogenesis to liver fibrogenesis and then the progression of CLDs. The interested reader can refer to more comprehensive reviews - such as articles with more details on the basic principles and mechanisms involved in angiogenesis as well as the analysis of the role of angiogenesis in liver regeneration or hepatocellular carcinoma[10-14].
HEPATIC ANGIOGENESIS AND ITS RELATIONSHIPS BETWEEN CHRONIC INFLAMMATION AND FIBROGENIC PROGRESSION OF CLDs
CLDs are characterized by reiteration of liver injury due to a number of aetiological conditions, including chronic infection by viral agents [mainly hepatitis B virus (HBV) and hepatitis C virus (HCV)] as well as metabolic, toxic/drug-induced (with alcohol consumption being predominant) and autoimmune causes, resulting in persistent inflammation and progressive fibrogenesis. Chronic activation of the wound healing response is the major driving force for progressive accumulation of extracellular matrix (ECM) components, eventually leading to liver cirrhosis and hepatic failure. Oxidative stress and redox signalling, derangement of epithelial-mesenchymal interactions or, as recently proposed, the process of epithelial to mesenchymal transition represent additional mechanisms able to sustain fibrogenesis progression towards the final end-point of cirrhosis[11,12,15-17].
Along these lines, cirrhosis should be regarded as an advanced stage of fibrosis characterized by the formation of regenerative nodules of parenchyma, surrounded and separated by fibrotic septa, and associated with significant changes in angio-architecture. The suggestion that angiogenesis may significantly contribute to fibrogenesis and disease progression relies first on the fact that vascular remodelling, irrespective of aetiology, is a common finding in human cirrhotic livers[11,12,14,18,19]. Moreover, the formation of fibrotic septa, as well as capillarization of sinusoids, the latter due to early deposition of fibrillar ECM in the space of Disse, can result in an increased resistance to blood flow and oxygen delivery. These are the premises for hypoxia and the transcription of hypoxia-sensitive pro-angiogenic genes, usually modulated by the so-called hypoxia inducible factors (HIFs)[20-23]. In addition, it is well known that in CLDs the inflammatory response gains the role of a dynamic state relevant for the progression of fibrogenesis towards the end-point of cirrhosis[11-13,16]. Several mediators of the inflammatory response may stimulate other cells in the surrounding microenvironment to express vascular endothelial growth factor (VEGF) and other pro-angiogenic factors as well as to sustain angiogenesis[24]. Moreover, cytokines or mediators produced during CLDs such as hepatocyte growth factor (HGF), platelet-derived growth factor (PDGF) and nitric oxide (NO), can play a role in the development of angiogenesis[6,13,14]. In particular, one should consider that: (1) neo-vessels themselves can significantly contribute to perpetuation of the inflammatory response by expressing chemokines and adhesion molecules promoting the recruitment of inflammatory cells; (2) angiogenesis, early in the course of a CLD, may contribute to the transition from acute to chronic inflammation[25].
A relevant additional point, as recently proposed[20], is that depending on the specific pattern of fibrosis (post-necrotic or bridging fibrosis, pericellular or perisinusoidal fibrosis, biliary fibrosis or centrilobular fibrosis)[12,26], the extent of neo-angiogenesis, in addition to favouring disease progression, may also represent a key limiting factor for fibrosis reversibility. This is potentially relevant for post-necrotic or bridging fibrosis, a pattern which is mainly seen in patients with advanced fibrosis or cirrhosis by chronic HBV or HCV infection. In this pattern of fibrosis, which is characterized by the formation of bridging septa between portal and central vein areas, angiogenesis, vascular remodelling and altered angio-architecture are particularly impressive.
PATHOLOGICAL ANGIOGENESIS AND PRO-ANGIOGENIC CYTOKINES HAVE BEEN DETECTED IN HUMAN CLDs
Current evidence suggests that angiogenesis and fibrogenesis are detectable and develop in parallel in any clinical condition of CLDs that can progress towards the end-point of cirrhosis, irrespective of aetiology, as well as in the most widely used experimental animal models of CLDs[6,13,19,20].
Where clinical data are concerned, best relationships between angiogenesis and the pattern of fibrosis (i.e. bridging fibrosis)[12,26] are usually found during chronic viral infection by either HBV or HCV. This is documented by either the abundant presence of endothelial cells (ECs) and neovessels/capillary structures found in inflamed portal tracts[6] or by the over-expression of major pro-angiogenic molecules, including VEGF and Angiopoietin 1 (Ang-1) as well as their related receptors (VEGFR type II, Tie2) and HGF[27-31]. In these clinical settings, PDGF, which is released by periportal inflammatory cells as well as by sinusoidal and perisinusoidal cells, may also play a pro-angiogenic role[32]. In addition, selected viral proteins may have a multiple pro-angiogenic role like HBV-related X protein[33]. This protein has been involved in disruption of inter-endothelial junctions by operating through a src-kinase-dependent signalling pathway, as well as in the up-regulation of inducible nitric oxide synthase (iNOS) through involvement of nuclear factor-κB (NF-κB) transcription factor[34] or even by up-regulating membrane-type matrix metalloprotease (MT-MMP) expression, and then MMP-2 activation in hepatocytes[35].
Angiogenesis has also been detected in biopsies from patients affected by either primary biliary cirrhosis (PBC) or autoimmune hepatitis as formation of neo-vessels by ECs positive for CD-31 and vascular endothelial-cadherin[6]. These neo-vessels were located, particularly in PBC, mainly in portal areas in association with inflammatory infiltrate[6,36]. Once again, enhanced expression of angiogenic molecules such as VEGF, Ang-1, Ang-2, Tie-2 and endoglin has also been characterized in these PBC patients.
Similar data have been reported for the most widely used experimental animal models of CLDs that have been instrumental in unequivocally documenting that angiogenesis and fibrogenesis develop in parallel during progression towards cirrhosis[31,37-43].
Both human and experimental studies have also outlined that several peptide mediators other than VEGF, Ang-1 and HGF are likely to be involved in hepatic angiogenesis associated with the fibrogenic progression process in CLDs. Unequivocal data have been provided for the pro-angiogenic action of PDGF[32] as well as for leptin, an adipocytokine that has been suggested to exert a pro-fibrogenic effect in promoting the development from non-alcoholic fatty liver disease (NAFLD) to non-alcoholic steatohepatitis (NASH)[40,44]. Where leptin is concerned, it has been shown that leptin can up-regulate the expression of VEGF and Ang-1, as well as the pro-inflammatory chemokine monocyte chemoattractant protein 1 or MCP-1[44]. The role of angiogenesis in NASH development and fibrosis has been confirmed by a study performed on Zucker rats, animals that naturally develop leptin receptor mutations[40], receiving the steatogenous choline-deficient and amino acid defined (CDAA) diet.
THE EMERGING ROLE OF HYPOXIA IN FIBROGENIC CLDs
Another major message coming mainly from experimental models of CLDs is that VEGF overexpression is strictly associated with hypoxic areas and is mostly limited to hepatocytes as well as to HSC/MFs[31,37-43]. This concept fits well with the rational hypothesis that hypoxia is likely to represent the major stimulus for hepatic angiogenesis. The colocalization of hypoxic areas with VEGF overexpression and/or the association between VEGF expression and progression of fibrogenesis was first described in the model of bile duct ligation (BDL)[37] and then confirmed in the diethyl-nitrosamine (DEN) model of fibrosis[38], in the model of chronic treatment with CCl4[31,39,41] and in the choline-deficient and amino acid-defined diet rat model of NAFLD evolving into NASH and significant fibrosis[40]. Of relevance, a recent study, has outlined in a mechanistic way, the strict relationships between hypoxia, angiogenesis, inflammation and fibrogenesis by using liver conditional HIF-1α-deficient mice that were subjected to the BDL model of fibrosis[43]. In this study, it was clearly shown that the appearance of HIF-1α-positive hypoxic areas in the liver of BDL mice can take place as early as 3 d after surgery, before the development of detectable signs of fibrosis. In particular, within hypoxic areas HIF-1α was found to be activated in hepatocytes and Kupffer cells. HIF-1α -/- conditional mice subjected to BDL were characterized by a very significant decrease in collagen type I and α-SMA transcripts and protein levels, as well as of transcripts for PDGF-A, PDGF-B, plasminogen activator inhibitor-1 (PAI-1) and fibroblast growth factor 2 (FGF-2) as compared to wild type mice in which the typical scenario of biliary-type fibrosis and cirrhosis was associated with early and sustained up-regulation of HIF-1α[43]. The authors of this study proposed that hypoxic hepatocytes, following activation of HIF-1α and through the HIF-1α-dependent release of these growth factors and mediators, may significantly contribute to either initial repair and revascularization of injured parenchyma as well as to fibrosis progression by targeting profibrogenic MFs. In a more recent study from the same group, this hypothesis was further investigated by exposing to hypoxia cultured hepatocytes obtained from normal mice as well as from HIF-1α or HIF-1β deficient mice[23]. The overall scenario which emerged from the latter study indicated that hypoxic hepatocytes, through the involvement of HIF-1α and HIF-2α, can express and release PAI-1, VEGF and the vasoactive peptides adrenomedullin-1 (ADM-1) and ADM-2[23]. Hepatocytes exposed to hypoxia during CLD progression are then reasonably a relevant source of vasoactive mediators as well as of the master pro-angiogenic cytokine VEGF. This unavoidably underlines the relevance of the cross-talk between hypoxic hepatocytes and surrounding non-parenchymal cells like sinusoidal endothelial cells (SECs) and, as we will see later in this editorial, activated MFs. Indeed, VEGF has a well known major pro-angiogenic role by increasing vascular permeability as well as triggering endothelial cell proliferation and regulating neo-vessel lumen diameter[1-8,14,19].
Along these lines, it is worth mentioning that in the rat model of chronic administration of CCl4, hypoxic hepatocytes have also been shown to contribute to the expression of the master profibrogenic cytokine transforming growth factor-β1 (TGF-β1)[45]. This contribution has been reported to be relevant mainly in an advanced stage of fibrosis or cirrhosis, whereas TGF-β1 is mainly produced by MFs and activated macrophages during early fibrosis and middle stage fibrogenic progression[45].
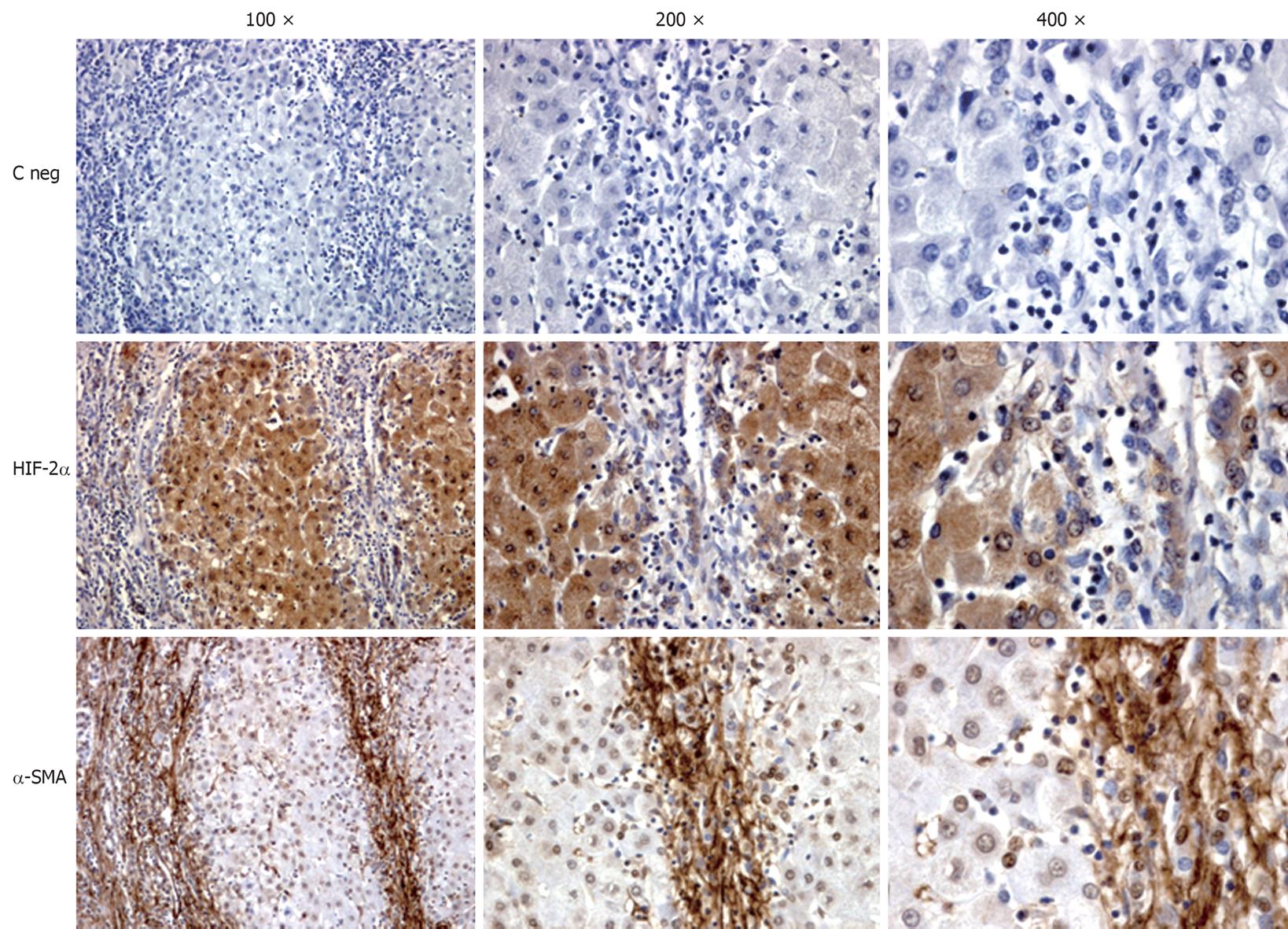
A strict association between hypoxia and liver fibrogenesis can also be easily appreciated in the liver of human HCV cirrhotic patients by detecting HIF-2α and α-SMA in serial sections (Figure 1). HIF-2α-positive staining in these human specimens is usually detectable in hepatocytes of pseudolobules as well as in some MFs at the interface between fibrotic septa and liver parenchyma or even within pseudolobules. Moreover, it is also evident that α-SMA-positive MFs are in close contact with HIF-2α positive hepatocytes. Along these lines, it has been suggested that hypoxia may result in VEGF- and Angiopoietin 1-dependent increased migration and chemotaxis of HSC/MFs, contributing to their recruitment towards the site of injury and alignment with both nascent and established fibrotic septa, a relevant pro-fibrogenic feature[31].
Figure 1 Immunohistochemical analysis performed on paraffin liver sections from patients with hepatitis C virus (HCV)-related liver cirrhosis (METAVIR F4).
Sections (2 μm thick) were incubated with specific antibodies raised against HIF-2α or α-SMA that positively stain cells exposed to hypoxia (HIF-2α) or myofibroblast-like cells (α-SMA). Primary antibodies were labelled by using EnVision, HRP-labelled System (DAKO) antibodies and visualized by 3’-diaminobenzidine substrate. Negative controls (C neg) were obtained by replacing the respective primary antibodies by isotype and concentrations matched irrelevant antibody. Original magnification is indicated.
Hypoxia has also been suggested to result in the down-regulation of HGF expression by HSCs and in the inhibition of c-met expression by hepatocytes[46], both events being able to significantly contribute to depressed liver regeneration during chronic liver injury.
THE ROLE OF PROFIBROGENIC CELLS IN LIVER ANGIOGENESIS
Hepatic stellate cells, due to their strategic location in the space of Disse and intimate contact with sinusoidal ECs, have been described to behave as liver specific pericytes and then to play a role in physiological angiogenesis[10]. Recent literature data suggest that HSC/MFs in CLDs are likely to represent a hypoxia-sensitive and cyto- and chemokine-modulated cellular crossroad between necro-inflammation, pathological angiogenesis and fibrogenesis. The latter statement is strongly suggested, in addition to exhaustive literature that has characterized the pro-fibrogenic and pro-inflammatory role of these cells, by a series of studies that outlined the following major concepts: (1) HSC and HSC/MFs can behave as pro-angiogenic cells able to react to conditions of hypoxia by up-regulating transcription and synthesis of VEGF, Ang-1 and their related receptors VEGFR-2 and Tie2[31,44,47,48]; the same behaviour has also been described for human HSC/MFs in which leptin was able to trigger a ERK1/2 and PI3-K-dependent nuclear translocation of HIF-1α[44]; (2) HSC/MFs also represent a cellular “target” for the action of VEGF and Angiopoietin 1; VEGF has been reported to be able to trigger HSC/MFs proliferation[39,49,50], increase deposition of ECM components[38,39,50], as well as increase migration and chemotaxis[31].
This is a scenario that is likely to be relevant in the progression of a CLD, as shown recently by in vivo morphological data obtained in human and rat fibrotic/cirrhotic livers[31]. α-SMA-positive MFs, able to express concomitantly VEGF, Ang-1 or the related receptors VEGFR-2 and Tie-2, are found at the leading edge of tiny and incomplete developing septa, but not in larger bridging septa. This distribution may reflect the existence of two different phases of the angiogenic process during CLDs: an early phase, occurring in developing septa, in which fibrogenesis and angiogenesis may be driven/modulated by HSC/MFs, and a later phase occurring in larger and more mature fibrotic septa where the chronic wound healing is less active and fibrogenic transformation more established. In such a late setting, pro-angiogenic factors are expressed only by endothelial cells, a scenario that is likely to favour stabilization of the newly formed vessels.
A very recent and elegant experimental study[51] has outlined another putative pro-angiogenic mechanism, that may have a role in vascular remodelling in cirrhosis. This mechanism is related to the action of so-called microparticles Hedgehog (Hh) ligands, which are known to be released during embryogenesis and to activate Hh signalling in endothelial cells. In this study, the authors showed that cholangiocytes and HSC/MFs can produce and then release, mainly in response to PDGF, Hh ligands in microparticles and that this event is relevant under conditions leading to experimental biliary cirrhosis (BDL model). The authors propose the following scenario: (1) in normal conditions the action of the low amount of Hh ligand released by rare immature ductular-type progenitors is counteracted by expression of Hh interacting protein (HIP) expressed by either quiescent HSC or fenestrated SEC; (2) under conditions of chronic injury, HIP expression is repressed and activation of ductular-type progenitor cells may result in PDGF-BB up-regulation and release; this, in turn, is likely to lead HSC/MFs and ductular cells to produce Hh ligands. Hh ligands, apart from promoting proliferation and survival of both cholangiocytes and HSC/MFs, may also promote changes in SEC gene expression resulting in capillarization of sinusoids and the release of vasoactive factors such as nitric oxide, then contributing to vascular remodelling in cirrhosis[51].
PATHOLOGICAL ANGIOGENESIS AS A POTENTIAL THERAPEUTIC TARGET IN CLDs
The analysis of the data and concepts presented in the previous sections, concerning the proposed relationships between angiogenesis, chronic wound healing and fibrogenesis and then disease progression, unavoidably leads to the following theoretical clinical goals: (1) detection of selected pro-angiogenic molecules (i.e. in serum or plasma) may serve as a non-invasive way to monitor both disease progression as well as the response to therapy; (2) anti-angiogenic therapy may be an effective tool for blocking or slowing down fibrogenic progression of CLDs.
We are indeed far from the first goal, and at present just a single study performed on 36 chronic HCV patients (vs 15 healthy controls) has tried to correlate circulating levels of molecules related to angiogenesis, disease progression and efficacy of standard pegylated interferon α-2b (IFN-α2b) plus ribavirin therapy. VEGF, Ang-2 and soluble Tie-2 (sTie-2) were determined in the serum before and after therapy and authors reported increased levels of VEGF and Ang-2 that were significantly decreased after therapy in these patients[52].
Where the efficacy of the angiogenic therapy is concerned, experimental data unequivocally indicate that anti-angiogenic therapy is indeed effective in preventing progressive fibrogenesis. Pioneering studies employed anti-angiogenic molecules or drugs like the semi-synthetic analogue of fumagillin TNP-470[53] or antibodies able to neutralize either VEGFR-1 (Flt-1) and/or VEGFR-2 (Flk-1)[39], both conditions being able to significantly inhibit angiogenesis, the number of α-SMA-positive cells and the development of fibrosis. The latter study also showed the in vivo predominance of VEGF interaction with VEGFR-2 to mediate angiogenesis during chronic liver injury. Neutralizing antibodies against VEGFR-2 were employed in other relevant studies, performed on a model of portal hypertensive rats, where VEGF expression and related angiogenesis were correlated to the development of porto-systemic collateral vessels and hyperdynamic splanchnic circulation[54,55]. These data suggest that the increase in portal blood flow, which is an important contributor to portal hypertension, depends not only on vasodilation, but also on the enlargement of the splanchnic vascular tree caused by angiogenesis.
More recently, positive results have been obtained in the chronic CCl4 rat model of CLD by employing Sunitinib, a tyrosine kinase receptor inhibitor able to target VEGF and PDGF receptors[41]. The treatment of cirrhotic animals with Sunitinib resulted in a significant decrease in hepatic vascular density, inflammatory infiltrate, abundance of α-SMA-positive mesenchymal cells, ECM deposition and even portal pressure.
Positive results have been also reported in another recent study in which mice undergoing BDL or chronic CCl4 treatment received an adenovirus expressing soluble Tie-2 (AdsTie-2), the receptor for Ang-1, resulting in the blocking of Ang-1 signalling and in a significant prevention of both angiogenesis and fibrosis[42].
A final and very recent experimental study reported a beneficial effect of Sorafenib[56], a receptor tyrosine kinase inhibitor already approved for the treatment of hepatocellular carcinoma[57,58], one of the most common complications of liver cirrhosis. In this study, oral administration of Sorafenib in rats with portal hypertension and cirrhosis (once a day for 2 wk) resulted in the inhibition of VEGF, PDGF and Raf kinase signalling; this, in turn, resulted in an approximately 80% decrease in splanchnic neovascularization and a very significant attenuation of hyperdynamic splanchnic and systemic circulations, as well as a significant decrease in the extent of portosystemic collaterals. Of relevance, Sorafenib treatment in cirrhotic rats also led to a 25% reduction in portal pressure and to a relevant improvement in liver injury, inflammation, fibrosis and angiogenesis.
The latter study has raised, in a more compelling way, the obvious question of whether anti-angiogenic therapy has an adequate rationale to be seriously considered for therapy in patients with cirrhosis and portal hypertension. As nicely pointed out by Shah and Bruix[59], any future clinical trial employing Sorafenib in cirrhotic patients should assess a number of critical issues, the first being the optimal dosage to be used which may be theoretically lower than the dosage used in patients with hepatocellular carcinoma (HCC). The adverse effects of Sorafenib may represent a relevant concern: it is already known that more than 40% of HCC patients are forced to interrupt treatment[57,58], and it has been shown that a major complication of angiogenic treatments employing Bevacizumab and Sunitinib in HCC patients is variceal bleeding[60,61]. Although available data indicate that Sorafenib may be relatively safe in this complication, caution is in any case necessary because it has been authoritatively suggested that an intense anti-angiogenic effect may lead to significant damage of the vasa vasorum needed to maintain the structure of varices[59].
Another note of caution has recently been provided by an experimental study performed in order to assess the anti-fibrotic potential of the inhibition of the vitronectin receptor integrin αvβ3, which has been shown to both promote angiogenesis by mediating migration and proliferation of SECs as well as to drive fibrogenic activation of HSCs[62]. These authors employed the specific inhibitor of integrin αvβ3, Cilengitide which was administered orally in two different animal models of liver fibrosis, BDL and chronic administration of thioacetamide (TAA). The relevant point was that this treatment was very effective in decreasing the overall formation of neo-vessels in both portal areas of BDL and septal areas of TAA fibrotic rats. Unfortunately, despite the anti-fibrogenic in vitro effect exerted by Cilengitide on cultured HSC/MFs, in vivo treatment with this inhibitor was associated in both models with a significant increase in liver collagen deposition and up-regulation of other profibrogenic genes and of matrix metalloproteinase-13, that is an overall worsening of liver fibrosis, with no relevant effect on inflammatory response[62].
The use of anti-angiogenic drugs and, in particular, those drugs which have already been approved for the treatment of HCC, may then represent an attractive alternative therapeutic tool to prevent or significantly slow down fibrosis progression towards cirrhosis, which also represents the main risk factor for liver cancer development, as well as the development of portal hypertension and its complications. However, a tempered final message is that angiogenesis inhibitors should be used with caution and carefully balanced in these patients, bearing in mind that angiogenesis unavoidably is a relevant event for wound healing and excessive blocking of angiogenesis may not represent the desired therapeutic objective. Clinical trials with an appropriate design and primary end-points are needed.
CONCLUSION
Hepatic angiogenesis has been unequivocally described in CLDs, irrespective of aetiology, and in the most reliable experimental models of liver fibrosis and cirrhosis. Angiogenesis and related changes in angio-architecture have been proposed to favour fibrogenic progression of the disease towards the end-point of cirrhosis. Moreover, in CLDs these changes are believed to be involved in the increase of vascular resistance and portal hypertension as well as in the decrease of parenchymal perfusion. At the same time, hepatic angiogenesis has been proposed to modulate the genesis of portal-systemic shunts and increase splanchnic blood flow, potentially affecting complications of cirrhosis. Hypoxia and HIFs-related cellular responses are emerging as crucial in the overall scenario of CLD progression. Several cellular and molecular mechanisms have been identified which regulate the cross-talk between angiogenesis and fibrogenesis as well as between the different hepatic cell populations. In this scenario a major role is played by hypoxic hepatocytes, sinusoidal endothelial cells as well as hepatic MFs. Where MFs are concerned, whatever their origin, they are currently believed to represent a crucial cellular cross-road at the intersection between inflammation, angiogenesis and fibrogenesis. Indeed, these profibrogenic and pro-inflammatory cells also represent a cellular target for the action of pro-angiogenic cytokines as well as an effective source of VEGF and Ang-1.
Finally, experimental anti-angiogenic therapy has proven to be very effective in limiting the fibrogenic progression of animal models of CLDs. The use of anti-angiogenic drugs, particularly of those that have already been approved for HCC therapy like Sorafenib, may then represent a rationale therapeutic option to limit the progression of human CLDs towards cirrhosis and its complications, including the development of HCC.
Peer reviewer: Devanshi Seth, PhD, Senior Scientist, Centenary Institute & Drug Health Services, RPAH & Clinical Senior Lecturer, Clinical School of Medicine, University of Sydney, Camperdown, NSW 2050, Australia
S- Editor Tian L L- Editor Webster JR E- Editor Ma WH