Published online Aug 7, 2010. doi: 10.3748/wjg.v16.i29.3674
Revised: April 1, 2010
Accepted: April 8, 2010
Published online: August 7, 2010
AIM: To evaluate tracking of magnetically labeled mesenchymal stem cells (MSCs) after intraportal transplantation.
METHODS: Mononuclear cells were isolated from bone marrow aspirates of pigs by density gradient centrifugation, cultured and expanded, after which, they were incubated with super paramagnetic iron oxide (SPIO). Prussian blue staining was performed to highlight intracellular iron. To establish swine models of acute liver injury, 0.5 g/kg D-galactosamine was administrated to 10 pigs, six of which were injected via their portal veins with SPIO-labeled MSCs, while the remaining four were injected with unlabeled cells. Magnetic resonance imaging (MRI) was performed with a clinical 1.5T MR scanner immediately before transplantation and 6 h, 3 d, 7 d and 14 d after transplantation. Prussian blue staining was again performed with the tissue slices at the endpoint.
RESULTS: Prussian blue staining of SPIO-labeled MSCs had a labeling efficiency of almost 100%. Signal intensity loss in the liver by SPIO labeling on the FFE (T2*WI) sequence persisted until 14 d after transplantation. Histological analysis by Prussian blue staining confirmed homing of labeled MSCs in the liver after 14 d; primarily distributed in hepatic sinusoids and liver parenchyma.
CONCLUSION: MSCs were successfully labeled with SPIO in vitro. MRI can monitor magnetically labeled MSCs transplanted into the liver.
- Citation: Shi XL, Gu JY, Han B, Xu HY, Fang L, Ding YT. Magnetically labeled mesenchymal stem cells after autologous transplantation into acutely injured liver. World J Gastroenterol 2010; 16(29): 3674-3679
- URL: https://www.wjgnet.com/1007-9327/full/v16/i29/3674.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i29.3674
In recent years, cell transplantation has had the advantages of lower cost, lower risk, and simpler manipulation of the procedure compared with orthotopic liver transplantation. A large body of evidence has suggested that mesenchymal stem cells (MSCs) could differentiate into liver-like cells with partial hepatic functions under appropriate environmental conditions in vivo and in vitro[1,2]. Given that autologous cell transplantation helps prevent immunological rejection, which is always a problem for orthotopic liver transplantation, MSCs can be regarded as seeding cells for transplantation in relation to liver diseases.
The major issue in liver cell transplantation is monitoring migration, distribution, and differentiation of the transplanted cells. Conventional tissue slicing is unable to distinguish between transplanted donor cells and the recipient cells, therefore, the tissue or organ has to be removed at certain time points and processed with special biochemical procedures to visualize the tagged cells. However, these tagging methods require in vitro preparation and examination of histological materials, which are unsuitable for noninvasive and repeated monitoring of in vivo transplanted cells under clinical conditions. Therefore, more recent research has focused on in vivo real-time tracking and detecting the fate of transplanted cells by using appropriate imaging technologies[3].
The present study had two purposes. First, we incubated swine autologous MSCs with super paramagnetic iron oxide (SPIO) in vitro, followed by stem cell transplantation performed via the portal vein in acutely injured liver models. Second, we investigated the characteristics of magnetically labeled swine MSCs by magnetic resonance imaging (MRI), as well as intrahepatic dynamic distribution.
Ten outbred white swine of either sex weighing 15-20 kg each were maintained under conventional conditions in the Laboratory Animal Center of the Affiliated Drum Tower Hospital of Nanjing University Medical School. All animal procedures were approved by the Animal Care Ethics Committee of Nanjing Drum Tower Hospital.
Porcine MSCs were isolated by bone marrow aspirates from the iliac crests of the animals as previously described, with slight modification[4]. Mononuclear cells were collected by gradient centrifugation over a Ficoll histopaque layer (20 min, 400 g, density 1.077 g/mL) (TBD, China) and seeded at a density of 1 × 106 cells/cm2 in growth medium that contained low-glucose Dulbecco’s modified Eagle’s medium (DMEM-LG; GIBCO, USA) supplemented with 10% fetal bovine serum (FBS; GIBCO, USA), penicillin (100 IU/mL) and streptomycin (100 μg/mL). The non-adherent cells were removed after the first 24 h and changed every 3-4 d thereafter. When the cells reached 80% confluence, they were detached using 0.25% Trypsin-EDTA (GIBCO, USA) and re-plated at a density of 1 × 104 cells/cm2 for expansion. Surface marker identification of the cultured MSCs was performed with a FACScan (Becton Dickinson, Franklin Lakes, NJ, USA) by fluorescein isothiocyanate (FITC)-labeled monoclonal antibody staining to CD45 (Antigenix America, Huntington Station, NY, USA) and phycoerythrin (PE)-conjugated antibodies against CD29 (VMRD, Pullman, WA, USA), CD44 and CD90 (Becton Dickinson). Isotypic antibodies served as the control.
MSCs were labeled with Feridex (Advanced Magnetics, Cambridge, MA, USA), as previously described[5]. Briefly, the polyamine poly-l-lysine (PLL) hydrobromide (Sigma, St Louis, MO, USA) was used as the transfection agent. A stock solution of PLL (1.5 mg/mL) was added to DMEM at a dilution of 1:1000 and mixed with Feridex (50 μg/mL) for 60 min at room temperature on a rotating shaker. MSCs of passage 5 were added to the culture medium that contained the Feridex-PLL complex, so that the final concentrations of Feridex and PLL were 25 μg/mL and 0.75 μg/mL, respectively. The cells were placed into six-well plates (Corning, NY, USA) overnight at 37°C in a 95% air/5% CO2 atmosphere.
After being incubated overnight with the Feridex-PLL complex, the MSCs were washed three times to remove excessive contrast agent. For Prussian blue staining, which indicates the presence of iron, the coverslip samples were fixed with 4% paraformaldehyde for 30 min, washed, incubated for another 30 min with 2% potassium ferrocyanide in 6% hydrochloric acid, washed again, and counterstained with nuclear fast red.
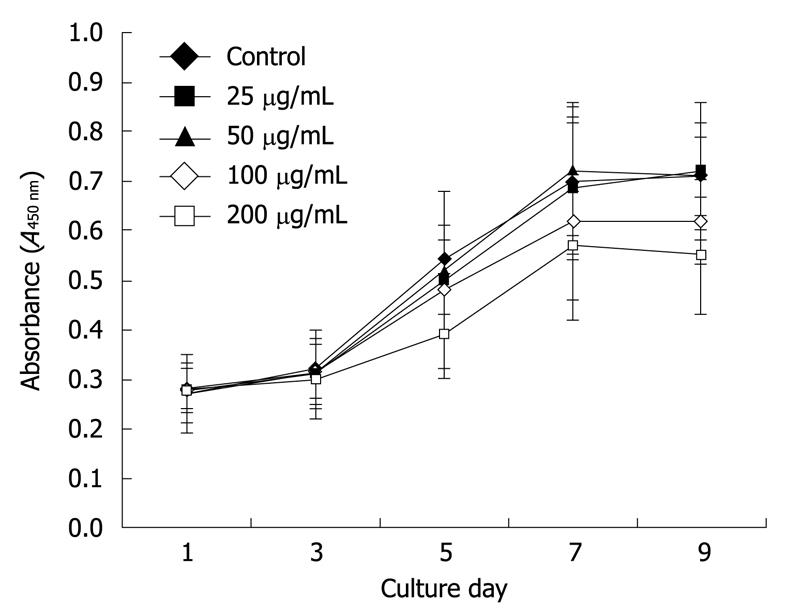
Firstly, MSCs were inoculated in 96-well plates at 1 × 104 cells per well at 37°C in a 95% air/5% CO2 atmosphere. Twenty-four hours later, final concentrations of Feridex in the Feridex-PLL complex (25, 50, 100 and 200 μg/mL) were added to each well with 11 other duplicates and incubated overnight. The remaining cells, which were not labeled with the complex and served as control cells, were kept under identical conditions. The magnetically labeled and non-labeled cells were then maintained in fresh culture medium for 2 d and washed twice. Ten microliters of cholecystokinin octapeptide (CCK-8; Dojindo Laboratories, Kumamoto, Japan) was added per well for 4 h. The absorbance was then measured at a wavelength of 450 nm.
Under general anesthesia with mechanical ventilation via an endotracheal tube, animals were administered a dose of 0.5 g/kg of D-galactosamine (D-Gal; Sigma) dissolved in 5% glucose solution, via the external jugular vein. Venous blood samples were drawn 6, 12 and 24 h after the operation for biochemical analysis.
Animals were randomly assigned to either control (n = 4) or experimental (liver injured) groups (n = 6). The abdomens of the liver-injured animals were opened to expose the portal vein, and approximately 1 × 107 labeled MSCs suspended in 2 mL DMEM were slowly injected into the portal vein. A 30-gauge needle was used for the procedure. The pinhole at the injection site was pressed for hemostasis. Thereafter, the laparotomy incision was enclosed in layers. The control group underwent the identical procedure except that the injected cells were unlabeled.
Animals underwent MRI of the liver immediately before, and 3, 7 and 14 d after injection of cells. MRI was performed with a 1.5-T imaging device (Philips Medical Systems, Eindhoven, the Netherlands). The pig was anesthetized and placed supine on a plastic flat plate. The scanning sequence was as follows: (1) SE: T1WI, TR 120 s, TE 14 ms; (2) FSE: T2WI, TR 3000 ms, TE 96 ms; and (3) FFE: T2*WI, TR 485 ms, TE 14.0 ms, flip angle, 18°.
Two weeks after cell transplantation, animals were sacrificed for histological examination. Liver tissues taken from both the control and experimental groups were fixed with 4% paraformaldehyde, embedded in paraffin, cut into 5-mm sections and stained with hematoxylin and eosin (HE) as well as Prussian blue for examination under a light microscope.
Data were shown as mean ± SE. The two-tailed unpaired Student’s t test was used to evaluate the statistical significance of differences which was set with a P value less than 0.05.
Twenty-four hours after first seeding, MSCs could be seen in newly formed colonies. Observed under the microscope, the MSCs rapidly grew fibroblast-like cells with a single nucleus. After the first passage, they looked like spindles or asters with a slim body. At passage 5, however, most of the miscellaneous cells were eliminated, and the remaining uniform fibroblast-like cells were MSCs. The expression of different cell surface markers, including CD29, CD44, CD45 and CD90, of MSCs from passage 5 was determined by flow cytometry; the results of which showed that > 90% of MSCs of passage 5 were positive for CD29, CD44 and CD90, but negative for CD45 (data not shown).
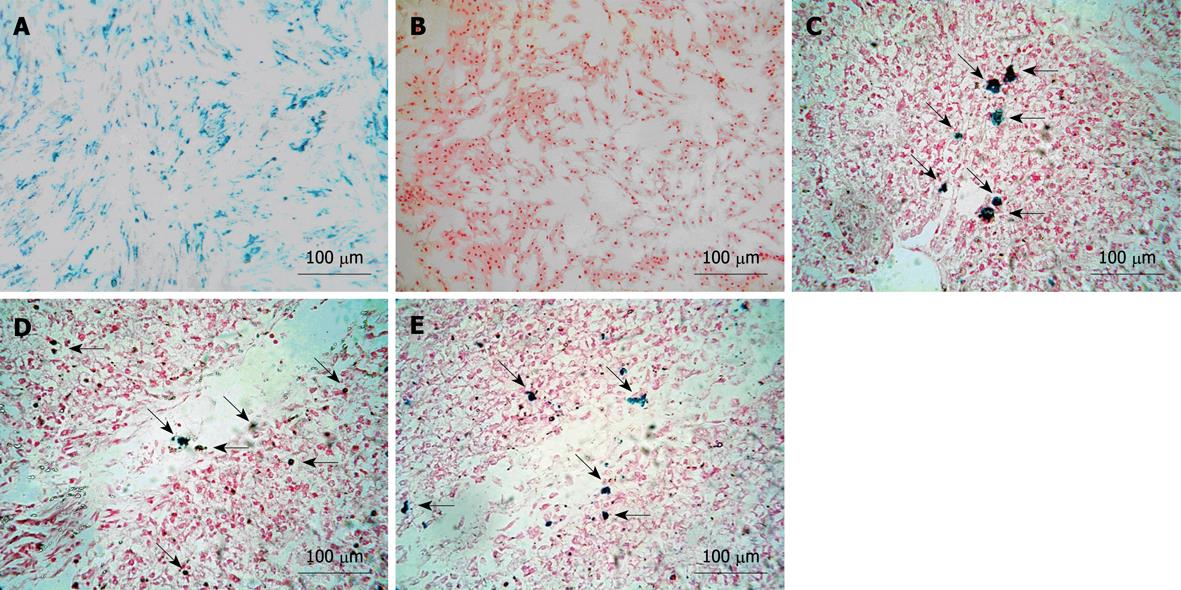
MSCs stained with Prussian blue showed results (blue particles) in all labeled cells. The labeling rate was approximately 100%, which was calculated under a light microscope via counting the numbers of positive cells in five random fields (Figure 1A). In contrast, no blue particles were observed in the unlabeled group (Figure 1B).
The growth curve of CCK-8 with Feridex-PLL labeled MSCs showed that the cellular proliferation of the 25 and 50 μg/mL subgroups were not significantly influenced by different concentration (P > 0.05). Figure 2 shows that MSCs labeled with higher concentrations of complex were somewhat inhibited in proliferation, which indicated that < 50 μg/mL Feridex-PLL would be suitable for MSC labeling in future transplantation.
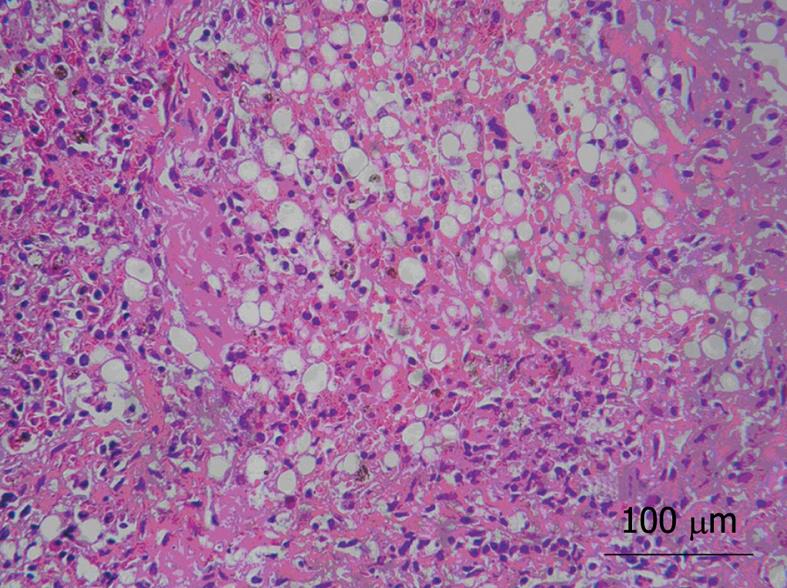
Acute liver injury was effectively induced in all animals. Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) and bilirubin levels were all significantly and progressively elevated 6 h after D-Gal induction (Table 1, P < 0.05). The porcine model clinically presented listlessness, loss of appetite and xanthochromia. Liver tissue samples from the injured model group after 24 h injection of D-Gal demonstrated severe hepatic necrosis in 60%-70% of the lobule, sinusoidal congestion, vacuolization, trabecular fragmentation, and granulocytic infiltration (Figure 3). Twenty-four hours after administration of D-Gal, the animals were ready for transplantation (Table 1).
| Pre-injection | 6 h after injection | 24 h after injection | |
| ALT (U/L) | 31.2 ± 2.6 | 87.5 ± 13.2 | 181.9 ± 12.8 |
| AST (U/L) | 28.9 ± 3.8 | 134.0 ± 7.8 | 564.8 ± 89.7 |
| TBIL (μmol/L) | 3.2 ± 0.45 | 26.7 ± 3.2 | 43.0 ± 2.9 |
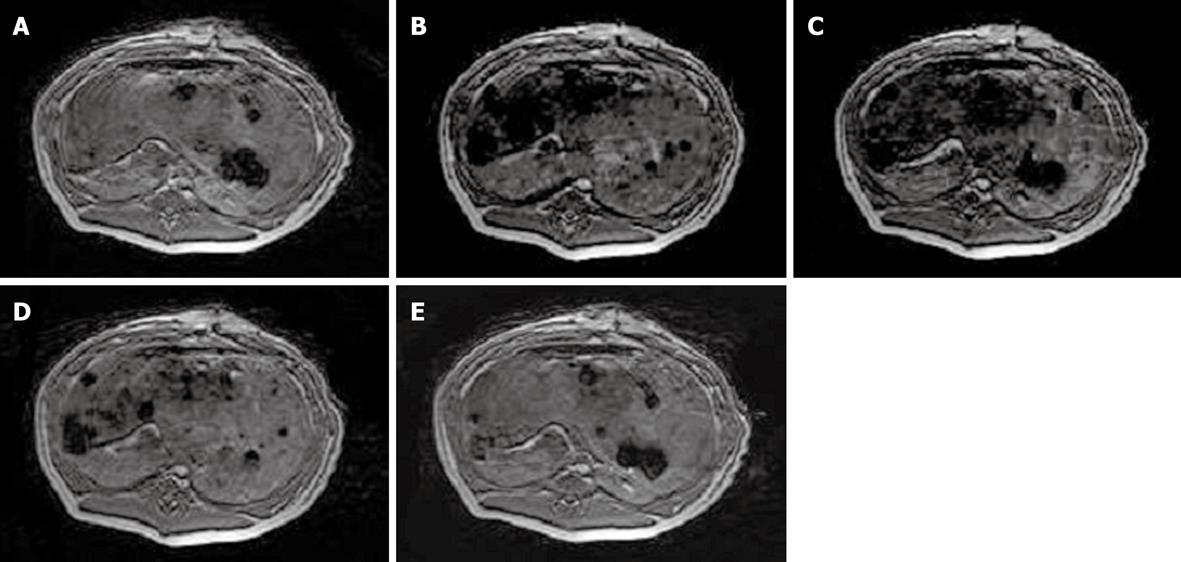
Signal intensity decreased 6 h after intrahepatic transplantation of labeled MSCs on the FFE sequence, but gradually approached close to normal on day 14 (Figure 4). For the control group, there was no visible difference at each time point after transplantation compared with before.
The Prussian blue staining demonstrated several blue-positive cells scattered in and around the sinusoids in the experimental group on day 3 and 7 and the endpoint of experiment (Figure 1C-E), which indicated the presence of the magnetically labeled MSCs transplanted into the liver.
Bone marrow-derived MSCs are multipotent adult stem cells of mesodermal origin with the potential for self renewal. Because MSCs have the ability to differentiate into cells of multiple organs/systems such as hepatocytes, osteoblasts, chondrocytes, adipocytes and myocytes under appropriate stimuli[1,2,6-9], they have generated considerable interest for their potential use in regenerative medicine and tissue engineering. Given the ease of their isolation, extensive expansion rate and differentiation potential, as well as their immunosuppressive properties, MSCs may be suitable candidates for seed cells for hepatocyte transplantation, in addition to being potential carrier cells for gene therapy[10], which holds a promising future in the treatment of acute or chronic liver failure and metabolic liver diseases. Despite their important potential, however, the detailed processes involved in MSC implantation, migration, and differentiation in the liver remain to be elucidated.
Previous tissue slicing experiments have been unable to monitor the dynamic changes of transplanted cells in the living body. Accordingly, a noninvasive and living labeling technique is needed with respect to MSC intrahepatic transplantation. With the advent of molecular imaging technologies, in vivo real-time tracking and detecting the fate of transplanted stem cells may become a reality[3,11,12]. Cells for transplantation have been labeled with MR contrast agents since the beginning of the 1990s. In this regard, MRI appears most promising for dynamically monitoring in vivo cell migration after transplantation, due to its well known properties of relative long-term imaging, high spatial resolution, and sharp contrast[13]. Currently, SPIO MRI contrast agents have been most widely used for tracking transplanted cells in various organs because of their strong signal attenuation properties[14-18]. In particular, dextran-coated SPIO nanoparticles have been approved by the US Food and Drug Administration for use in hepatic reticuloendothelial cell imaging, and ultrasmall SPIOs are in phase III clinical trials for use as blood pool agents or for use with lymphography[19-21]. However, such contrast agents cannot be used to label efficiently stem cells in vitro in their native unmodified form[22]. By conjugating antigen-specific internalizing monoclonal antibodies to the surface dextran coating, cells can be magnetically labeled during their normal expansion in culture medium. In the present study, we used PLL as a transfection agent to magnetically label MSCs in vitro by the establishment of a Feridex-PLL complex through electrostatic interactions. This encourages cellular membrane endocytosis and transportation of Feridex into endosomes without requiring novel synthesis or covalent binding of proteins or antibodies to the dextran coating. Our results indicated that the labeling efficiency in our study approximated to 100% as expected. Subsequent analysis of the inhibitory effects of different concentrations of Feridex-PLL complex on MSCs revealed < 50 μg/mL Feridex was a relatively safe and effective dose for MSC labeling, which was suitable for transplantation study.
So far, most studies concerning MRI of grafted stem cells have been applied to animal brains, spines and hearts[17,23,24]. MRI techniques offer the possibility of tracking labeled cells in vivo noninvasively and repeatedly during extended study periods. The potential of MRI for future clinical interventions within the realm of regenerative cell therapy has been elegantly demonstrated in previous studies, and MRI fluoroscopy has been used to guide the delivery of MR-labeled adult stem cells into damaged organs. In our study, migration and retention of porcine MSCs after intraportal transplantation were demonstrated by using in vivo MRI. Significant signal intensity loss was observed in labeled MSCs on FFE sequences 6 h after transplantation. Thereafter, it gradually approached normal levels on day 14. The loss of signal could be attributed to either biodegradation of the contrast agent, the process of cellular division, or cellular migration to neighboring organs. To confirm the long-term results, Prussian blue staining was performed to demonstrate positive cells in liver tissue slices at the endpoint of the experiment. Furthermore, the dispersed distribution of labeled MSCs confirmed that the acute injured liver model may offer an ideal microenvironment for cell recruitment and implantation.
In summary, the present study incubated porcine MSCs with Feridex-PLL complex in vitro, and in vivo real-time tracking and detecting of magnetically labeled MSCs were manipulated by MRI in models of acute liver injury. Future research will focus on the optimized numbers of transplanted MSCs, distribution beyond injured liver, as well as safety and therapeutic effects concerning liver regeneration after MSC intraportal transplantation. In addition, our newly established co-culture system for hepatocytes and MSCs for cell transplantation and bioartificial liver devices should also be monitored[25].
In recent years, cell transplantation has had the advantages of lower cost, lower risk, and simpler manipulation of the procedure compared with orthotopic liver transplantation. Autologous cell transplantation helps prevent immunological rejection, which is always a problem for orthotopic liver transplantation. Moreover, a large body of evidence has suggested that mesenchymal stem cells (MSCs) differentiate into liver-like cells with partial hepatic functions under appropriate environmental conditions in vivo and in vitro. Therefore, MSCs could be regarded as seeding cells for transplantation in relation to liver diseases.
One of the major issues in liver cell transplantation is monitoring migration, distribution, and differentiation of the transplanted cells. Present tagging methods require in vitro preparation and examination of histological materials, which are unsuitable for noninvasive and repeated monitoring of in vivo transplanted cells under clinical conditions. Therefore, more recent research has focused on in vivo real-time tracking and detecting the fate of transplanted cells by using appropriate imaging technologies.
The authors sought to label MSCs in vitro with super paramagnetic iron oxide (SPIO) and to monitor the labeled cells with magnetic resonance imaging (MRI). Through this research, they found a new invasive and repeatable monitoring method.
This method of labeling and monitoring cells could be applied to research in cell transplantation. In future, it could be considered as an invasive and repeatable monitoring method for clinical cell transplantation.
SPIO is an MRI contrast agent that shows a high signal during imaging.
In this study, authors successfully showed the use of swine mesenchymal stem cells in an acute liver injury model. The study should be considered as a brief report as it is a nice piece of information.
Peer reviewer: Shashi Bala, PhD, Post doctoral Associate, Department of Medicine, LRB 270L, 364 Plantation street, UMass Medical School, Worcester, MA 01605, United States
S- Editor Tian L L- Editor Kerr C E- Editor Lin YP
| 1. | Shi XL, Mao L, Xu BY, Xie T, Zhu ZH, Chen JH, Li L, Ding YT. Optimization of an effective directed differentiation medium for differentiating mouse bone marrow mesenchymal stem cells into hepatocytes in vitro. Cell Biol Int. 2008;32:959-965. |
| 2. | Sgodda M, Aurich H, Kleist S, Aurich I, König S, Dollinger MM, Fleig WE, Christ B. Hepatocyte differentiation of mesenchymal stem cells from rat peritoneal adipose tissue in vitro and in vivo. Exp Cell Res. 2007;313:2875-2886. |
| 4. | Nakamura Y, Wang X, Xu C, Asakura A, Yoshiyama M, From AH, Zhang J. Xenotransplantation of long-term-cultured swine bone marrow-derived mesenchymal stem cells. Stem Cells. 2007;25:612-620. |
| 5. | Arbab AS, Bashaw LA, Miller BR, Jordan EK, Lewis BK, Kalish H, Frank JA. Characterization of biophysical and metabolic properties of cells labeled with superparamagnetic iron oxide nanoparticles and transfection agent for cellular MR imaging. Radiology. 2003;229:838-846. |
| 6. | Heino TJ, Hentunen TA. Differentiation of osteoblasts and osteocytes from mesenchymal stem cells. Curr Stem Cell Res Ther. 2008;3:131-145. |
| 7. | Yokoyama M, Miwa H, Maeda S, Wakitani S, Takagi M. Influence of fetal calf serum on differentiation of mesenchymal stem cells to chondrocytes during expansion. J Biosci Bioeng. 2008;106:46-50. |
| 8. | Scavo LM, Karas M, Murray M, Leroith D. Insulin-like growth factor-I stimulates both cell growth and lipogenesis during differentiation of human mesenchymal stem cells into adipocytes. J Clin Endocrinol Metab. 2004;89:3543-3553. |
| 9. | Li X, Yu X, Lin Q, Deng C, Shan Z, Yang M, Lin S. Bone marrow mesenchymal stem cells differentiate into functional cardiac phenotypes by cardiac microenvironment. J Mol Cell Cardiol. 2007;42:295-303. |
| 10. | Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol. 2004;32:414-425. |
| 11. | Swijnenburg RJ, van der Bogt KE, Sheikh AY, Cao F, Wu JC. Clinical hurdles for the transplantation of cardiomyocytes derived from human embryonic stem cells: role of molecular imaging. Curr Opin Biotechnol. 2007;18:38-45. |
| 12. | Shah K, Weissleder R. Molecular optical imaging: applications leading to the development of present day therapeutics. NeuroRx. 2005;2:215-225. |
| 13. | Delikatny EJ, Poptani H. MR techniques for in vivo molecular and cellular imaging. Radiol Clin North Am. 2005;43:205-220. |
| 14. | Engberink RD, Blezer EL, Hoff EI, van der Pol SM, van der Toorn A, Dijkhuizen RM, de Vries HE. MRI of monocyte infiltration in an animal model of neuroinflammation using SPIO-labeled monocytes or free USPIO. J Cereb Blood Flow Metab. 2008;28:841-851. |
| 15. | Lee SM, Lee SH, Kang HY, Baek SY, Kim SM, Shin MJ. Assessment of musculoskeletal infection in rats to determine usefulness of SPIO-enhanced MRI. AJR Am J Roentgenol. 2007;189:542-548. |
| 16. | Oude Engberink RD, van der Pol SM, Döpp EA, de Vries HE, Blezer EL. Comparison of SPIO and USPIO for in vitro labeling of human monocytes: MR detection and cell function. Radiology. 2007;243:467-474. |
| 17. | Fukuda Y, Ando K, Ishikura R, Kotoura N, Tsuda N, Kato N, Yoshiya S, Nakao N. Superparamagnetic iron oxide (SPIO) MRI contrast agent for bone marrow imaging: differentiating bone metastasis and osteomyelitis. Magn Reson Med Sci. 2006;5:191-196. |
| 18. | Kim SH, Lee JM, Han JK, Lee JY, Kang WJ, Jang JY, Shin KS, Cho KC, Choi BI. MDCT and superparamagnetic iron oxide (SPIO)-enhanced MR findings of intrapancreatic accessory spleen in seven patients. Eur Radiol. 2006;16:1887-1897. |
| 19. | Harisinghani MG, Saini S, Weissleder R, Hahn PF, Yantiss RK, Tempany C, Wood BJ, Mueller PR. MR lymphangiography using ultrasmall superparamagnetic iron oxide in patients with primary abdominal and pelvic malignancies: radiographic-pathologic correlation. AJR Am J Roentgenol. 1999;172:1347-1351. |
| 20. | Gandhi SN, Brown MA, Wong JG, Aguirre DA, Sirlin CB. MR contrast agents for liver imaging: what, when, how. Radiographics. 2006;26:1621-1636. |
| 21. | Mack MG, Balzer JO, Straub R, Eichler K, Vogl TJ. Superparamagnetic iron oxide-enhanced MR imaging of head and neck lymph nodes. Radiology. 2002;222:239-244. |
| 22. | Lewin M, Carlesso N, Tung CH, Tang XW, Cory D, Scadden DT, Weissleder R. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol. 2000;18:410-414. |
| 23. | Wu X, Hu J, Zhou L, Mao Y, Yang B, Gao L, Xie R, Xu F, Zhang D, Liu J. In vivo tracking of superparamagnetic iron oxide nanoparticle-labeled mesenchymal stem cell tropism to malignant gliomas using magnetic resonance imaging. Laboratory investigation. J Neurosurg. 2008;108:320-329. |
| 24. | He G, Zhang H, Wei H, Wang Y, Zhang X, Tang Y, Wei Y, Hu S. In vivo imaging of bone marrow mesenchymal stem cells transplanted into myocardium using magnetic resonance imaging: a novel method to trace the transplanted cells. Int J Cardiol. 2007;114:4-10. |
| 25. | Gu J, Shi X, Zhang Y, Chu X, Hang H, Ding Y. Establishment of a three-dimensional co-culture system by porcine hepatocytes and bone marrow mesenchymal stem cells in vitro. Hepatol Res. 2009;39:398-407. |