Published online Jul 28, 2010. doi: 10.3748/wjg.v16.i28.3529
Revised: May 4, 2010
Accepted: May 11, 2010
Published online: July 28, 2010
AIM: To investigate the immune response of peripheral blood mononuclear cells (PBMCs) and dendritic cells (DCs) that were stimulated by probiotic preparations.
METHODS: PBMCs were isolated, cultured, and stimulated with Bio-Three (a mixture of Bacillus mesentericus, Clostridium butyricum and Enterococcus faecalis; 105, 106 and 107 CFU/mL for 24 h). Cytokine production of (1) circulating PBMCs; (2) PBMCs stimulated by probiotic preparation; (3) monocyte-derived DCs; and (4) DC and T cell co-culture was determined by enzyme-linked immunosorbent assay. Phenotypic analysis of circulating PBMCs was also investigated by flow cytometry. Blood was obtained from individuals who consumed Bio-Three (109 CFU/d B. mesentericus, C. butyricum and E. faecalis) for 2 wk, or those who did not take probiotics orally.
RESULTS: In culture supernatants, interferon-γ (IFN-γ) and interleukin (IL)-10 production increased, but IL-4 and tumor necrosis factor-α (TNF-α) production by PBMCs decreased after 1 and 2 wk of probiotic treatment. Flow cytometry was also performed on day 14 and detected enhanced expression of CD11b, HLA-DR, CD4, CD45RA, CD25, CD44 and CD69 in response to Bio-Three. Furthermore, IL-10 and IL-12 were upregulated in supernatants of monocyte-derived DCs, and IFN-γ and IL-10 were enhanced in supernatants of CD4+ T cells co-cultured with DCs.
CONCLUSION: Bio-Three appeared to stimulate the Th1 immune response, downregulate pro-inflammatory cytokines (TNF-α) and upregulate anti-inflammatory cytokine (IL-10). Probiotics could be effective in activation of PBMCs and DCs.
- Citation: Hua MC, Lin TY, Lai MW, Kong MS, Chang HJ, Chen CC. Probiotic Bio-Three induces Th1 and anti-inflammatory effects in PBMC and dendritic cells. World J Gastroenterol 2010; 16(28): 3529-3540
- URL: https://www.wjgnet.com/1007-9327/full/v16/i28/3529.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i28.3529
The use of probiotics to promote human health has been proposed for many years. Mechanisms of probiotic actions include effects on luminal microbial ecology and immune modulation, particularly through balance control of pro-inflammatory and anti-inflammatory cytokines[1-4]. Currently, species of lactobacillus and bifidobacteria are most widely used to prevent and treat allergy and intestinal disorders; other strains such as Bacillus, Clostridium, Streptococcus, Escherichia coli and Saccharomyces have received increased attention.
To date, the most extensively studied and best documented probiotic application is for the treatment of acute infectious diarrhea, prevention of antibiotic-associated diarrhea, and allergic diseases[5-7]. Many other benefits are largely unproven, including therapeutic use in necrotizing enterocolitis, irritable bowel syndrome, constipation, inflammatory bowel diseases, pouchitis, and Helicobacter pylori infection[5-7].
It has been proposed that many effects of probiotics are mediated via immune modulation[3]. There are host-specific and strain-specific differences in the activities of probiotic bacteria. Some strains can enhance or eliminate the activity of other strains in vivo[1,2,8]. Previously, most studies that have reported the beneficial effect of probiotics have been on single strain preparations. Few have examined the effect of multiple strain preparations (e.g. VSL#3)[9,10]. Our study was undertaken to investigate whether Bio-Three (a mixture of Bacillus mesentericus, Clostridium butyricum and Enterococcus faecalis) affects immune regulation in human peripheral blood mononuclear cells (PBMCs).
The primary effectors of the human gut are antigen-presenting cells [APCs, including monocytes, macrophages, and dendritic cells (DC)], which provide nonspecific innate immune protection. APCs are responsible for detecting microbes through Toll-like receptors (TLRs) and presenting their antigenic structures to T cells. This triggering process of APCs initiates a signal transduction cascade that leads to the release of cytokines and initiation of the acquired immune response[2,11,12]. Among the APCs, DCs are the most potent. These differentiate from CD14+ monocytes in vitro in response to granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4[10,13,14]. DCs activated by microbes further stimulate the development of T helper type 1 (Th1) and T helper type 2 (Th2) cells or regulatory T cells[1,15].
Several cytokines are involved in immune modulation. Tumor necrosis factor-α (TNF-α) and IL-6 are pro-inflammatory cytokines that are involved in systemic inflammation and the acute phase reaction[12]. IL-12 together with interferon-γ (IFN-γ) causes a shift towards a Th1 response, which favors the development of cell-mediated and cytotoxic immunity[2]. Prostaglandin E2 (PGE2), together with IL-4, causes a shift towards a Th2 immune response, which favors the production of antibodies and the induction of IgE and allergic responses[2]. IL-10 (an anti-inflammatory cytokine) enhances the generation of regulatory T (Treg) cells[1,2]. Treg cells seem to suppress or regulate effector T cell function through production of cytokines such as IL-10 and transforming growth factor-β (TGF-β).
The purpose of this study was to determine whether a probiotic combination (Bio-Three) had immunomodulatory effects (altered the phenotype of circulating lymphocytes or monocytes) in human PBMCs. We observed the expression of specific cytokines and changes in PBMC phenotypes.
The study protocol was approved by the Institutional Review Board of Chang Gung Memorial Hospital, Taiwan, China.
Blood cells were separated from platelet-rich plasma and suspended in RPMI 1640 medium. Human PBMCs were isolated by centrifugation of buffy coats on Lymphoprep (Nycomed, Oslo, Norway) gradients. After washing, cells were resuspended at a concentration of 1 × 106 cells/mL in RPMI 1640 medium that contained 10% heat-inactivated fetal bovine serum.
All experiments were performed with cells obtained from 14 blood donor volunteers. Subjects were eligible if they were in good general health and were not currently taking medications, probiotics, and other supplements. These blood donors were divided randomly into two groups: group A (n = 7) consumed the probiotic preparation Bio-Three 5 × 108 CFU/dose twice daily (total 109 CFU/d) for 2 wk, and group B (n = 7), the negative control, did not consume probiotics. Peripheral blood samples were obtained from each subject by venipuncture on day 7 (week 1) and day 14 (week 2).
The probiotic preparation Bio-Three was a lyophilized mixture that consisted of three different bacteria (B. mesentericus, C. butyricum and E. faecalis), at a concentration of 3 × 108 live bacteria/packet. 3 × 108 live bacteria were reconstituted in 3 mL sterile PBS without additives, and serial dilutions (1:10) were made in sterile PBS for addition to cell cultures.
The concentration of PBMCs (obtained from groups A and B) was adjusted to 105 cells/mL in complete medium, and the cells were transferred to 24-well plates. Some wells were collected for cytokine detection [by enzyme-linked immunosorbent assay (ELISA)] after 12 h culture at 37°C. The remaining wells were then stimulated with Bio-Three for 24 h at 37°C in an atmosphere that contained 5% CO2. Initial dose-response experiments were performed by co-culturing 105 (1:1), 106 (1:10), and 107 CFU (1:100) of bacteria per mL (host cells: bacteria ratio), respectively. Culture supernatants were collected, and triplicates were pooled and kept at -20°C until analyzed by ELISA. The remaining cells in the culture plates were mixed with TRIzol Reagent (Gibco Life Technologies, Carlsbad, CA, USA) and stored at -20°C for gene expression analysis. Repeated thawing and freezing were avoided.
Concentrations of IFN-γ, IL-4, TNF-α, IL-10, and IL12 p70 in the supernatants of cell cultures were determined by ELISA. All antibodies and standards were purchased from Pharmingen (San Diego, CA, USA). Costar plates (Invitrogen, San Diego, CA, USA) were coated with the following capture monoclonal antibodies (mAbs): anti-IFN-γ (MQ2-13A5), anti-IL-4 (8D4-8), anti-IL-12 p70 (20C2), anti-TNF-α (MAb1), and anti-IL-10 (JES3-9D7). Standard curves were generated using recombinant human IFN-γ, IL-4, IL-12 p70, TNF-α, and IL-10, respectively. The following biotinylated antibodies were used for detection: anti-IFN-γ (MQ2-39C3), anti-IL-4 (MP4-25D2), anti-IL-12 p40/p70 (C8.6), anti-TNF-α (MAb11), and anti-IL-10 (JES3-12G8). Samples, standards, biotinylated antibodies, and streptavidin-horseradish peroxidase were diluted in high-performance ELISA dilution buffer (Sanquin, Amsterdam, Netherlands).
Cytokine mRNA expression in PBMCs was evaluated by real-time polymerase chain reaction (PCR). Total cellular RNA was isolated from frozen cultured PBMCs using TRIzol (Gibco Life Technologies) according to the manufacturer’s instructions. cDNA was synthesized and used as templates for PCR using specific primers for human IFN-γ (forward: 5'-GCATCGTTTTGGGTTCTCTTGGCTGTTACTGC; reverse: 5'-CTCCTTTTTCGCTTCCCTGTTTTAGCTGCTGG), IL-4 (forward: 5'-TCTCACCTCCCAACTGCTTCC; reverse: 5'-CGTTTCAGGAATCGGATCAGC), TNF-α (forward: 5'-AGCCAGTAGCTCATGTTGTAGCAA; reverse: 5'-GGCACTATCAGCTGGTTGTCTGT), IL-10 (forward: 5'-GCTGGAGGACTTTAAGGGTTACCT; reverse: 5'-CTTGATGTCTGGGTCTTGGTTCT), IL-12 (forward: 5'-TGGATGCTATTCACAAGCTCAAGT; reverse: 5'-TGGTTTGATGATGTCTCTGATGAAG), and β-actin (forward: 5'-GCATGGAGTCCTGTGGCAT; reverse: 5'-CTAGAAGCATTTGCGGTGG). All experimental samples were amplified in duplicate. The results were normalized to β-actin expression.
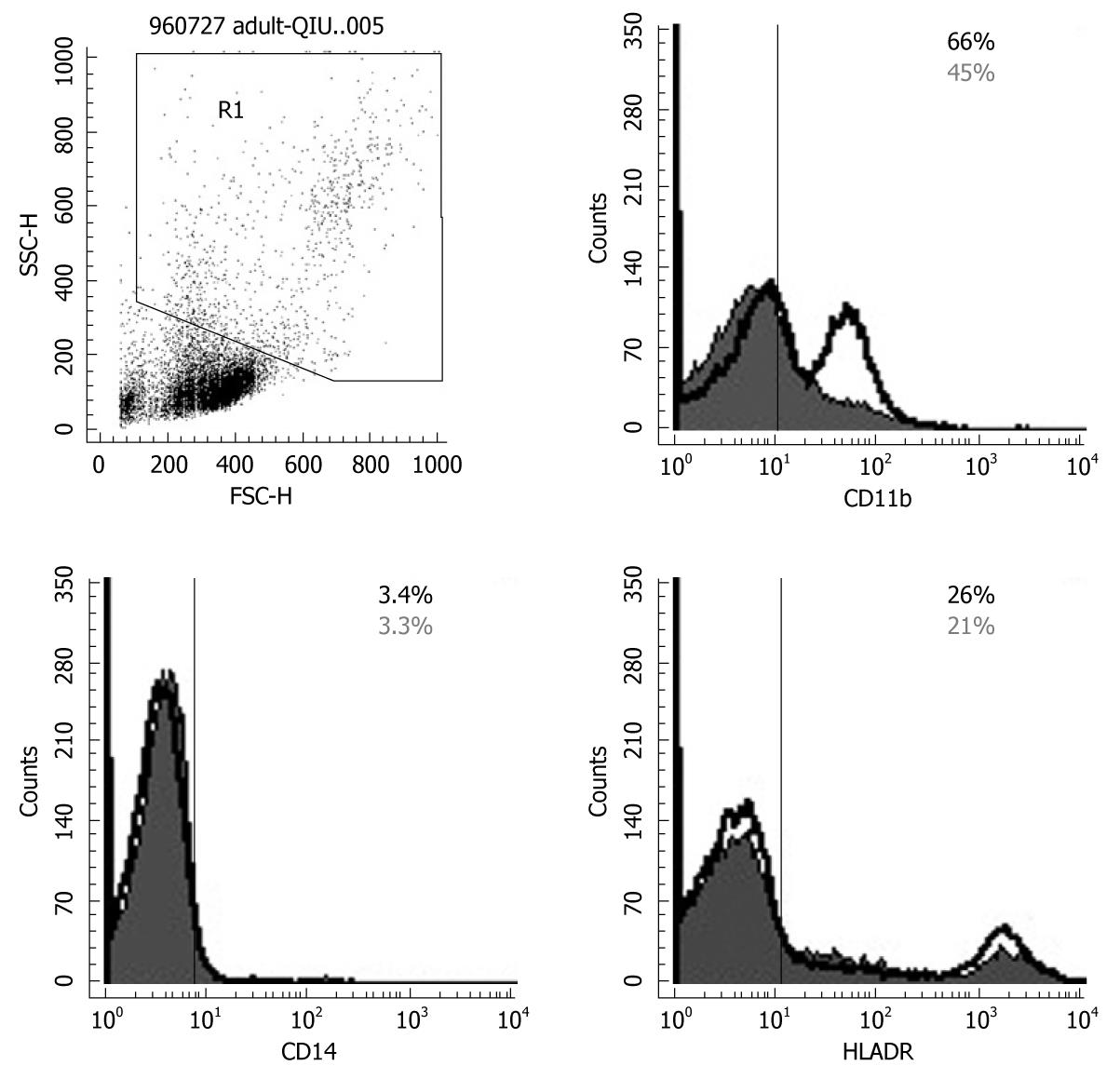
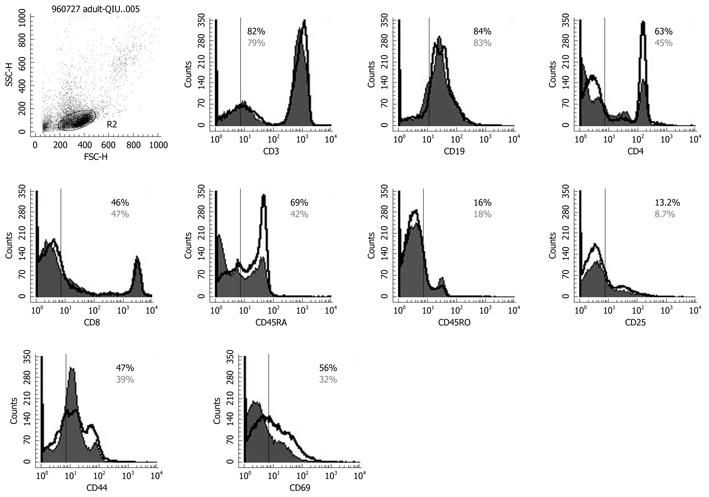
Flow cytometry was performed on day 14 after enrollment and detected the expression of immune phenotype distributions. White blood cells were stained using a panel of mAbs directed against surface antigens expressed by PBMCs, lymphocytes, monocytes and the appropriate species-specific IgG isotype controls. After blocking with FCγIII/IIR antibody (CD16/CD32; Pharmingen), the cells were stained with mAbs directed against CD4, CD45RA, CD45RO, CD14 fluorescein isothiocyanate, together with one of the activation markers: CD3, CD8, CD19, CD25, CD44, CD69, or CD11b, HLA-DR (all phycoerythrin-conjugated; Pharmingen). We analyzed 10 000-20 000 cells using a FACSCalibur system (Becton-Dickinson, Franklin Lakes, NJ, USA) equipped with CellQuest software (San Jose, CA, USA).
CD14-positive monocytes were then purified from the mononuclear cells by magnetic cell sorting by using positive selection according to the manufacturer’s protocol (Miltenyi Biotec, Bergisch Gladbach, Germany). Monocytes (106 cells/mL) were cultured in six-well plates in endotoxin-free RPMI 1640 medium supplemented with 250 U/mL recombinant IL-4 (R&D Systems, Abingdon, UK) and 250 U/mL recombinant GM-CSF (R&D Systems). The cells were cultured in the presence of 5% CO2 at 37°C for 7 d. Fresh medium that contained IL-4 and GM-CSF was added every second day. This procedure resulted in generation of immature DCs that were positive for CD11b but negative for CD14.
PBMCs were isolated by centrifugation of buffy coats on Lymphoprep gradients. CD4+ T cells were separated using negative selection affinity columns (R&D Systems), according to the manufacturer’s instructions. After separation, the T cells were washed and resuspended in RPMI 1640 culture medium supplemented with 5% heat-inactivated human AB serum, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 2 mmol/L L-glutamine. Purified CD4+ T cells (1 × 106/mL) were stimulated by the combination of immobilized anti-CD3 (1 μg/mL) and soluble anti-CD28 (5 μg/mL) mAbs (Pharmingen). Subsequently, purified CD4+ T cells were incubated with the above DCs at a ratio of 2.5 × 105/mL DCs to 106/mL T cells. After 48 h of co-culture, concentrations of IFN-γ, IL-4, IL-10, and IL12 p70 in the culture supernatants were determined by ELISA.
Statistical analysis was performed using a paired samples t test to reveal significant between-group differences in cytokine production. In all cases, P < 0.05 was considered as significant. Statistical calculations were performed using the GraphPad Software Prism 3.03 (San Diego, CA, USA) and SPSS for Windows 12.0 (Chicago, IL, USA).
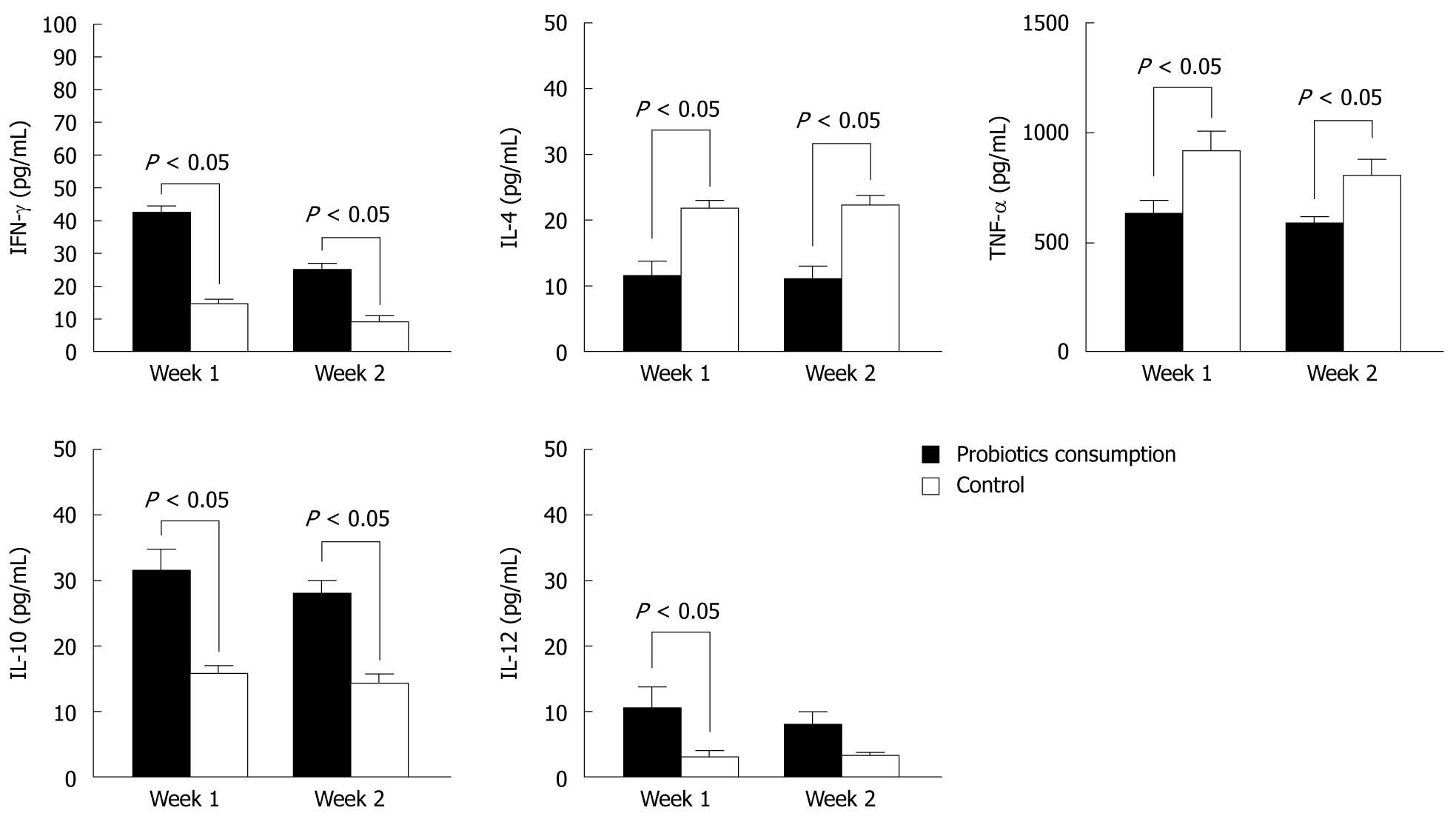
To determine the cytokine production of PBMCs, we examined the supernatants of cells isolated from blood donors. Group A consumed Bio-Three and group B was a negative control. In Figure 1, IFN-γ, IL-10 and IL-12 levels were upregulated in group A, but IL-4 and TNF-α levels were downregulated at 1 and 2 wk after Bio-Three consumption. This indicates that this probiotic preparation enhances cytokines associated with Th1 (IFN-γ, IL-12) and anti-inflammatory (IL-10) responses. In contrast, it might reduce cytokine production associated with Th2 (IL-4) and pro-inflammatory (TNF-α) responses.
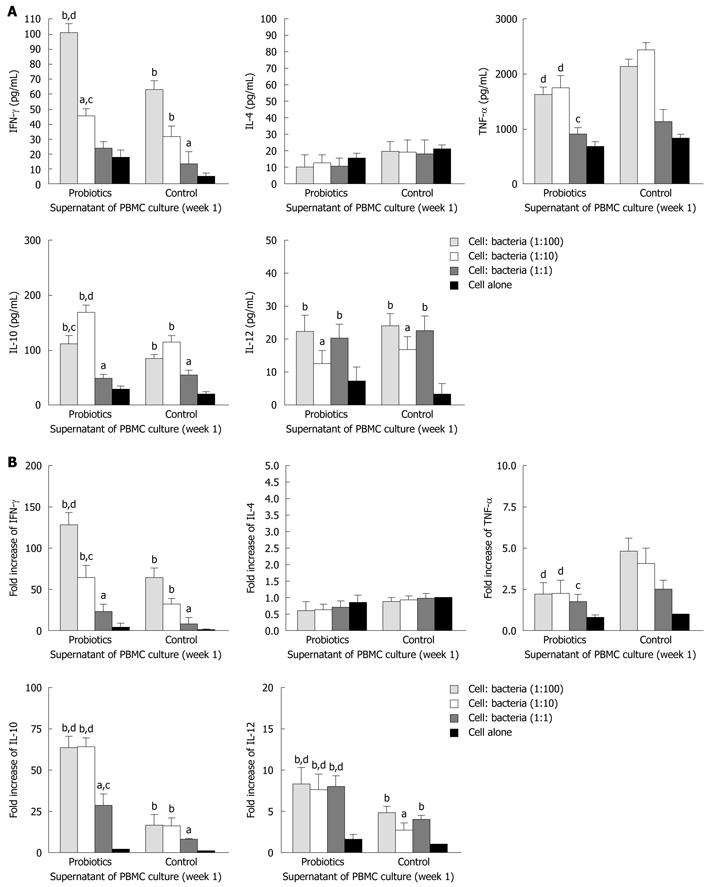
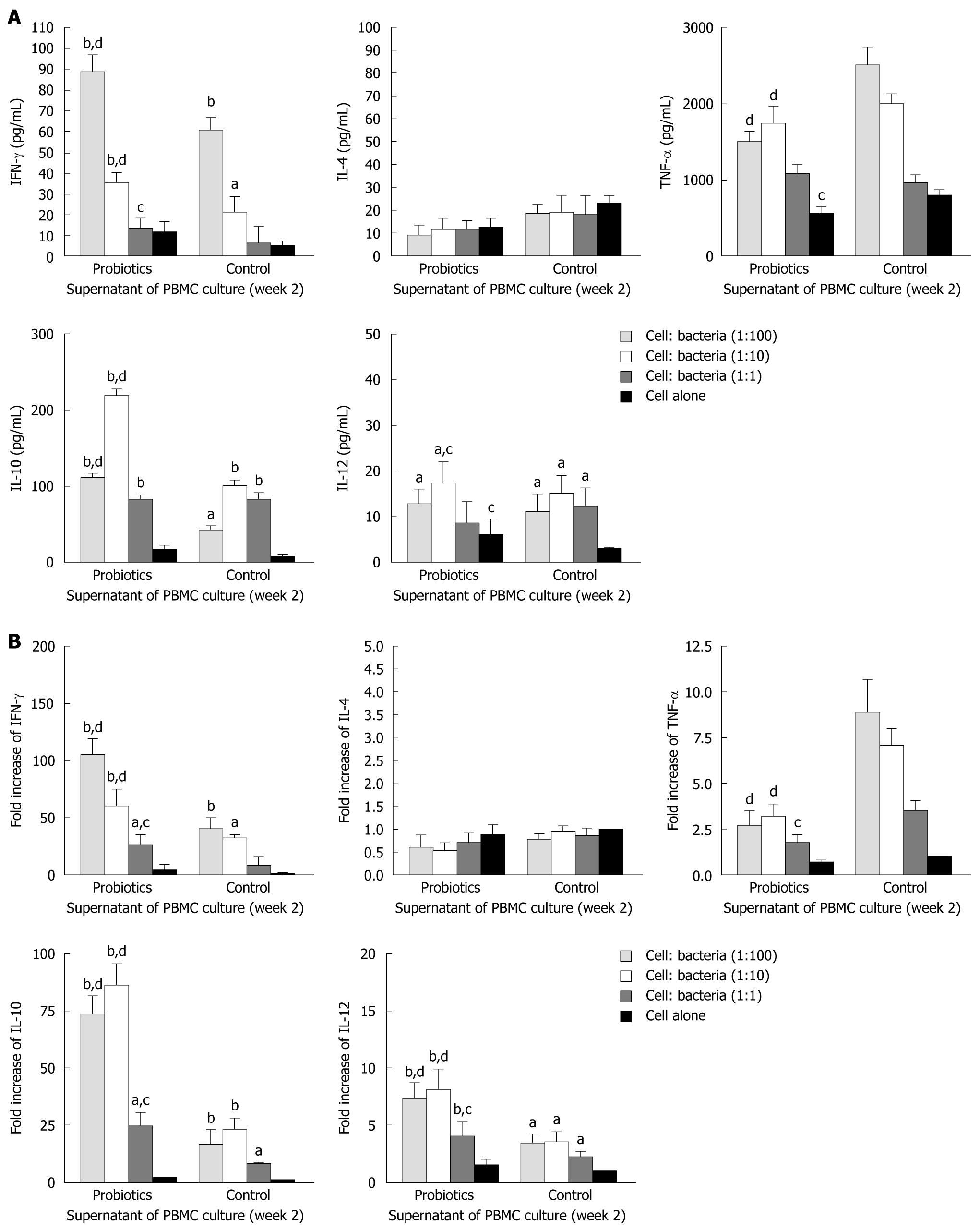
To explore the effects of probiotic bacteria on PBMCs, we performed stimulation experiments with different bacteria at concentrations of 105, 106 and 107 CFU/mL.
The level of IFN-γ was significantly higher in group A (probiotics) than group B (negative control) (Figures 2 and 3). Moreover, the level of IFN-γ revealed a dose-dependent effect of Bio-Three. There was no significant effect on IL-4 level, although it seemed slightly lower in the probiotic group. In contrast, TNF-α level was decreased at weeks 1 and 2 in the probiotic group (Figures 2 and 3). TNF-α level was significantly lower in response to restimulation with 107 CFU/mL probiotic bacteria after 2 wk of Bio-Three consumption. The probiotic group showed increased IL-10 production, which was maximal following restimulation with 106-107 CFU/mL probiotic bacteria at week 2. The level of IL-12 was low in both groups, which suggested that Bio-Three had no significant effect on IL-12 production (Figures 2 and 3). After stimulation with Bio-Three and co-culture in vitro, the IL-12 level in supernatants of PBMCs was upregulated (Figures 2 and 3). PBMCs can be activated by in vivo stimulation (probiotic consumption) and probiotic bacteria re-stimulation in vitro, which enhanced IFN-γ and IL-10 production, as well as downregulated TNF-α production.
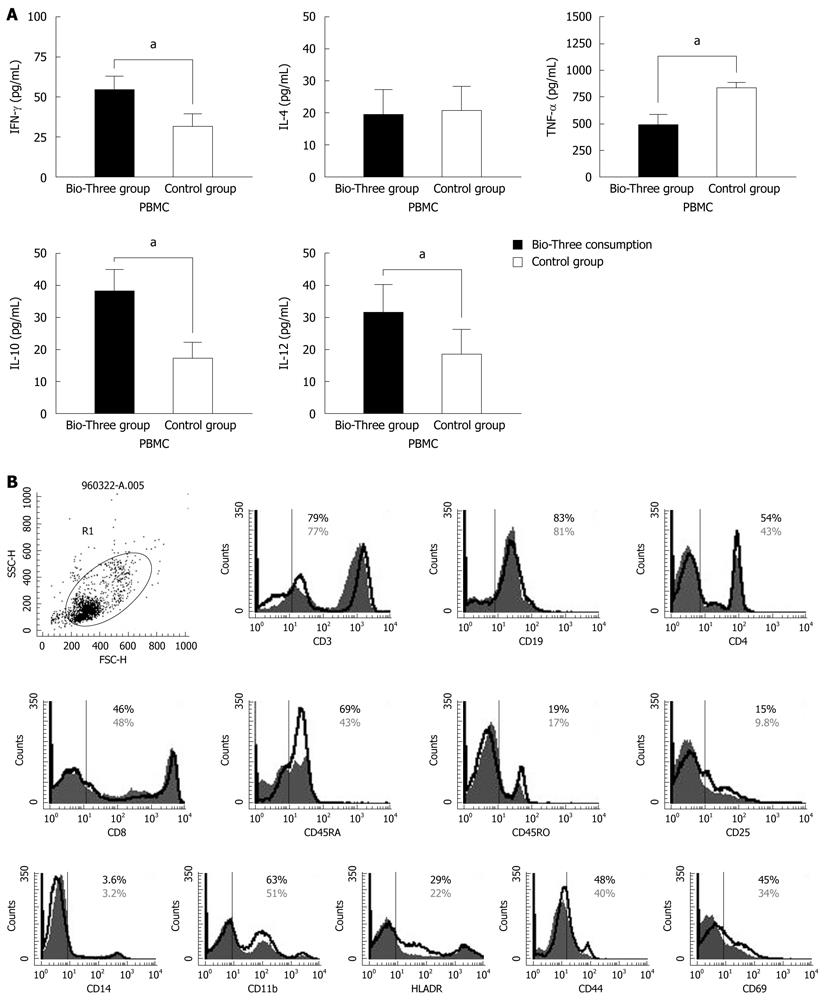
Initial concentrations of IFN-γ, IL-4, TNF-α, IL-10, and IL12 p70 in the supernatants of isolated human PBMCs were determined (Figure 4A) by ELISA. To determine the effect of probiotics (Bio-Three) on PBMCs, phenotypic analysis of immune responses was also studied. Figure 4B shows that probiotics might alter the expression of some T cell and DC surface phenotypes compared with controls, such as CD4+ (54.2% ± 3.6% vs 43.4% ± 3.0%), CD45RA+ (69.1% ± 4.2% vs 43.3% ± 3.6%), CD25+ (15.1% ± 2.6% vs 9.8% ± 2.3%), CD44+ (48.3% ± 3.8% vs 40.1% ± 3.2%), CD69+ (45.6% ± 2.4% vs 34.3% ± 2.7%), CD11b+ (63.6% ± 4.5% vs 51.8% ± 2.8%) and HLA-DR (29.9% ± 2.7% vs 22.3% ± 2.3%). Probiotics can enhance expression of CD4, CD45RB, CD44, CD69, CD25, CD11b and HLA-DR, which indicates alternation of co-stimulation with markers of Th cells and DCs.
To determine the effect of Bio-Three on circulating monocytes and APCs (such as DCs), phenotypic analysis of CD14, CD 11b and HLA-DR was also studied. Probiotics can also enhance the expression of CD11b, and HLA-DR in gating monocytes and granulocytes (Figure 5), which indicates alternation of co-stimulation with marker of DCs. However, the expression of CD14 was similar in the probiotics and control groups.
To determine the effect of Bio-Three on circulating lymphocytes, phenotypic analysis of T cells and B cells was also studied. Figure 6 shows that probiotics can alter the expression of some T-cell surface phenotypes compared with controls, such as CD4+ (63.2% ± 4.6% vs 45.6% ± 3.1%), CD45RA+ (69.2% ± 3.9% vs 42.3% ± 2.6%), CD25+ (13.2% ± 2.5% vs 8.7% ± 2.1%), CD44+ (47.3% ± 3.6% vs 39.8% ± 3.8%) and CD69+ (56.6% ± 2.3% vs 32.1% ± 2.6%). Flow cytometry showed that probiotic treatment was associated with altered expression of CD4 (Th cells), CD45RA (naïve T cells), CD25 (Treg cells), CD44 and CD69 (T-cell activation markers) on lymphocytes, but there was no significant effect on the expression of CD3, CD8 or CD45RO.
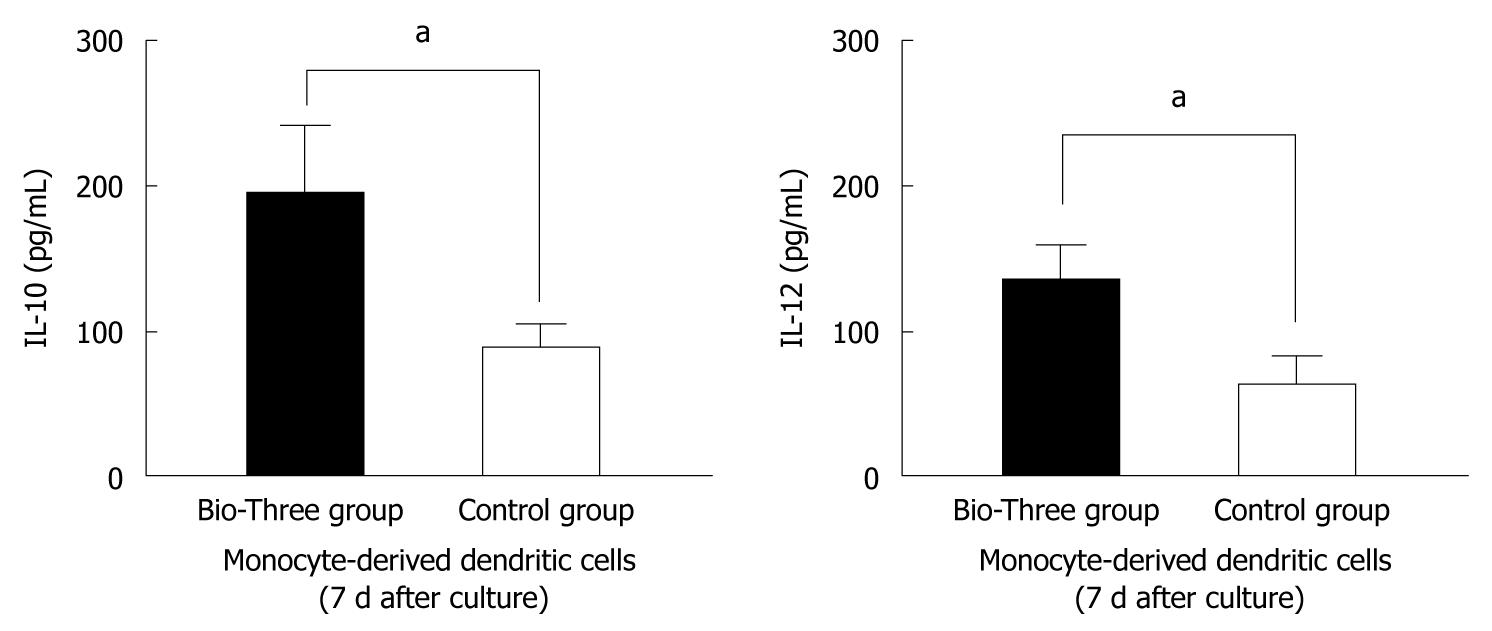
To determine the effect of probiotics on DCs, we isolated monocytes and cultured them at 37°C for 7 d. In the culture supernatants from monocyte-derived DCs, IL-10 and IL-12 levels were upregulated in the probiotic group compared to the control group (Figure 7). This indicates that Bio-Three can stimulate monocyte-derived DCs to produce more IL-10 and IL-12.
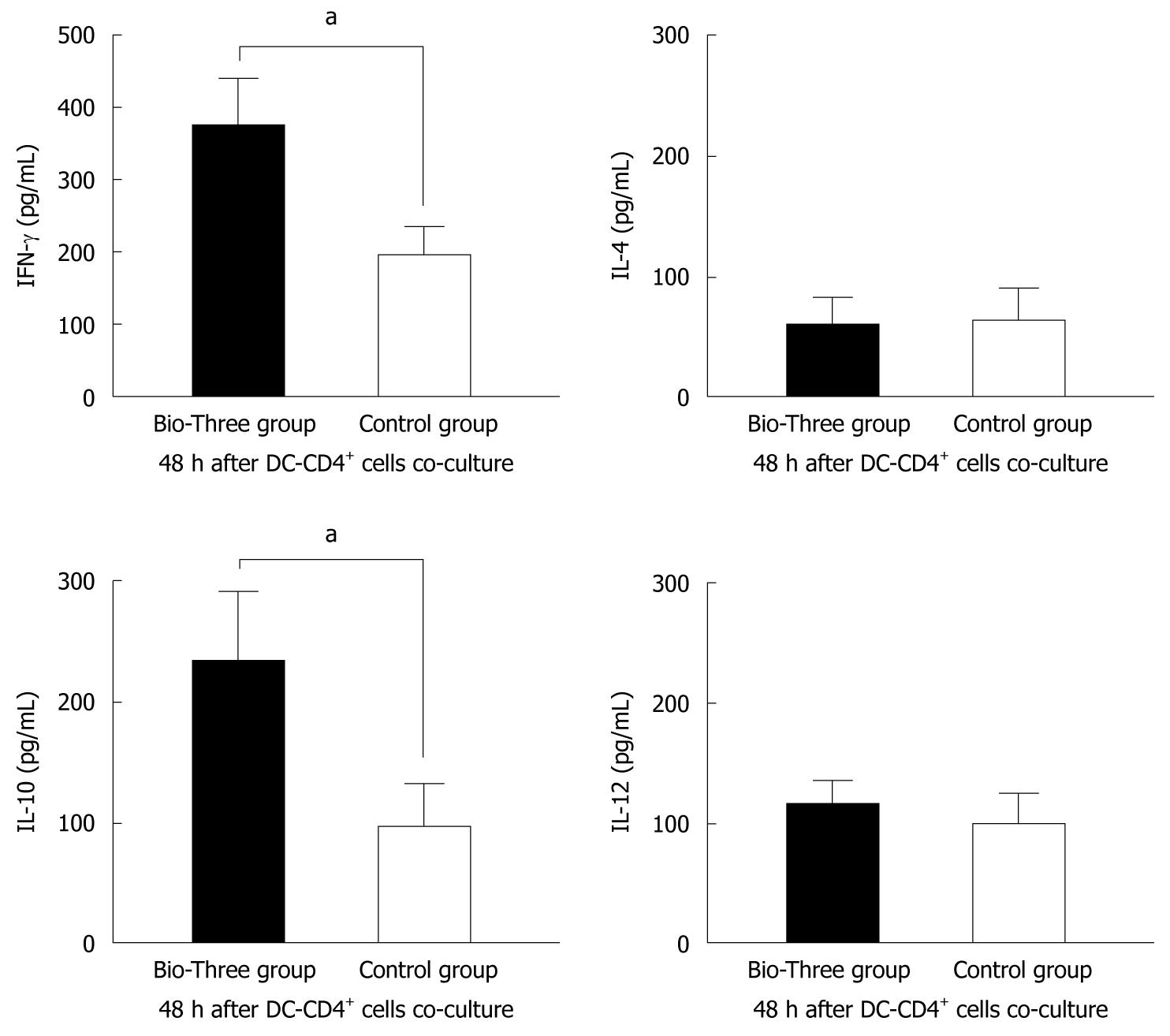
To explore the effect of probiotic consumption on DC and CD4+ T cell differentiation, we use a cell culture model to study cytokine levels in the supernatants of CD4+ T cells co-cultured with monocyte-derived DCs. Bio-Three upregulated IFN-γ (associated with Th1) and IL-10 (associated with Treg cells) levels in the supernatants of DCs and CD4+ T cells co-cultured for 48 h (Figure 8).
The role of intestinal microflora as a modulator of the immune response has been studied intensively in recent years. Lactobacillus species are the most well-studied both in vitro and in vivo, and could have clinical importance in inducing phagocytosis and IgA secretion, modifying T-cell responses, enhancing Th1 responses, and attenuating Th2 responses[2,15-17]. However, how intestinal microbes interact with the mucosal immune system remains unclear[15]. The ability of different strains of Lactobacillus to induce production of key cytokines such as IL-12 and IL-10 varies markedly[2,8].
The functionally different CD4+ Th cell subsets known as Th1 and Th2 have different cytokine profiles. The Th1/Th2 paradigm is relevant to the pathogenesis of several pathological conditions and provides the rationale for the development of new strategies for treating and preventing diseases. The development of polarized Th1 or Th2 responses depends on environmental factors [e.g. dose of antigen, nature of the immunogen, and cytokines (IL-4, IL-12 or interferons) at the time of antigen presentation], or on other undefined factors that mainly affect so-called natural immunity[18]. Th1-dominated responses are potentially effective in eradicating infectious agents, including those hidden within the host cells.
In our study, probiotics upregulated IFN-γ levels and moderately downregulated IL-4 in the supernatant of cultured PBMCs, which suggests that probiotics enhance the Th1 immune response and suppress the Th2 immune response.
The effects of consuming probiotics other than lactic acid bacteria are relatively unexplored. Bacillus strains are used in the treatment of diarrhea[19]. Additionally, they have antimicrobial activities, induce secretory IgA, IFN-γ, IL-12, IL-10 and TGF-β production, stimulate CD4+ T cell proliferation, and suppress IL-4 levels[20,21]. The clinical and immunomodulatory effects of Clostridium and Enterococcus species are well documented in animal models. Heat-inactivated C. butyricum enhanced IFN-γ production, polyclonal antibody formation, and phagocytosis in a mouse model[22,23]. Moreover, culture supernatants of C. butyricum TO-A downregulate TLR4 expression in human colonic epithelial cells[24]. Feeding E. faecium SF68 to mice has been documented to antagonize Giardia intestinalis infection and increase the percentage of CD4+ T cells in the Peyer’s patches and spleen[25]. It has also been suggested that adequate E. faecium after antibiotic treatment improves the intestinal ecosystem, and thereby prevents the shift to Th2 immunity in neonatal mice[26].
The present study focused on the probiotic mixture Bio-Three of B. mesentericus, C. butyricum and E. faecalis. We speculated that each species of Bio-Three would modify the immune function differently, thus leading to more complex effects. To date, few studies have examined the clinical effects of Bio-Three. One study has found that Bio-Three prevents enterohemorrhagic Escherichia coli O157: H7 infection in rabbits[27]. Another has found that Bio-Three is effective in patients with ulcerative colitis that is refractory to conventional therapy[28]. Administration of Bio-Three to infants changes the composition of intestinal flora and decreases serum endotoxin produced by potentially pathogenic microorganisms[29], and probably reduces infectious complications after pancreaticoduodenectomy[30].
In the present study, Bio-Three induced IL-10 production and increased the number of Treg cells (CD4+ and CD25+ T lymphocytes). IL-10 modulates immune responses by probiotic bacteria[2,15,31] by inhibiting synthesis of IL-2, IL-12 and TNF-α produced by cells such as APCs and Th1 cells[10,12,32]. Increased IL-10 production might explain why Bio-Three inhibits TNF-α production, which can be harmful at high levels[3,33].
IL-12 plays a central role in promoting the Th1 response[2,10]. Specific strains of Lactobacillus enhance IL-12 production by human mononuclear cells[34,35]. However, in our study, this effect was limited probably because of strains in Bio-Three were not stimulatory. Other explanations include inhibition by IL-10, shorter time of stimulation in vitro (24 h), and inadequate dose (around 109 organisms/d). In previous studies, daily doses of 1010-1011 (but not < 109) organisms of probiotic lactic acid bacteria conferred physiological benefits[36,37]. In the present study, IL-12 level in both groups was low, therefore, the influence of Bio-Three dose on IL-12 production should be further investigated. Notably, not all probiotics species can induce IL-12 production.
We used a previously published method (105, 106 and 107 CFU/mL bacteria co-cultured with PBMCs (105/mL) for 1 d (the ratio of bacteria:PBMC was 1:1, 10:1 and 100:1) to investigate how the concentration of bacteria affects cytokine production[35,38,39]. A prior study has demonstrated that Lactobacillus dose-dependently stimulates PBMC expression of cytokines[39]. In our system, the optimum dose was around 106-107 CFU/mL, which was almost statistically significant (P < 0.05). However, cytokine production was less at the highest concentration (108 CFU/mL bacteria, data not shown), which suggested that stimulation of PBMCs was not fully dose-dependent. This inconsistency between the responses to Bio-Three and Lactobacillus may have been due to species differences. Moreover, the possibility that highly concentrated cell debris induces apoptosis, deletion, or cell death of PBMCs should be considered.
In our experiment, CD4+, CD45RA+ and CD25+ T lymphocytes were upregulated, whereas CD14+ cells showed no significant change. DCs are CD11b+ cells that can differentiate from CD14+ monocytes[10,40]. The expression of CD11b+ cells in our study suggested that they were increased by exposure to Bio-Three. DCs regulate the development of T cell responses, especially the polarization of such responses[1,2]. It has been demonstrated that exposure to probiotic bacteria upregulates markers of DC maturation, such as HLA-DR and members of the B7 family (CD80, CD86)[10,31,41-44]. High but not low doses of probiotic organisms induce DC maturation, which suggests that different intracellular signaling pathways are activated by high doses[10,45].
As described above, the level of HLA-DR (a marker of immune stimulation), which participates in DC signaling, is usually enhanced. In the present study, Bio-Three had an enhancing effect on expression of HLA-DR. Furthermore, co-culture with DCs and CD4+ T cells, showed upregulation of IFN-γ (associated with Th1 cells) and IL-10 (regulatory cytokine) in the supernatants.
To date, the real pathways of probiotic immunomodulatory effects are not fully understood, and some types of immune cells that are primed by probiotics might be the connection between in vivo and in vitro stimulation. We proposed that PBMCs are involved in the in vivo sensitizing effect of Bio-Three and further in vitro stimulatory effects. The monocyte-derived DC transformation might play a key role in the mechanism of probiotic immunomodulation. DCs are the most potent APCs that are primed by probiotic bacteria, and they are the principal stimulators of naïve T cells to drive further immune responses.
In conclusion, we found that probiotics (Bio-Three) increased expression of IFN-γ (associated with Th1 cells), increased IL-10 production (anti-inflammatory cytokine), and decreased TNF-α level. The optimum concentration of Bio-Three for cytokine production was around 106-107 CFU/mL. It is reasonable to speculate that Bio-Three redirected the immune system toward an anti-inflammatory phenotype, rather than an aggressive immune response, even at a relatively low dose (109 organisms/d for 2 wk). Furthermore, the short term in vivo (2 wk) and in vitro (24 h) exposure was probably sufficient to promote a Th1 cell response and HLA-DR expression on DCs.
There is increasing evidence that probiotic bacteria influence host immune function, but the immune response in human peripheral blood after probiotic consumption is little known. Probiotic products, however, are usually consumed by the general population, but not much is known about the effects that they have on the immune system in healthy adults.
It is not fully clarified how probiotics exert their beneficial effects, but one of the most probable mechanisms is the modulation of host immune responses. The possible action mechanism could be the ability to induce cytokines that further regulate innate and adaptive immune responses.
The present study was designed to explore the immune response of peripheral blood mononuclear cells (PBMCs) in vivo after stimulation by a probiotic preparation, such as cytokine production and phenotypic analysis of circulating PBMCs. The authors also investigated cytokine production of (1) PBMCs stimulated by probiotic preparation; (2) monocyte-derived dendritic cells (DCs); and (3) co-cultured DCs and T cells.
The authors found that the probiotic preparation Bio-Three (Bacillus mesentericus, Clostridium butyricum and Enterococcus faecalis) could direct immune responses to either a Th1 or anti-inflammatory response. More detailed information on the cytokine patterns elicited by probiotic bacteria could help in designing probiotic preparations for specific preventative or therapeutic purposes.
This is a well-written paper with promising results that could be the basis of forthcoming new research on the therapeutic and immunomodulatory effects of probiotics.
Peer reviewers: Sung-Gil Chi, Professor, School of Life Sciences and Biotechnology, Korea University, #301, Nok-Ji Building, Seoul 136-701, South Korea; Ferenc Sipos, MD, PhD, Cell Analysis Laboratory, 2nd Department of Internal Medicine, Semmelweis University, Szentkirályi u. 46., Budapest 1088, Hungary
S- Editor Wang JL L- Editor Kerr C E- Editor Lin YP
| 1. | Madsen K. Probiotics and the immune response. J Clin Gastroenterol. 2006;40:232-234. |
| 2. | Vaarala O. Immunological effects of probiotics with special reference to lactobacilli. Clin Exp Allergy. 2003;33:1634-1640. |
| 3. | Isolauri E, Sütas Y, Kankaanpää P, Arvilommi H, Salminen S. Probiotics: effects on immunity. Am J Clin Nutr. 2001;73:444S-450S. |
| 4. | Kalliomäki MA, Isolauri E. Probiotics and down-regulation of the allergic response. Immunol Allergy Clin North Am. 2004;24:739-752, viii. |
| 5. | Michail S, Sylvester F, Fuchs G, Issenman R. Clinical efficacy of probiotics: review of the evidence with focus on children. J Pediatr Gastroenterol Nutr. 2006;43:550-557. |
| 6. | Lemberg DA, Ooi CY, Day AS. Probiotics in paediatric gastrointestinal diseases. J Paediatr Child Health. 2007;43:331-336. |
| 8. | Christensen HR, Frøkiaer H, Pestka JJ. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J Immunol. 2002;168:171-178. |
| 9. | Bibiloni R, Fedorak RN, Tannock GW, Madsen KL, Gionchetti P, Campieri M, De Simone C, Sartor RB. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol. 2005;100:1539-1546. |
| 10. | Drakes M, Blanchard T, Czinn S. Bacterial probiotic modulation of dendritic cells. Infect Immun. 2004;72:3299-3309. |
| 11. | Karlsson H, Larsson P, Wold AE, Rudin A. Pattern of cytokine responses to gram-positive and gram-negative commensal bacteria is profoundly changed when monocytes differentiate into dendritic cells. Infect Immun. 2004;72:2671-2678. |
| 12. | Karlsson H, Hessle C, Rudin A. Innate immune responses of human neonatal cells to bacteria from the normal gastrointestinal flora. Infect Immun. 2002;70:6688-6696. |
| 13. | Rodriguez AV, Baigorí MD, Alvarez S, Castro GR, Oliver G. Phosphatidylinositol-specific phospholipase C activity in Lactobacillus rhamnosus with capacity to translocate. FEMS Microbiol Lett. 2001;204:33-38. |
| 14. | Randolph GJ, Inaba K, Robbiani DF, Steinman RM, Muller WA. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 1999;11:753-761. |
| 15. | Niers LE, Timmerman HM, Rijkers GT, van Bleek GM, van Uden NO, Knol EF, Kapsenberg ML, Kimpen JL, Hoekstra MO. Identification of strong interleukin-10 inducing lactic acid bacteria which down-regulate T helper type 2 cytokines. Clin Exp Allergy. 2005;35:1481-1489. |
| 16. | Pochard P, Gosset P, Grangette C, Andre C, Tonnel AB, Pestel J, Mercenier A. Lactic acid bacteria inhibit TH2 cytokine production by mononuclear cells from allergic patients. J Allergy Clin Immunol. 2002;110:617-623. |
| 17. | Pessi T, Sütas Y, Hurme M, Isolauri E. Interleukin-10 generation in atopic children following oral Lactobacillus rhamnosus GG. Clin Exp Allergy. 2000;30:1804-1808. |
| 18. | Del Prete G. The concept of type-1 and type-2 helper T cells and their cytokines in humans. Int Rev Immunol. 1998;16:427-455. |
| 19. | Mazza P. The use of Bacillus subtilis as an antidiarrhoeal microorganism. Boll Chim Farm. 1994;133:3-18. |
| 20. | Ciprandi G, Tosca MA, Milanese M, Caligo G, Ricca V. Cytokines evaluation in nasal lavage of allergic children after Bacillus clausii administration: a pilot study. Pediatr Allergy Immunol. 2004;15:148-151. |
| 21. | Urdaci MC, Bressollier P, Pinchuk I. Bacillus clausii probiotic strains: antimicrobial and immunomodulatory activities. J Clin Gastroenterol. 2004;38:S86-S90. |
| 22. | Murayama T, Mita N, Tanaka M, Kitajo T, Asano T, Mizuochi K, Kaneko K. Effects of orally administered Clostridium butyricum MIYAIRI 588 on mucosal immunity in mice. Vet Immunol Immunopathol. 1995;48:333-342. |
| 23. | Wang GR, Chen HY, Chen CH, Yeh MY, Mikami Y. Immunopotentiating activity of Clostridium butyricum in mice. Proc Natl Sci Counc Repub China B. 1996;20:101-109. |
| 24. | Isono A, Katsuno T, Sato T, Nakagawa T, Kato Y, Sato N, Seo G, Suzuki Y, Saito Y. Clostridium butyricum TO-A culture supernatant downregulates TLR4 in human colonic epithelial cells. Dig Dis Sci. 2007;52:2963-2971. |
| 25. | Benyacoub J, Pérez PF, Rochat F, Saudan KY, Reuteler G, Antille N, Humen M, De Antoni GL, Cavadini C, Blum S. Enterococcus faecium SF68 enhances the immune response to Giardia intestinalis in mice. J Nutr. 2005;135:1171-1176. |
| 26. | Sudo N, Yu XN, Aiba Y, Oyama N, Sonoda J, Koga Y, Kubo C. An oral introduction of intestinal bacteria prevents the development of a long-term Th2-skewed immunological memory induced by neonatal antibiotic treatment in mice. Clin Exp Allergy. 2002;32:1112-1116. |
| 27. | Tachikawa T, Seo G, Nakazawa M, Sueyoshi M, Ohishi T, Joh K. [Estimation of probiotics by infection model of infant rabbit with enterohemorrhagic Escherichia coli O157:H7]. Kansenshogaku Zasshi. 1998;72:1300-1305. |
| 28. | Tsuda Y, Yoshimatsu Y, Aoki H, Nakamura K, Irie M, Fukuda K, Hosoe N, Takada N, Shirai K, Suzuki Y. Clinical effectiveness of probiotics therapy (BIO-THREE) in patients with ulcerative colitis refractory to conventional therapy. Scand J Gastroenterol. 2007;42:1306-1311. |
| 29. | Urao M, Fujimoto T, Lane GJ, Seo G, Miyano T. Does probiotics administration decrease serum endotoxin levels in infants? J Pediatr Surg. 1999;34:273-276. |
| 30. | Nomura T, Tsuchiya Y, Nashimoto A, Yabusaki H, Takii Y, Nakagawa S, Sato N, Kanbayashi C, Tanaka O. Probiotics reduce infectious complications after pancreaticoduodenectomy. Hepatogastroenterology. 2007;54:661-663. |
| 31. | Hart AL, Lammers K, Brigidi P, Vitali B, Rizzello F, Gionchetti P, Campieri M, Kamm MA, Knight SC, Stagg AJ. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut. 2004;53:1602-1609. |
| 32. | de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209-1220. |
| 33. | Henderson B, Poole S, Wilson M. Microbial/host interactions in health and disease: who controls the cytokine network? Immunopharmacology. 1996;35:1-21. |
| 34. | Mohamadzadeh M, Olson S, Kalina WV, Ruthel G, Demmin GL, Warfield KL, Bavari S, Klaenhammer TR. Lactobacilli activate human dendritic cells that skew T cells toward T helper 1 polarization. Proc Natl Acad Sci USA. 2005;102:2880-2885. |
| 35. | Miettinen M, Matikainen S, Vuopio-Varkila J, Pirhonen J, Varkila K, Kurimoto M, Julkunen I. Lactobacilli and streptococci induce interleukin-12 (IL-12), IL-18, and gamma interferon production in human peripheral blood mononuclear cells. Infect Immun. 1998;66:6058-6062. |
| 36. | Donnet-Hughes A, Rochat F, Serrant P, Aeschlimann JM, Schiffrin EJ. Modulation of nonspecific mechanisms of defense by lactic acid bacteria: effective dose. J Dairy Sci. 1999;82:863-869. |
| 37. | Schiffrin EJ, Brassart D, Servin AL, Rochat F, Donnet-Hughes A. Immune modulation of blood leukocytes in humans by lactic acid bacteria: criteria for strain selection. Am J Clin Nutr. 1997;66:515S-520S. |
| 39. | Helwig U, Lammers KM, Rizzello F, Brigidi P, Rohleder V, Caramelli E, Gionchetti P, Schrezenmeir J, Foelsch UR, Schreiber S. Lactobacilli, bifidobacteria and E. coli nissle induce pro- and anti-inflammatory cytokines in peripheral blood mononuclear cells. World J Gastroenterol. 2006;12:5978-5986. |
| 40. | Drakes ML, Lu L, Subbotin VM, Thomson AW. In vivo administration of flt3 ligand markedly stimulates generation of dendritic cell progenitors from mouse liver. J Immunol. 1997;159:4268-4278. |
| 41. | Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245-252. |
| 42. | Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1:311-316. |
| 43. | Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255-258. |
| 44. | Chen CC, Chiu CH, Lin TY, Shi HN, Walker WA. Effect of probiotics Lactobacillus acidophilus on Citrobacter rodentium colitis: the role of dendritic cells. Pediatr Res. 2009;65:169-175. |
| 45. | Smits HH, Engering A, van der Kleij D, de Jong EC, Schipper K, van Capel TM, Zaat BA, Yazdanbakhsh M, Wierenga EA, van Kooyk Y. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol. 2005;115:1260-1267. |