Published online Jul 21, 2010. doi: 10.3748/wjg.v16.i27.3450
Revised: April 28, 2010
Accepted: May 5, 2010
Published online: July 21, 2010
AIM: To evaluate survival and recurrence after radiofrequency ablation (RFA) for the treatment of small hepatocellular carcinoma (HCC) using a meta-analysis.
METHODS: Literature on RFA vs surgical resection for the treatment of small HCC published between January 1990 and December 2008 was retrieved. A meta-analysis was conducted to estimate pooled survival and recurrence ratios. A fixed or random effect model was established to collect the data.
RESULTS: The differences in overall survival at 1-year, 3-years and at end of follow-up were not statistically significant between the RFA and surgery groups (P > 0.05). There were no differences in 1-year and 3-year recurrences between the RFA and surgery groups (P > 0.05). However, recurrence in the RFA group was lower than that in the surgery group up to the end of follow-up (P = 0.03). Survival was not significantly different. There was a significant difference in recurrences at the end of follow-up after RFA compared with surgical resection.
CONCLUSION: RFA did not decrease the number of overall recurrences, and had no effect on survival when compared with surgical resection in a selected group of patients.
- Citation: Liu JG, Wang YJ, Du Z. Radiofrequency ablation in the treatment of small hepatocellular carcinoma: A meta analysis. World J Gastroenterol 2010; 16(27): 3450-3456
- URL: https://www.wjgnet.com/1007-9327/full/v16/i27/3450.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i27.3450
The preferred treatment for hepatocellular carcinoma (HCC) is surgical resection which has a good long-term effect. In recent years, radiofrequency ablation (RFA) has emerged as the latest oriented treatment, especially for HCC, and has become an important treatment following surgical resection and has established its place in the treatment algorithm of liver tumors. The treatment of HCC in patients with chronic liver disease is a major challenge. With the intention of avoiding hepatic failure which can appear after hepatic resection, percutaneous ablative treatments have been proposed. RFA ablation has progressively reached consensus due to its efficacy, tolerability and low-risk[1]. RFA is much less invasive, involves a short hospital stay and has an extremely low associated mortality; however, long-term results are difficult to ascertain, because the majority of reports concern evaluation of the percentage of success in terms of tumor necrosis and few data are available on the overall and disease-free survival of patients[2-6]. Clear evidence is still needed for RFA to be accepted as an alternative to surgery for resectable HCC on cirrhosis. Few studies have focused on a comparison between the results of surgery and RFA. In order to reduce research bias and differences, we used a meta-analysis to compare survival and recurrences following RFA compared with surgical resection for the treatment of small HCC. This article may provide a reference for clinical practice.
We carried out an exhaustive Medline, PubMed, CBM and CNKI search of the world literature comparing survival and recurrences following RFA compared with surgical resection for the treatment of small HCC, between the period January 1990 to December 2008 using the key words (radiofrequency, radio-frequency or radio frequency), (surgical resection or hepatectomy) and (liver or hepatic or hepatocellular) in English, French, German, Italian, Spanish, Danish, Dutch, Korean and Chinese. All abstract supplements from published literature were searched manually. Relevant papers were also identified from the reference lists of previous papers which were obtained through the search, and from abstracts from recent international meetings.
In the case of overlap between 2 reports, only the most detailed report was included. Only series with a minimum follow-up of 12 mo were included. Reports about treatments obtained with noncommercial electrodes and treatments with palliative intent (intentional partial debulking) were excluded. When appropriate, authors were contacted to obtain more details about the cases they reported.
In addition, we chose some Chinese articles, as there are many patients with small HCC in China. A good meta-analysis requires these data.
Data were extracted by two or three independent observers using standardized forms. The recorded data included the number of patients, overall survival and recurrence. The quality of all selected articles was ranked in accordance with the score of the non-randomized controlled clinical trial quality evaluation standard (Table 1).
| Author | Yr | Study design | RFA (cases) | Hepatectomy (cases) | Journal | Quality evaluation score1 |
| Peng et al[8] | 2008 | Retrospective study | 251 | 183 | Zhongguo Shiyong Waike Zazhi | 7 |
| Vivarelli et al[9] | 2004 | Cohort study | 58 | 40 | Ann Surg | 7 |
| Zhang et al[10] | 2007 | Retrospective study | 15 | 29 | Disan Junyi Daxue Xuebao | 7 |
| Zhou et al[11] | 2007 | Retrospective study | 47 | 40 | Gandan Waike Zazhi | 7 |
| Guglielmi et al[12] | 2008 | Retrospective study | 109 | 91 | J Gastrointest Surg | 7 |
| Montorsi et al[13] | 2005 | Cohort study | 79 | 79 | J Gastrointest Surg | 7 |
| Hong et al[14] | 2005 | Cohort study | 55 | 93 | J Clin Gastroenterol | 9 |
| Wakai et al[15] | 2006 | Retrospective study | 21 | 85 | World J Gastroenterol | 7 |
| Cho et al[16] | 2005 | Retrospective study | 99 | 61 | Korean J Hepatol | 9 |
| Gao et al[17] | 2007 | Retrospective study | 53 | 34 | Zhongguo Yixue Yingxiang Jishu Zazhi | 9 |
Inclusion criteria for this study were as follows: (1) A solitary HCC smaller than 5 cm in diameter or multiple (no more than three) HCC smaller than 5 cm in total diameter; (2) No extrahepatic metastasis; (3) No radiologic evidence of invasion into the major portal/hepatic vein branches; (4) Good liver function with Child-Pugh Class A or B, with no history of encephalopathy, ascites refractory to diuretics or variceal bleeding; (5) No previous treatment of HCC; (6) Patient should be suitable for treatment with either surgical resection or RFA; and (7) No recurrences where no tumor was found by spiral computed tomography and serum α-fetoprotein level when assessed every 3 mo after treatment during the follow-up period.
Meta-analysis was performed using fixed-effect or random-effect methods, depending on the absence or presence of significant heterogeneity. Statistical heterogeneity between trials was evaluated by the Cochran χ2 test and was considered significant when P < 0.10. In the absence of statistically significant heterogeneity, the Mantel-Haenszel method in the fixed-effect model was used for the meta analysis. Otherwise, the DerSimonian and Laird method in the random-effect model was selected.
The odds ratio (OR) with 95% confidence interval (CI) was used to assess treatment efficacy. The combined result was an average OR and 95% CI weighted according to the standard error of the OR of the trial. P < 0.05 was considered statistically significant. We used funnel plots to assess the publication bias, and tested for funnel plot asymmetry using Egger’s test and Begg’s test. All analyses were performed with STATA version 9.0 (Stata Co., College Station, TX, USA) and Review Manager version 4.2.2 (RevMan, Cochrane Collaboration, Oxford, England).
According to exclusion and selected criteria of historical data, 10 studies were selected for the meta analysis, including 787 cases of RFA and 735 cases of surgical resection. However, one publication[7] was removed, because the number of cases continued to expand in another publication[8]. Among the 10 articles selected, 4 (40%) were from China, and corresponded to the high incidence of Hepatitis B virus-associated HCC in China. The characteristics of the 10 clinical trials included are shown in Table 1.
The comparison of survival and recurrence following RFA vs surgical resection for the treatment of small HCC using the meta-analysis is shown in Figures 1, 2, and 3 [8-17].
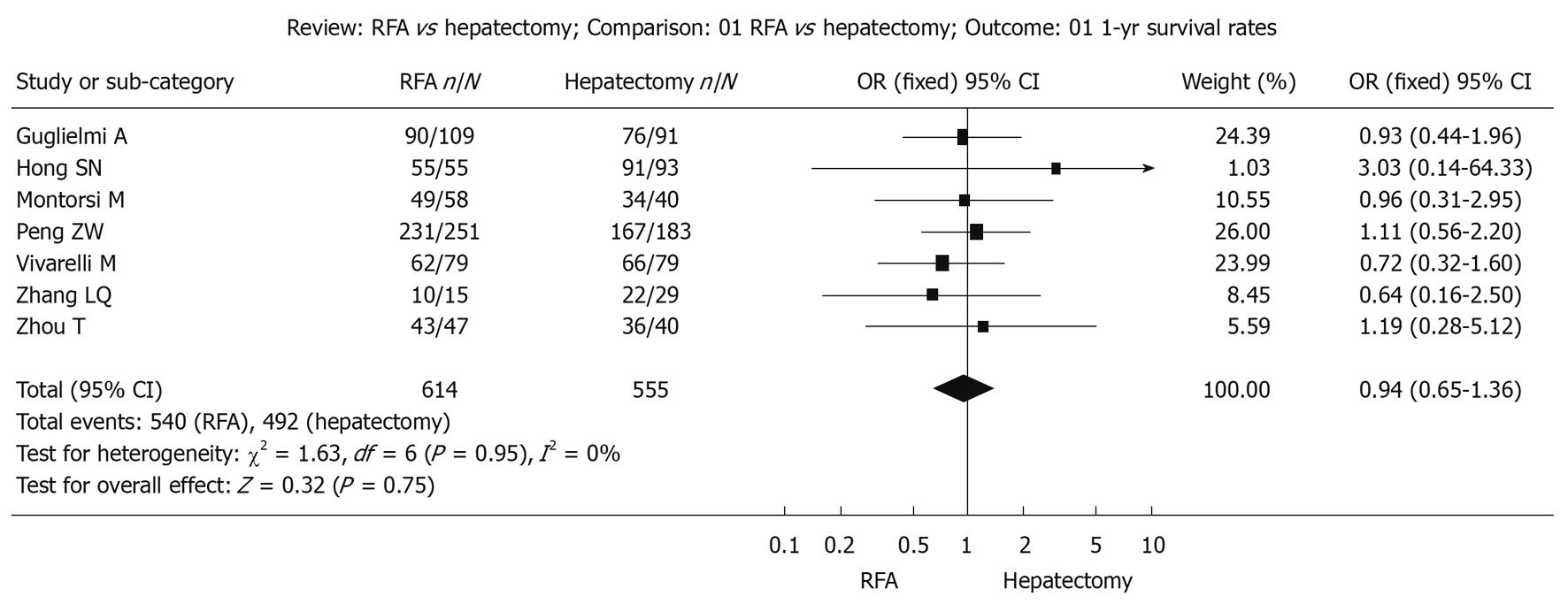
Survival during follow-up 1 year after treatment: The χ2 test of heterogeneity was highly significant (P = 0.95). Accordingly, a fixed-effect model was used. There was no difference in the 1-year overall survival rate between the RFA group (87.9%) and the surgical resection group (88.6%) with a combined OR of 0.94 (95% CI: 0.65 to 1.36, P = 0.75, Figure 1).
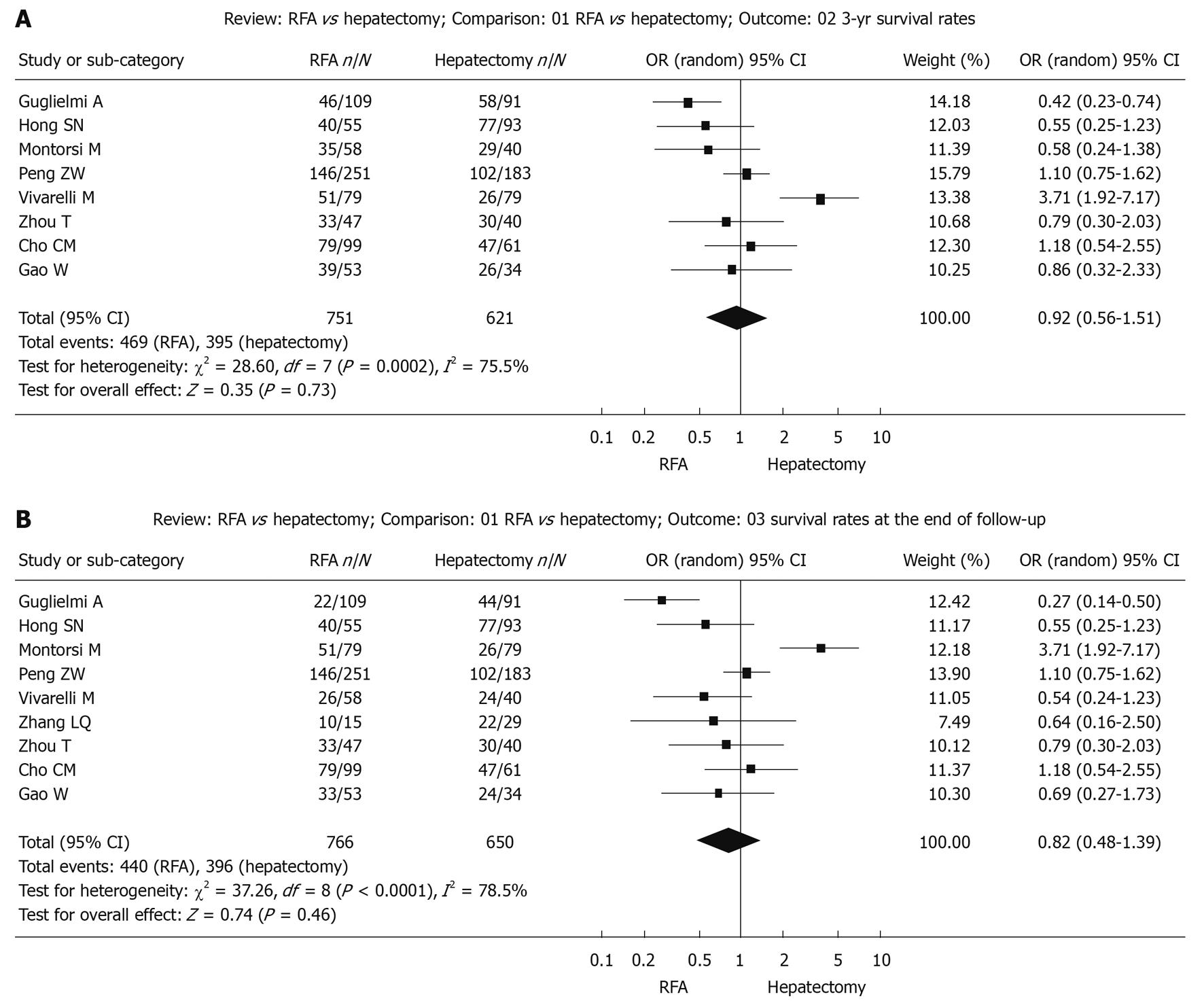
Survival during follow-up 3-year after treatment: The χ2 test of heterogeneity was highly significant (P = 0.0002). Accordingly, a random-effect model was used. There was no difference in the 3-year overall survival rate between the RFA group (62.5%) and the surgical resection group (63.6%) with a combined OR of 0.92 (95% CI: 0.56 to 1.51, P = 0.73, Figure 2A).
Survival up to the end of the follow-up period: The χ2 test of heterogeneity was highly significant (P < 0.0001). Accordingly, a random-effect model was used. There was no difference in overall survival rate at the end of follow-up after treatment with RFA (57.4%) compared with surgical resection (60.9%) with a combined OR of 0.82 (95% CI: 0.48 to 1.39, P = 0.46, Figure 2B).
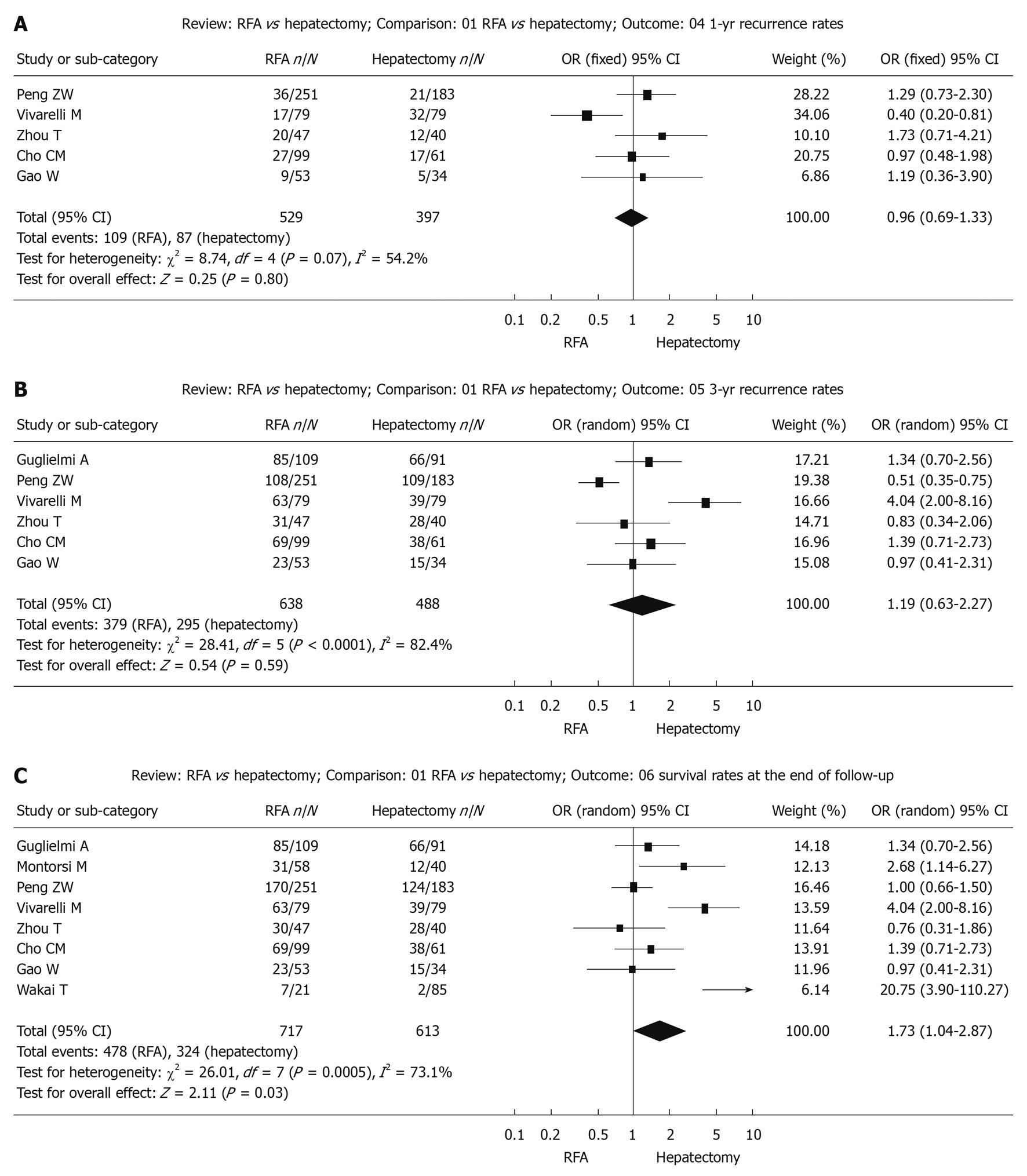
Recurrence during follow-up 1-year after treatment: The χ2 test of heterogeneity was highly significant (P = 0.07). Accordingly, a fixed-effect model was used. There was no difference in recurrence rate during follow-up 1-year after treatment between the RFA group (20.6%) and the surgical resection group (20.9%) with a combined OR of 0.96 (95% CI: 0.69 to 1.33, P = 0.80, Figure 3A).
Recurrence during follow-up 3-year after treatment: The χ2 test of heterogeneity was highly significant (P < 0.0001). Accordingly, a random-effect model was used. There was no difference in recurrence rate during follow-up 3-years after treatment between the RFA group (59.4%) and the surgical resection group (60.4%) with a combined OR of 1.19 (95% CI: 0.63 to 2.27, P = 0.59, Figure 3B).
Recurrence up to the end of the follow-up period: The χ2 test of heterogeneity was highly significant (P = 0.0005). Accordingly, a random-effect model was used. The recurrence rate up to the end of the follow-up period was significantly higher in the RFA group (66.7%) than in the surgical resection group (52.9%) with a combined OR of 1.73 (95% CI: 1.04 to 2.87, P = 0.03, Figure 3C).
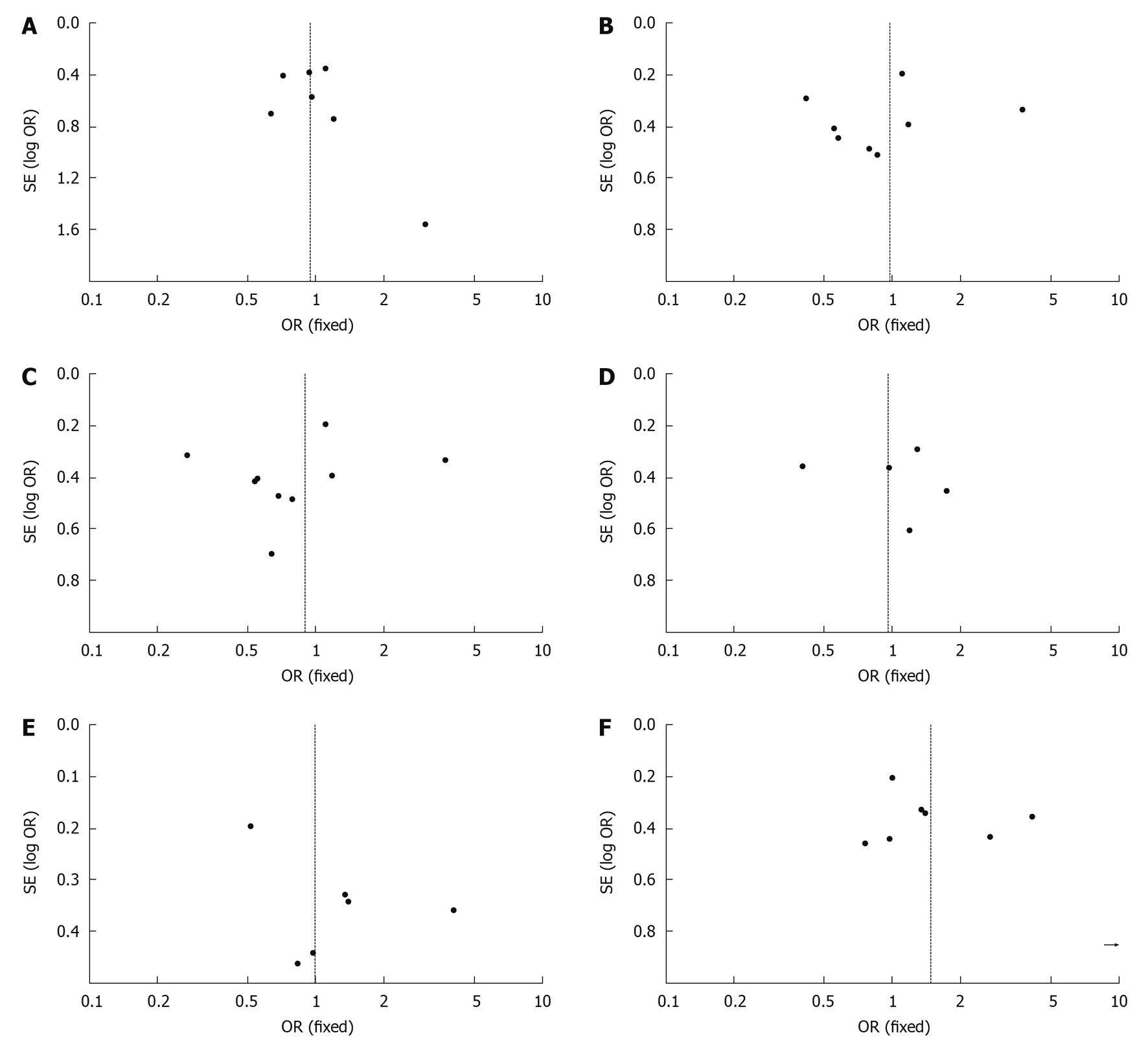
Publication bias may exist when no significant findings remain unpublished, thus artificially inflating the apparent magnitude of an effect.
Survival and recurrences following RFA or surgical resection for the treatment of small HCC were calculated by the fixed-effect model and random-effect model, respectively. The results were similar and the combined results were highly reliable.
Funnel plots of the study results are shown in Figure 4A-F. The funnel plots on survival and recurrence following RFA or surgical resection for the treatment of small HCC showed basic symmetry, which suggested no publication bias.
Hepatocellular carcinoma (HCC) is one of most common malignant tumors of the liver. According to the general condition of patients, tumor location and size and liver function status, surgery can include radical tumor resection, or liver surgery such as local excision. However, there are factors that limit the use of surgical resection. RFA is a relatively new treatment and is now performed more widely, because it results in large coagulated necrosis of the tumor, requires fewer treatment sessions, and achieves higher survival rates[18,19].
RFA has the potential to enhance the long-term survival rate of liver cancer patients worldwide and is of significant importance[20]. Research has indicated that more than 90% of the tumor can be completely destroyed and tumor recurrence in situ is effectively inhibited following RFA, which also achieved satisfactory short-term efficacy[21]. Long-term survival following RFA treatment was satisfactory in liver cancer patients as was liver function in those with A-class[22]. The efficacy of RFA was also shown to be related to Child-Pugh grading[23]. Compared with surgery, RFA did not cause significant liver function damage, had a lower rate of complications and was more affordable in terms of treatment costs. The results of this study showed that RFA did not decrease overall recurrences, but had no effect on survival in comparison with surgical resection (i.e. compared with surgical resection, RFA showed no significant difference in the short-term survival rate).
This review has some limitations. Funnel plots can be suggestive of publication bias with lack of negative small RCTs. However, a firm conclusion about bias is difficult to reach as the asymmetry of the funnel plot is minimal. In addition, funnel plots can show asymmetry for reasons other than publication bias. Therefore, our pooled OR might be an overestimate of the true effect. Due to data constraints, this meta-analysis could not analyze the quality of life score and was unable to carry out stratified analyses of other possible confounding factors. If the method is to be more effective, then larger samples and randomized controlled studies with longer follow-up are required[24]. Chinese article should also be chosen, because there are many patients with small HCC in China. A good meta-analysis requires these data. However, the conclusions of this study also need more detailed data to confirm the results. The search language was limited. The integrity of the data was affected to a certain extent.
In conclusion, with the development of RFA, when conditions permit and under technically assured circumstances, RFA can be performed percutaneously, laparoscopically or during laparotomy, and can partially replace surgical resection. For patients who do not have the opportunity or are unwilling to accept surgical treatment, RFA is an acceptable means of palliative care.
Over the last decade, radiofrequency thermal ablation (RFA) has established its place in the treatment algorithm of liver tumors. This meta-analysis was designed to evaluate survival and recurrence following RFA for the treatment of small hepatocellular carcinoma (HCC).
The study evaluated survival and recurrence following RFA for the treatment of HCC using a meta analysis of all relevant controlled studies.
The authors made a comprehensive search of studies dealing with small HCC treated with RFA. The studies were analyzed to determine survival and recurrence after RFA in these patients.
RFA is an effective technique for the treatment of small HCC and offers an alternative treatment method. This meta-analysis shows that RFA did not decrease overall recurrences, but had no effect on survival in comparison with surgical resection in a selected group of patients. Larger samples and randomized controlled studies with longer follow-up are required.
This is an interesting report of RFA vs surgical resection for HCC.
Peer reviewer: Toru Ishikawa, MD, Department of Gastroenterology, Saiseikai Niigata Second Hospital, Teraji 280-7, Niigata, Niigata 950-1104, Japan
S- Editor Tian L L- Editor Webster JR E- Editor Ma WH
| 1. | Befeler AS, Di Bisceglie AM. Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology. 2002;122:1609-1619. |
| 2. | Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210:655-661. |
| 3. | Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, Gazelle GS. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761-768. |
| 4. | Bonny C, Abergel A, Gayard P, Chouzet S, Ughetto S, Slim K, Rosenfeld L, Guillon R, Poincloux L, Bommelaer G. [Radiofrequency ablation of hepatocellular carcinoma in patients with cirrhosis]. Gastroenterol Clin Biol. 2002;26:735-741. |
| 5. | Jiang HC, Liu LX, Piao DX, Xu J, Zheng M, Zhu AL, Qi SY, Zhang WH, Wu LF. Clinical short-term results of radiofrequency ablation in liver cancers. World J Gastroenterol. 2002;8:624-630. |
| 6. | Curley SA, Izzo F, Ellis LM, Nicolas Vauthey J, Vallone P. Radiofrequency ablation of hepatocellular cancer in 110 patients with cirrhosis. Ann Surg. 2000;232:381-391. |
| 7. | Chen MS, Li JQ, Liang HH, Lin XJ, Guo RP, Zheng Y, Zhang YQ. [Comparison of effects of percutaneous radiofrequency ablation and surgical resection on small hepatocellular carcinoma]. Zhonghua Yixue Zazhi. 2005;85:80-83. |
| 8. | Peng ZW, Xu L, Chen MS, Gao HJ, Liang HH, Zhang HJ, Lin XJ, Li JQ. Percutaneous radiofrequency ablation and surgical resection for small hepa tocellular carcinoma: a comparative study. Zhongguo Shiyong Waike Zazhi. 2008;28:633-636. |
| 9. | Vivarelli M, Guglielmi A, Ruzzenente A, Cucchetti A, Bellusci R, Cordiano C, Cavallari A. Surgical resection versus percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma on cirrhotic liver. Ann Surg. 2004;240:102-107. |
| 10. | Zhang LQ, Wang JM. Clinical control study of radio frequency ablation and operation on complications and survival rate of liver cancer. Disan Junyi Daxue Xuebao. 2007;29:457-459. |
| 11. | Zhou T, Chou YD, Kong WT, Zhang WW, Ding YT. Comparing the effect of Radiofrequency ablation and surgical resection for the treatment of small hepatocellular. Gandan Waike Zazhi. 2007;15:424-427. |
| 12. | Guglielmi A, Ruzzenente A, Valdegamberi A, Pachera S, Campagnaro T, D’Onofrio M, Martone E, Nicoli P, Iacono C. Radiofrequency ablation versus surgical resection for the treatment of hepatocellular carcinoma in cirrhosis. J Gastrointest Surg. 2008;12:192-198. |
| 13. | Montorsi M, Santambrogio R, Bianchi P, Donadon M, Moroni E, Spinelli A, Costa M. Survival and recurrences after hepatic resection or radiofrequency for hepatocellular carcinoma in cirrhotic patients: a multivariate analysis. J Gastrointest Surg. 2005;9:62-67; discussion 67-68. |
| 14. | Hong SN, Lee SY, Choi MS, Lee JH, Koh KC, Paik SW, Yoo BC, Rhee JC, Choi D, Lim HK. Comparing the outcomes of radiofrequency ablation and surgery in patients with a single small hepatocellular carcinoma and well-preserved hepatic function. J Clin Gastroenterol. 2005;39:247-252. |
| 15. | Wakai T, Shirai Y, Suda T, Yokoyama N, Sakata J, Cruz PV, Kawai H, Matsuda Y, Watanabe M, Aoyagi Y. Long-term outcomes of hepatectomy vs percutaneous ablation for treatment of hepatocellular carcinoma < or = 4 cm. World J Gastroenterol. 2006;12:546-552. |
| 16. | Cho CM, Tak WY, Kweon YO, Kim SK, Choi YH, Hwang YJ, Kim YI. [The comparative results of radiofrequency ablation versus surgical resection for the treatment of hepatocellular carcinoma.]. Korean J Hepatol. 2005;11:59-71. |
| 17. | Gao W, Chen MH, Yan K, Yang W, Sun Y, Xing BC. Therapeutic effect of radiofrequency ablation in unsuitable operative small hepatocellular carcinoma. Zhongguo Yixue Yingxiang Jishu Zazhi. 2007;23:254-257. |
| 18. | Lencioni R, Crocetti L. Radiofrequency ablation of liver cancer. Tech Vasc Interv Radiol. 2007;10:38-46. |
| 19. | Valls C, Ruiz S, Barrau V, Burdío F, Lladó L, Figueras J, Vilgrain V. [Radiofrequency ablation of hepatic tumors]. Radiologia. 2006;48:53-69. |
| 20. | Netto GJ, Altrabulsi B, Katabi N, Martin P, Burt K, Levy M, Sanchez E, Watkins DL, Jennings L, Klintmalm G. Radio-frequency ablation of hepatocellular carcinoma before liver transplantation: a histologic and ‘TUNEL’ study. Liver Int. 2006;26:746-751. |
| 21. | Kuvshinoff BW, Ota DM. Radiofrequency ablation of liver tumors: influence of technique and tumor size. Surgery. 2002;132:605-611; discussion 611-612. |
| 22. | Yang LT, Guo JM, Cheng XD, Zhou LX, Qian CW. Effect of Radiofrequency Ablation for Hepatocellular Carcinomas. Zhongguo Zhongliu. 2004;13:392-394. |
| 23. | Yan K, Wang YB, Chen MH, Gao W, Yang W, Dai Y, Yin SS. [Prognostic factors on outcome of radiofrequency ablation of 172 primary hepatic tumors]. Zhonghua Yixue Zazhi. 2005;85:2322-2326. |
| 24. | Lin SM, Chu CM. Percutaneous tumor ablation or surgical resection for small hepatocellular carcinoma? J Gastroenterol Hepatol. 2007;22:1561-1564. |