Published online Jun 21, 2010. doi: 10.3748/wjg.v16.i23.2918
Revised: April 1, 2010
Accepted: April 8, 2010
Published online: June 21, 2010
AIM: To investigate whether a noninvasive measurement of tissue strain has a potential usefulness for management of nonalcoholic steatohepatitis (NASH).
METHODS: In total 26 patients, 23 NASHs and 3 normal controls were enrolled in this study. NASH was staged based on Brunt criterion. At a region of interest (ROI), a shear wave was evoked by implementing an acoustic radiation force impulse (ARFI), and the propagation velocity was quantified.
RESULTS: Shear wave velocity (SWV) could be reproducibly quantified at all ROIs in all subjects except for 4 NASH cases, in which a reliable SWV value was not calculated at several ROIs. An average SWV of 1.34 ± 0.26 m/s in fibrous stage 0-1 was significantly slower than 2.20 ± 0.74 m/s and 2.90 ± 1.01 m/s in stages 3 and 4, respectively, but was not significantly different from 1.79 ± 0.78 m/s in stage 2. When a cutoff value was set at 1.47 m/s, receiver operating characteristic analysis showed significance to dissociate stages 3 and 4 from stage 0-1 (P = 0.0092) with sensitivity, specificity and area under curve of 100%, 75% and 94.2%, respectively. In addition, the correlation between SWV and hyaluronic acid was significant (P < 0.0001), while a tendency toward negative correlation was observed with serum albumin (P = 0.053).
CONCLUSION: The clinical implementation of ARFI provides noninvasive repeated evaluations of liver stiffness at an arbitrary position, which has the potential to shed new light on NASH management.
- Citation: Osaki A, Kubota T, Suda T, Igarashi M, Nagasaki K, Tsuchiya A, Yano M, Tamura Y, Takamura M, Kawai H, Yamagiwa S, Kikuchi T, Nomoto M, Aoyagi Y. Shear wave velocity is a useful marker for managing nonalcoholic steatohepatitis. World J Gastroenterol 2010; 16(23): 2918-2925
- URL: https://www.wjgnet.com/1007-9327/full/v16/i23/2918.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i23.2918
Nonalcoholic fatty liver diseases (NAFLD) are a pandemic over the globe, especially in the developed countries depending on a high calorie diet and sedentary life style. Nonalcoholic steatohepatitis (NASH) is a subgroup of NAFLD, in which chronic necroinflammatory response causes fiber accumulation in the liver[1]. Regardless of the etiology, accumulation of fiber in the liver is correlated with a deterioration of functional liver reserve. By semiquantifying the density of fibrosis in the liver, histological evaluation is a standard strategy used to confirm the presence of chronic necroinflammation, and to evaluate a degree of the pathophysiological extent of liver damage[2]. Unfortunately, however, it is impractical to conduct liver biopsy in a large population of NAFLD and to repeat it through a course of NASH.
Several markers have been reported to be useful for the prediction of liver fibrosis, which can be categorized into three groups. One is a physical marker such as FibroScan®: transient elastography (TE)[3] and a digital quantification using contrast magnetic resonance imaging (MRI)[4]. The others are serum markers and can be further divided into two groups; direct and indirect markers. Serum markers are affected in both the inside and outside of the liver, while MRI may not be affordable for a large NAFLD cohort. TE is reported to be inaccurate for cases with higher body mass index (BMI) and hepatic steatosis[5].
Acoustic radiation force is a method exploiting energy in transmitted acoustic pulses to deliver a localized, precise force sufficient in magnitude to induce subtle tissue displacements[6]. The induced displacements can be tracked using conventional ultrasonic means and are indicative of local tissue mechanical properties[7]. Acoustic radiation force-based methods have been demonstrated for differentiating tissue structure via a mechanical property in various applications including the liver[8]. Here we report quantification of shear wave velocity (SWV) comparing with histology and serum biomarkers in NASH cases under ultrasound imaging coupled with the first clinical implementation of acoustic radiation force impulse (ARFI). The usefulness and limitations of SWV measurement are discussed.
Twenty six cases, consisting of 23 patients suffering from NASH and 3 normal controls, were enrolled in this study. NASH was diagnosed when each of the following requirements were met; (1) abnormal values of alanine aminotransferase; (2) no positive results for hepatitis B surface antigen (HBsAg), anti-hepatitis C virus (anti-HCV), and anti-mitochondrial antibody; (3) histological findings consistent with NASH; and (4) neither suspicious drug usage, alcohol abuse, hereditary diseases nor any other clinical manifestations causing liver cell damage. Three normal controls were patients with each of chronic cholecystitis, chronic pancreatitis, and gallbladder polyps, who fulfilled all requirements for NASH diagnosis except for abnormal values of the transaminase and histological abnormalities, which were not evaluated. Two expert pathologists independently evaluated liver biopsy specimens and judged fibrous staging and inflammatory grading on the basis of Brunt criterion[9]. Informed consent was obtained from each patient, and the study protocol conformed to ethical guidelines of the 2008 Declaration of Helsinki as reflected in a prior approval by Niigata University Graduate School of Medical and Dental Sciences Human Research Committee.
HBsAg and anti-HCV antibody were detected by a chemiluminescence immunoassay using ARCHITECT HBsAg QT and ARCHITECT HCV (Abbott Japan Co. Ltd., Chiba, Japan), respectively. Anti-mitochondrial antibody was quantified in sera using commercial kits of AMA; FluoroAID-1 test (MBL Co. Ltd., Nagoya, Japan). Hyaluronic acid was measured using a latex agglutination method, in which normal controls measure less than 50 ng/mL.
SWV was measured using an ACUSON S2000 ultrasound system (SIEMENS Medical Solutions Inc., CA, USA). A single ultrasonic transducer array is used to both apply temporally short localized radiation forces within tissue and to track the resulting tissue dynamic response through time. During a regular ultrasound observation, region-of-interest (ROI) is set as a rectangle, and acoustic pulses are radiated to make tissues displace downward beside ROI. Once tissues are displaced, the shear stress starts to restore the tissue to its previous position and generate shear waves, which travel perpendicular to the direction of the acoustic push pulse in the process. The machine continuously monitors tissue positions in ROI and automatically calculates the speed of the wave in m/s unit. Fundamentally, SWV can be measured at any portion in the liver as long as the ROI can be placed under ultrasound imaging except for a position where the acoustic pulse cannot effectively displace tissues, such as deeper than 5 cm from a probe, or inside a vasculature, cyst or gallbladder. Using an ACUSON S2000, one can switch from regular ultrasound imaging to SWV measurements seamlessly and repeatedly with neither special preparation nor technique.
Categorical data was compared using ANOVA analysis among four fibrosis stages. Fisher’s exact test was employed to compare the frequency in a contingency table of each factor consisting of BMI-Age-Alanine aminotransferase (ALT)-Triglyceride (TG) (BAAT) score[10]. Correlations between SWV and variable parameters were quantified by calculating the Spearman correlation coefficient. In order to judge the clinical usefulness of fibrosis indicators, an area under a receiver operating characteristic curve (AUROC) was calculated. All analyses were performed using GraphPad Prism 4 software (GraphPad Software, Inc. La Jolla, USA), and a two-sided P-value less than 0.05 was considered statistically significant.
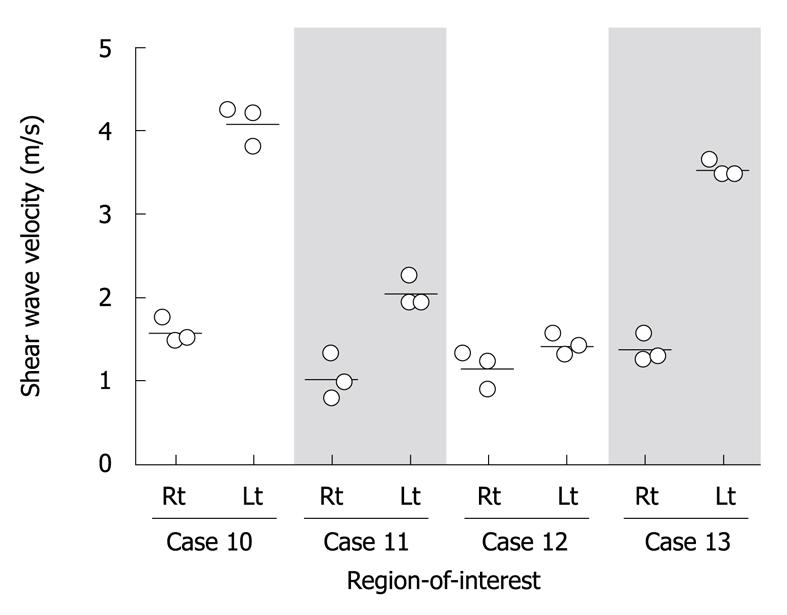
Aiming to evaluate reliability in SWV measurement, ROI was placed at each of the right and left lobes, and SWV was independently measured three times at the same lobe in four patients with fibrosis stage 3, cases 10 to 13. SWV was reproducibly determined at each ROI as the average coefficient of variance was 11.9% ± 8.0% (Figure 1). The speeds, however, largely varied between right and left lobes. These results strongly indicate that a repeated measurement may not be necessary at the same ROI for compensation of an implementation error, but is required by applying various ROIs throughout the liver in order to evaluate an average rigidity of a whole liver.
According to inconsistent SWVs between the left and right lobes, SWV measurements were performed by placing four ROIs, which were located in each of the four segments; right posterior, right anterior, left medial and left lateral segments. Among the 23 NASH cases, a reliable SWV could not be calculated in 4 cases; listed as cases 23 to 26 in Table 1. Case 24 carried massive ascites and the others had a BMI of over 30 kg/m2. In terms of BMI, SWV could be measured in all cases with BMI less than 30 kg/m2, whereas SWV could not be measured in 3 out of 10 cases with BMI of more than 30 kg/m2. None of the ROIs that could not return a reliable SWV value were located in the right segments, but in the left. The 4 cases missing at least one of four SWV values were excluded from further analyses.
| No. | Diagnosis | Age (yr) | Gender | BMI (kg/m2) | Stage1 | Grade1 | SWV (m/s) | ALB (g/dL) | AST (IU/L) | ALT (IU/L) | TG (mmol/L) | Plt (× 104/mm3) | PT (%) | HA (ng/mL) | BAAT | NFS |
| 1 | Chr. cholecystitis | 73 | F | 20.8 | 1.08 | 4.4 | 22 | 21 | 1.31 | 17.9 | 1 | -1.2 | ||||
| 2 | Chr. pancreatitis | 77 | M | 16.9 | 1.23 | 4.4 | 22 | 10 | 0.43 | 18.3 | 1 | -0.3 | ||||
| 3 | Gall bladder polyp | 80 | M | 17.1 | 1.36 | 4.0 | 21 | 20 | 0.72 | 19.7 | 1 | -1.3 | ||||
| 4 | NASH | 14 | M | 22.2 | 1 | 1 | 1.69 | 4.9 | 102 | 255 | 1.74 | 19.1 | 100 | 11 | 2 | -4.4 |
| 5 | NASH | 18 | M | 35.1 | 2 | 3 | 1.73 | 4.9 | 46 | 84 | 1.83 | 24.0 | 104 | 13 | 3 | -3.5 |
| 6 | NASH | 61 | F | 27.7 | 2 | 2 | 2.97 | 4.3 | 61 | 70 | 0.93 | 20.6 | 104 | 161 | 2 | -1.5 |
| 7 | NASH | 41 | F | 40.8 | 2 | 3 | 1.05 | 4.6 | 83 | 156 | 1.43 | 24.9 | 100 | 11 | 2 | -2.1 |
| 8 | NASH | 15 | M | 27.9 | 2 | 2 | 1.14 | 5.4 | 95 | 185 | 0.93 | 27.2 | 116 | 10 | 1 | -4.0 |
| 9 | NASH | 39 | F | 20.8 | 2 | 3 | 2.06 | 3.3 | 72 | 28 | 4.17 | 17.1 | 75 | 29 | 1 | -0.1 |
| 10 | NASH | 53 | M | 26.6 | 3 | 2 | 2.55 | 4.3 | 91 | 129 | 2.27 | 25.3 | 108 | 102 | 3 | -1.5 |
| 11 | NASH | 51 | F | 26.6 | 3 | 3 | 1.57 | 4.3 | 49 | 75 | 1.78 | 31.4 | 126 | 60 | 3 | -2.4 |
| 12 | NASH | 56 | F | 30.5 | 3 | 2 | 1.63 | 4.2 | 53 | 65 | 0.89 | 9.1 | 82 | 34 | 3 | 0.1 |
| 13 | NASH | 43 | M | 31.6 | 3 | 1 | 2.24 | 4.4 | 36 | 33 | 1.26 | 7.8 | 87 | 92 | 1 | 1.2 |
| 14 | NASH | 20 | M | 22.2 | 3 | 3 | 2.03 | 4.7 | 156 | 243 | 2.57 | 17.7 | 74 | 17 | 2 | -3.6 |
| 15 | NASH | 46 | M | 31.1 | 3 | 2 | 3.80 | 4.6 | 28 | 26 | 2.46 | 20.6 | 100 | 412 | 2 | -0.6 |
| 16 | NASH | 66 | F | 24.1 | 3 | 3 | 2.17 | 3.9 | 128 | 68 | 1.22 | 19.8 | 97 | 231 | 2 | -0.3 |
| 17 | NASH | 11 | M | 21.8 | 3 | 3 | 1.60 | 4.7 | 39 | 68 | 0.86 | 27.1 | 95 | 14 | 0 | -5.3 |
| 18 | NASH | 75 | F | 34.0 | 4 | 1 | 4.01 | 3.7 | 43 | 40 | 1.39 | 11.7 | 86 | NT | 2 | 2.5 |
| 19 | NASH | 82 | M | 23.9 | 4 | 2 | 3.35 | 3.7 | 67 | 48 | 0.83 | 12.3 | 72 | 259 | 1 | 2.1 |
| 20 | NASH | 70 | F | 22.1 | 4 | 2 | 1.95 | 4.7 | 44 | 33 | 1.60 | 18.4 | 79 | 92 | 1 | -0.1 |
| 21 | NASH | 71 | F | 32.0 | 4 | 2 | 3.48 | 2.9 | 33 | 21 | 0.78 | 11.2 | 62 | NT | 2 | 3.3 |
| 22 | NASH | 71 | M | 27.0 | 4 | 2 | 1.73 | 2.9 | 36 | 22 | 0.73 | 6.4 | 59 | NT | 1 | 2.4 |
| 23 | NASH | 74 | F | 31.1 | 4 | |||||||||||
| 24 | NASH | 75 | F | 24.1 | 4 | |||||||||||
| 25 | NASH | 73 | F | 30.2 | 3 | |||||||||||
| 26 | NASH | 17 | M | 37.8 | 3 | |||||||||||
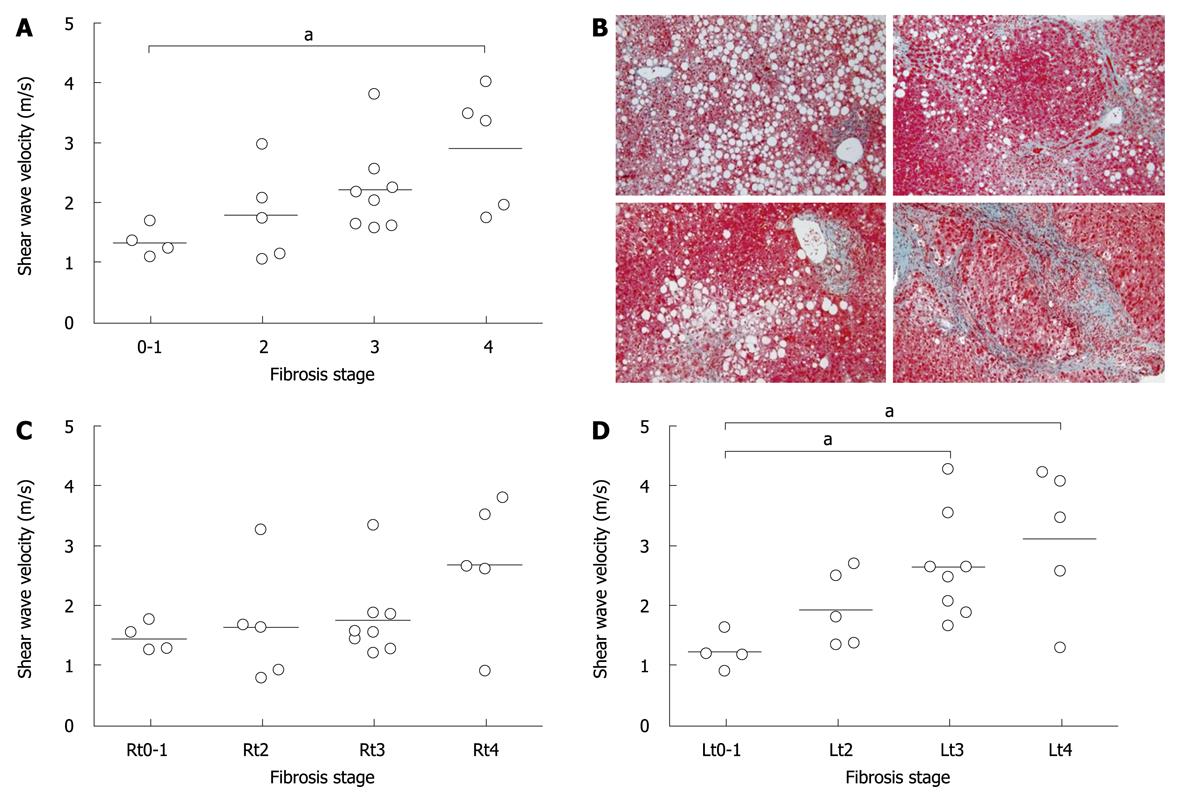
An average of 4 SWVs in each case was compared among the fibrosis stages. When controls were designated as a stage 0, SWV was significantly correlated with fibrosis stages (P = 0.0018, r = 0.63). As only one case with fibrosis stage 1 was enrolled, 3 controls were combined with stage 1 and subjected to further analysis as one group of stage 0-1. The velocities in stages 0-1, 2, 3, and 4 were 1.34 ± 0.26, 1.79 ± 0.78, 2.20 ± 0.74, and 2.90 ± 1.01, respectively, and were significantly different among groups (Figure 2A, P = 0.043). Dunn’s multiple comparison revealed that there was a significant difference between stages 0-1 and 4 (P < 0.05). When the velocity was compared in the right or left lobe, there was no significant difference in the right lobe (P = 0.31, Figure 2C), but there was a difference in the left lobe (P = 0.02, Figure 2D), in which SWVs were significantly different not only between stages 0-1 and 4, but also between 0-1 and 3 (P < 0.05, P < 0.05, respectively). There was no significant difference in SWV among inflammation activity grades (P = 0.12).
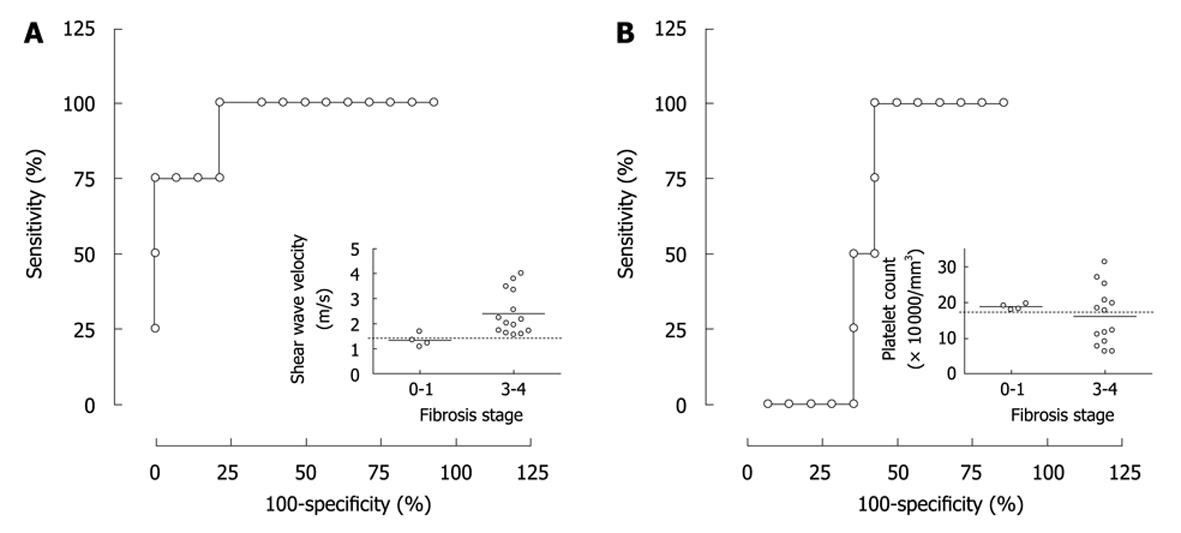
Because it was suggested that SWV has a potential to discriminate fibrosis stage 3 or more from stage 0-1, a probability of the discrimination was evaluated using ROC analysis. SWV showed sensitivity and specificity of 100% and 75%, respectively when a speed of 1.47 m/s was selected as a cut-off value leading to a significant AUROC of 94.2% (Figure 3A, P = 0.0092). In contrast, platelet counts did not lead to a significant AUROC to distinguish stages 3 and 4 from 0-1 in our population (Figure 3B, P = 0.52, AUROC = 61%). Because several combinatorial systems were reported for identifying liver fibrosis in NAFLD, BAAT[10] and NAFLD fibrosis score[11] were calculated (Table 1). In the results, AUROCs of BAAT and NAFLD fibrosis score were 76.9% and 72.1%, respectively, and neither of these systems were significant in this setting (P = 0.16 and P = 0.18, respectively). Furthermore, none of the factors making up BAAT score; BMI, age, ALT and TG, showed a different frequency between fibrosis stage 0-1 and stages 3 and 4 (P = 1.0, 0.26, 1.0 or 1.0, respectively).
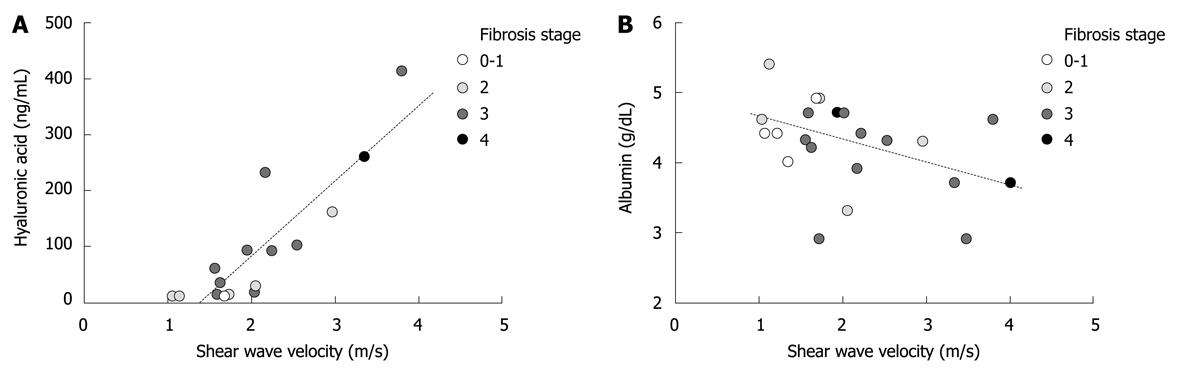
Next, a correlation was analyzed between SWV and serum markers. Hyaluronic acid was measured in sixteen out of nineteen NASH cases and showed a tight positive correlation with SWV (Figure 4A, P < 0.0001, r = 0.84). In addition, albumin concentration in the serum tended to be correlated with SWV in a negative fashion (Figure 4B, P = 0.053, r = -0.42). On the other hand, prothrombin time, age and BMI were not significantly associated with SWV (P = 0.18, P = 0.42 and P = 0.46, respectively).
Fundamentally, tissue displacements generated by acoustic push pulse used in ARFI are inversely related to tissue stiffness, while tissue recovery response is related to tissue viscoelastic properties[12]. In addition, SWV is positively correlated with the major mechanical properties indicating material rigidity[13]. Thus, assuming the liver is a linear, isotropic, elastic body, SWV is an indicative factor of tissue rigidity. Theoretically, the stiffer the tissue, the faster the shear wave will be propagated. Consistently, in this study SWV was correlated with histological progression of fibrosis and representative serum markers for fibrosis such as hyaluronic acid and collagen type IV (data not shown) as well. Although ARFI imaging has been used for delineating tissue structure via mechanical properties in numerous applications including chronic liver diseases[14,15], this is the first report to quantify SWV by implementing ARFI in the clinic and show a significant correlation with fiber deposition focusing on the NASH liver.
As a noninvasive methodology to quantify tissue stiffness in the liver, TE had been applied in the clinic[16]. TE mounts an ultrasound transducer on the axis of a vibrator, which transmits vibrations to propagate elastic shear waves. The major differences between TE and ARFI implemented SWV measurement are a real-time imaging capability, target size and target position. TE utilizes a vibration at audibility range and targets tissues without imaging supports as a 10 mm wide and 40 mm long cylinder, which is required to lie between 25 and 65 mm below the skin and to be free from large vascular structures. Given those requirements, the area available for TE is limited to a portion of right lobe, and practically it is difficult to compare the stiffness at different parts of the liver. On the other hand, S2000 radiates the push pulse at MHz order to excite tissue displacement and scrutinizes SWV at ROI, which is substantially smaller (7 mm × 10 mm) and can be precisely placed at any position throughout the liver under real-time image guidance as long as it is not deeper than 5 cm from the probe. Because the interface evokes ultrasound-guided liver biopsy, “acoustic biopsy” may be coined for this technology. The major advantage of acoustic biopsy is the feasibility to be able to perform as many in time and space without a risk to patients. In this study SWV measurements were performed in four different segments at the same time point and revealed that SWV largely varied in different portions of the liver, while reproducible values were obtained at each ROI. Although the small number of subjects employed in this study limited the implication, these data support the idea that fibrogenesis and/or fibrolysis heterogeneously take place in the liver[17]. It must give us critical insight into the process of liver fibrosis to take acoustic biopsies from a wide area of the liver at different time points.
In terms of measurement failures, it was reported that TE frequently failed to compute liver stiffness in patients with massive ascites or BMI over 28 kg/m2[5], and SWV measurements showed a similar tendency. One hypothesis is that the tissue displacement cannot be efficiently induced by radiation of acoustic push pulse through massive ascites or dense subcutaneous fat layers. However, it is noteworthy that SWV could be measured in right lobes of the four failed cases. All ROIs that returned ineffective SWV were located in left lobes. Although the exact reasons are not clear, oscillation of the liver due to heart beat may be an important factor to hamper accurate measurements. It has been shown that SWV in stiffer livers is associated with larger variability and smaller overall displacements[8], which suggest susceptibility of this technology to motion artifacts. A prospective study is required to clarify whether SWV in right lobes can be a useful indicator for liver fibrosis in those cases.
As for variability of SWV between right and left lobes, a considerable sampling variability was reported in liver biopsy specimens from patients with chronic hepatic diseases[18]. The concordance between two biopsy specimens that were simultaneously obtained from the same individual was reported to be moderate to low in terms of perisinusoidal fibrosis and other histological pathologic features of NAFLD[19]. The interlobar difference of SWV that was observed in this study seems to be consistent with the sampling variability suggesting nonhomogenous progression of NAFLD. Because sequential biopsies from multiple sites are practically impossible, longitudinal measurements of SWV at numerous locations of the liver in a large NAFLD cohort would be able to elucidate distribution of the pathological involvement in time and space.
Evaluation of liver fibrosis is aimed at two aspects of NASH management; one as a diagnostic tool and the other as a scrutiny of disease progression. Although histopathological examination is the current gold standard for evaluation of liver fibrosis, there are serious concerns for this strategy such as its invasive nature, sampling errors and diagnostic variability among observers[2]. To circumvent these limitations, surrogate serum markers have been reported such as BAAT score[10], NAFLD fibrosis score[11], hyaluronic acid[20], platelet count[21], and NashTest[22]. TE and MRI were also reported to show satisfying sensitivity and specificity to surrogate histological evaluation[4,16]. All noninvasive methodologies, however, have been shown to be effective in predicting advanced diseases rather than fibrogenic evolution, which is a hallmark to distinguish NASH from other NAFLD. Furthermore, it is impractical to perform an MRI study on a great number of NAFLD patients. Because SWV was not significantly different between fibrosis stages 0-1 and 2, and because the cohort in this study did not include NAFLD cases without fiber accumulation, the significance of SWV measurement in NASH diagnosis is also unclear. A further analysis should be conducted on a larger sample group including NAFLD cases without liver fibrosis to elucidate this issue.
As far as disease progression is concerned, platelet count, which has been confirmed as a useful surrogate marker for fibrosis in chronic hepatitis C[23], did not show significant AUROC in our cohort. A regulation of platelet count may be different between NASH and chronic hepatitis C. Although the NAFLD fibrosis score clearly distinguished fibrosis stage 4 from stages 0-1 (data not shown), AUROC was not able to distinguish stages 3 and 4 from stage 0-1, and there was no significant correlation between progression of liver fibrosis and the score, either. In BAAT, neither the score nor each factor used to calculate the score could discriminate fibrosis stages 0-1 from stages 3 and 4. Values of serum markers can be affected by extra hepatic conditions and/or a type of background diseases[24]. Furthermore, fibrosis markers have to be investigated over time to see pathophysiological progression. Serum markers at different time points would be affected by difference of systemic situation. Thus, it is most suspicious that the different populations among studies might cause different discrimination abilities in those indirect serum markers. As for the NashTest, it requires 13 parameters including α2-macroglobulin, apolipoprotein A1, and haptoglobin, which are not covered by public health insurance in Japan for patients with liver dysfunction, thus it is not affordable for significant numbers of patients. On the other hand, SWV tended to get faster along the progression of fibrosis in a stepwise fashion. Because a standard histopathological analysis has the same difficulty in discriminating adjacent stages of fibrosis[18], the usefulness in stepwise discrimination should be evaluated by a longitudinal study in a larger cohort.
There are a few reports that mention liver stiffness in association with biochemical properties of functional liver reserve. Kawamoto et al[25] revealed positive correlations of TE with prothrombin time, platelet count and pre-albumin. Masuzaki et al[26] also reported a tendency of positive correlations with prothrombin activity, platelet count, and albumin concentration. In both reports the subjects were patients with chronic viral hepatitis type B and/or C. Interestingly, in our NASH cohort, prothrombin activity and platelet count were not significantly correlated with SWV, whereas serum albumin showed a tendency towards negative correlation. Although it is not difficult to suppose that TE/SWV and the direct markers for fibrosis will show positive correlation regardless of viral hepatitis or NASH, as in the case of hyaluronic acid, the indirect markers would be regulated in a different way depending on the disease responsible. The association between SWV and biochemical properties may implicate a significance of SWV measurement as a prognostic indicator.
The substitution of histopathological analysis of liver biopsy with a surrogate noninvasive marker would have a tremendous impact on both public health and health economics. Our data suggest that SWV measurements in implementation of ARFI have considerable potential to confirm or to exclude cirrhosis. Furthermore, this technology provides a repeated data acquisition for tissue mechanical properties from precise points throughout the liver. The actual longitudinal evaluation of fibrosis at different areas over the liver in a larger NAFLD population may prove not only useful for staging of NASH but also the process of fiber accumulation and value as a prognostic indicator.
Nonalcoholic steatohepatitis (NASH) is manifestation of metabolic syndrome in the liver and is a pandemic over the globe especially in the developed countries, based on a high calorie diet and sedentary life style. As in the other types of chronic hepatitis, collagen fibers continuously accumulate in the liver through the course of NASH toward cirrhosis and hepatocellular carcinoma development. Histological evaluation is a current gold standard for quantification of the fiber deposition. A tiny biopsy specimen, however, leads to considerable variability and is practically difficult to obtain repeatedly from multiple sites. Several surrogate markers have been developed for evaluation of fiber accumulation in a noninvasive way. Unfortunately, however, so far no methodology can satisfy both specificity for the liver and applicability for multiple sites.
The significance of serum surrogate markers for histological evaluation of fibrosis has been reported, with examples including BMI-Age-Alanine aminotransferase-Triglyceride score, nonalcoholic fatty liver diseases (NAFLD) fibrosis score, hyaluronic acid, platelet count, and NashTest. Transient elastography and magnetic resonance imaging were also reported to show satisfying sensitivity and specificity to surrogate the histological evaluation. None of the serum markers, however, are specific for the liver but are affected by systemic involvement. Transient elastography requires a relatively large area to measure tissue rigidity, which prevents multifocal measurements. Magnetic resonance imaging is too laborious and expensive to target a large NAFLD cohort.
Using ACUSON S2000 ultrasound system, the research team led by Suda T from Department of Gastroenterology and Hepatology of Niigata University investigated the efficacy of shear wave velocity (SWV) measurement in the evaluation of NASH pathophysiology. ACUSON S2000 is the world’s first ultrasound system implementing acoustic radiation force impulse to enable SWV measurement in the liver. The analyses revealed significant positive correlation between SWV and the histological fibrous stages and suggested the possibility to distinguish mild fibrosis from severe fibrosis or cirrhosis with substantial accuracy. Furthermore, the correlations of SWV with biochemical markers such as hyaluronic acid or albumin were presented. This is the first report suggesting that SWV can be reproducibly measured in NASH and varies through the liver.
Due to the non-invasive and easily accessible nature of SWV measurement, this technology makes it possible to conduct a longitudinal evaluation of liver rigidity at multiple sites of the liver in a large cohort. The actual longitudinal evaluation in a larger NAFLD population may prove not only useful for fibrous staging of NASH but also for the process of fiber accumulation and value as a prognostic indicator.
SWV is the speed of a transverse wave propagating perpendicular to the direction of tissue displacement. Assuming the liver is a linear, isotropic, elastic body, SWV is an indicative factor of tissue rigidity. Theoretically, the stiffer the liver, the faster the shear wave will be propagated. Acoustic radiation force impulse is a phenomenon due to energy transfer associated with acoustic frequency higher than tissue attenuation capability. The energy results in a momentum transfer generating a force that causes tissue displacement.
This is a well-conducted study that evaluates the usefulness of SWV in quantifying liver fibrosis in patients with NASH.
Peer reviewers: Philip Abraham, Professor, Consultant Gastroenterologist and Hepatologist, P. D. Hinduja National Hospital and Medical Research Centre, Veer Savarkar Marg, Mahim, Mumbai 400 016, India; Mercedes Susan Mandell, MD, PhD, Department of Anesthesiology, University of Colorado Health Sciences Ctr., 12401 E. 17th Ave, B113 Aurora, CO 80045, United States; Matilde Bustos, MD, PhD, Hepatology and Gene Therapy Area, CIMA (Center for Applied Medical Research), Avda Pio XII, 55, 31008 Pamplona, Spain
S- Editor Wang JL L- Editor O’Neill M E- Editor Ma WH
| 1. | Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology. 2008;134:1682-1698. |
| 2. | Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49:1017-1044. |
| 3. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. |
| 4. | Aguirre DA, Behling CA, Alpert E, Hassanein TI, Sirlin CB. Liver fibrosis: noninvasive diagnosis with double contrast material-enhanced MR imaging. Radiology. 2006;239:425-437. |
| 5. | Foucher J, Castéra L, Bernard PH, Adhoute X, Laharie D, Bertet J, Couzigou P, de Lédinghen V. Prevalence and factors associated with failure of liver stiffness measurement using FibroScan in a prospective study of 2114 examinations. Eur J Gastroenterol Hepatol. 2006;18:411-412. |
| 6. | Sarvazyan AP, Rudenko OV, Swanson SD, Fowlkes JB, Emelianov SY. Shear wave elasticity imaging: a new ultrasonic technology of medical diagnostics. Ultrasound Med Biol. 1998;24:1419-1435. |
| 7. | Nightingale K, Soo MS, Nightingale R, Trahey G. Acoustic radiation force impulse imaging: in vivo demonstration of clinical feasibility. Ultrasound Med Biol. 2002;28:227-235. |
| 8. | Palmeri ML, Wang MH, Dahl JJ, Frinkley KD, Nightingale KR. Quantifying hepatic shear modulus in vivo using acoustic radiation force. Ultrasound Med Biol. 2008;34:546-558. |
| 9. | Brunt EM. Nonalcoholic steatohepatitis: definition and pathology. Semin Liver Dis. 2001;21:3-16. |
| 10. | Ratziu V, Giral P, Charlotte F, Bruckert E, Thibault V, Theodorou I, Khalil L, Turpin G, Opolon P, Poynard T. Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117-1123. |
| 11. | Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846-854. |
| 12. | Nightingale KR, Palmeri ML, Bouchard R, Trahey GE. Acoustic radiation force impulse imaging: a parametric analysis of factors affecting image quality. IEEE Ultrasonics Symposium 2003. |
| 13. | Palmeri ML, Sharma AC, Bouchard RR, Nightingale RW, Nightingale KR. A finite-element method model of soft tissue response to impulsive acoustic radiation force. IEEE Trans Ultrason Ferroelectr Freq Control. 2005;52:1699-1712. |
| 14. | Lupsor M, Badea R, Stefanescu H, Sparchez Z, Branda H, Serban A, Maniu A. Performance of a new elastographic method (ARFI technology) compared to unidimensional transient elastography in the noninvasive assessment of chronic hepatitis C. Preliminary results. J Gastrointestin Liver Dis. 2009;18:303-310. |
| 15. | Takahashi H, Ono N, Eguchi Y, Eguchi T, Kitajima Y, Kawaguchi Y, Nakashita S, Ozaki I, Mizuta T, Toda S. Evaluation of acoustic radiation force impulse elastography for fibrosis staging of chronic liver disease: a pilot study. Liver Int. 2010;30:538-545. |
| 16. | Yoneda M, Yoneda M, Fujita K, Inamori M, Tamano M, Hiriishi H, Nakajima A. Transient elastography in patients with non-alcoholic fatty liver disease (NAFLD). Gut. 2007;56:1330-1331. |
| 17. | Goldstein NS, Hastah F, Galan MV, Gordon SC. Fibrosis heterogeneity in nonalcoholic steatohepatitis and hepatitis C virus needle core biopsy specimens. Am J Clin Pathol. 2005;123:382-387. |
| 18. | Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449-1457. |
| 19. | Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898-1906. |
| 20. | Suzuki A, Angulo P, Lymp J, Li D, Satomura S, Lindor K. Hyaluronic acid, an accurate serum marker for severe hepatic fibrosis in patients with non-alcoholic fatty liver disease. Liver Int. 2005;25:779-786. |
| 21. | Ono Y, Shiratori T, Okudaira M, Imamura T, Teratani F, Kanai N, Kato H, Yoshida S, Shiina S, Omata M. Platelet count reflects stage of chronic hepatitis C. Hepatol Res. 1999;15:192-200. |
| 22. | Poynard T, Ratziu V, Charlotte F, Messous D, Munteanu M, Imbert-Bismut F, Massard J, Bonyhay L, Tahiri M, Thabut D. Diagnostic value of biochemical markers (NashTest) for the prediction of non alcoholo steato hepatitis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006;6:34. |
| 23. | Lackner C, Struber G, Liegl B, Leibl S, Ofner P, Bankuti C, Bauer B, Stauber RE. Comparison and validation of simple noninvasive tests for prediction of fibrosis in chronic hepatitis C. Hepatology. 2005;41:1376-1382. |
| 24. | Stauber RE, Lackner C. Noninvasive diagnosis of hepatic fibrosis in chronic hepatitis C. World J Gastroenterol. 2007;13:4287-4294. |
| 25. | Kawamoto M, Mizuguchi T, Katsuramaki T, Nagayama M, Oshima H, Kawasaki H, Nobuoka T, Kimura Y, Hirata K. Assessment of liver fibrosis by a noninvasive method of transient elastography and biochemical markers. World J Gastroenterol. 2006;12:4325-4330. |
| 26. | Masuzaki R, Tateishi R, Yoshida H, Goto E, Sato T, Ohki T, Goto T, Yoshida H, Kanai F, Sugioka Y. Comparison of liver biopsy and transient elastography based on clinical relevance. Can J Gastroenterol. 2008;22:753-757. |