Published online May 14, 2010. doi: 10.3748/wjg.v16.i18.2244
Revised: February 4, 2010
Accepted: February 11, 2010
Published online: May 14, 2010
AIM: To study the effects of Roux-en-Y gastric bypass (RYGB) on the expression of pancreatic duodenal homeobox-1 (PDX-1) and pancreatic β-cell regeneration/ neogenesis, and their possible mechanisms in diabetics.
METHODS: Three groups of randomly selected non-obese diabetic Goto-Kakizaki (GK) rats were subjected to RYGB, sham-RYGB and sham-operation (sham-op) surgery, respectively. The rats were euthanized at post-operative 1, 2, 4 and 12 wk. Their pancreases were resected and analyzed using reverse transcription polymerase chain reaction to detect the mRNA of PDX-1. Anti-PDX-1 immunohistochemical (IHC) staining and Western blotting were used to detect the protein of PDX-1. Double IHC staining of anti-Brdu and -insulin was performed to detect regenerated β-cells. The index of double Brdu and insulin positive cells was calculated.
RESULTS: In comparison with sham-RYGB and sham-op groups, a significant increase in the expressions of PDX-1 mRNA in RYGB group was observed at all experimental time points (1 wk: 0.378 ± 0.013 vs 0.120 ± 0.010, 0.100 ± 0.010, F = 727.717, P < 0.001; 2 wk: 0.318 ± 0.013 vs 0.110 ± 0.010, 0.143 ± 0.015, F = 301.509, P < 0.001; 4 wk: 0.172 ± 0.011 vs 0.107 ± 0.012, 0.090 ± 0.010, F = 64.297, P < 0.001; 12 wk: 0.140 ± 0.007 vs 0.120 ± 0.010, 0.097 ± 0.015, F = 16.392, P < 0.001); PDX-1 protein in RYGB group was also increased significantly (1 wk: 0.61 ± 0.01 vs 0.21 ± 0.01, 0.15 ± 0.01, F = 3031.127, P < 0.001; 2 wk: 0.55 ± 0.00 vs 0.15 ± 0.01, 0.17 ± 0.01, F = 3426.455, P < 0.001; 4 wk: 0.39 ± 0.01 vs 0.18 ± 0.01, 0.22 ± 0.01, F = 882.909, P < 0.001; 12 wk: 0.41 ± 0.01 vs 0.20 ± 0.01, 0.18 ± 0.01, F = 515.833, P < 0.001). PDX-1 mRNA and PDX-1 protein production showed no statistical significance between the two sham groups. Many PDX-1 positive cells could be found in the pancreatic islets of the rats in RYGB group at all time points. In addition, the percentage of Brdu-insulin double staining positive cells was higher in RYGB group than in the other two groups (1 wk: 0.22 ± 0.13 vs 0.03 ± 0.06, 0.03 ± 0.06, P < 0.05; 2 wk: 0.28 ± 0.08 vs 0.00 ± 0.00, 0.03 ± 0.06, P < 0.05; 4 wk: 0.24 ± 0.11 vs 0.07 ± 0.06, 0.00 ± 0.00, P < 0.001; 12 wk: 0.20 ± 0.07 vs 0.03 ± 0.06, 0.00 ± 0.00, P < 0.05).
CONCLUSION: RYGB can increase the expression of pancreatic PDX-1 and induce the regeneration of β-cells in GK rats. The associated regeneration of islet cells may be a possible mechanism that how RYGB could improve type 2 diabetes mellitus.
- Citation: Li Z, Zhang HY, Lv LX, Li DF, Dai JX, Sha O, Li WQ, Bai Y, Yuan L. Roux-en-Y gastric bypass promotes expression of PDX-1 and regeneration of β-cells in Goto-Kakizaki rats. World J Gastroenterol 2010; 16(18): 2244-2251
- URL: https://www.wjgnet.com/1007-9327/full/v16/i18/2244.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i18.2244
Type 2 diabetes mellitus (T2DM) is a chronic metabolic disease characterized by insulin resistance and progressive deterioration of islet functions[1]. The apoptosis of β-cells may be a major contributor to the failure of these cells at the late stages of T2DM[2]. An effective treatment for diabetes can improve the functions of β-cells and increase their numbers. Roux-en-Y gastric bypass (RYGB) is a commonly used surgical treatment for patients with morbid obesity. It could not only significantly and persistently reduce patients’ body weight, but also lower the serum levels of glucose, and glycosylated hemoglobin in 80%-100% of T2DM patients[2,3]. Furthermore, this treatment could also prevent impaired glucose intolerance from developing into diabetes among these patients[3]. Animal experiments have shown that RYGB treatment can improve the functions of pancreatic islets and the metabolism of glucose in both obese and non-obese diabetic rats[4]. These results suggest that RYGB surgery is effective in the treatment of diabetes.
Efforts have been made to understand the mechanism of RYGB treatment on T2DM patients, however, the exact mechanism still remains elusive[5,6]. RYGB treatment may be correlated to the changes of gastrointestinal hormones, which then influence the enteroinsular axis to regulate the endocrine function of the pancreas[7]. One of the most important intestinal hormones involved in this mechanism is glucagon-like peptide 1 (GLP-1). It has been found that RYGB surgery can increase GLP-1 levels in the sera of T2DM patients and in the experimental animals. This effect may last 1 year and in some cases, even up to 20 years after RYGB surgery[5,8]. This may be one of the reasons why the post-operative diabetes is well controlled in both human beings and experimental rodents.
Recently, it has also been reported that after RYGB surgery, some patients developed nesidioblastosis, a serious life-threatening postprandial hyperinsulinism with hypoglycemia[9-12]. These symptoms may be alleviated, after part of the pancreas is removed. Hypertrophy and hyperplasia pancreatic islets are common features seen in the resected specimens, and even multiple islet tumors detected in some specimens. Since the hyperplasia of islet cells are rarely found in the normal population[9,12,13], the above data suggest that RYGB may be associated with the regeneration and neogenesis of the pancreatic islets. Thus, RYGB surgery may occasionally cause the pathological overgrowth and hyperplasia of β-cells in the islets[14]. However, this hypothesis has not been supported by any animal experiments.
Pancreatic duodenal homeobox-1 (PDX-1) is known as the first and most important transcription factor expressed during the embryonic development of the pancreas in experimental animals. All pancreatic cells are derived from the precursor cells with expression of PDX-1[15]. The embryonic pancreas failed to develop in the PDX-1 knockout mice[16]. The mice with heterozygous mutations of PDX-1 gene could develop a normal pancreas, but may show abnormal glucose tolerance and diabetes in adulthood[17]. If PDX-1 is removed from mature β-cells by Cre-recombinase-mediated deletion, the expression of these genes is impaired and the loss of β-cells is accelerated[18]. The expression level of PDX-1 in the pancreas reach the peak in the first 10.5 d during the development of mouse embryos, and decline gradually afterwards. After the birth and in the adulthood, the expression of PDX-1 becomes very weak, which is specifically found only in 90% of β-cells and 10% of δ-cells in the islets[19].
PDX-1 is significantly re-expressed in proliferating cells in the ducts[20,21] and islets[22] during pancreas regeneration[23] and may serve as a marker of cells that regain their pluripotency to differentiate into all types of pancreatic cells. Subtotal pancreatectomy promoted the PDX-1 expression of duct epithelial cells in Sprague-Dawley rats[21], and the PDX-1 positive cells were also obvious in mouse model with pancreas injury[24]. PDX-1 has significant effect on cell proliferation and could induce a dramatic cell proliferation, which is similar to the formation of the pancreas during embryonic development[25]. Studies of animal models suggested that the down-regulation of PDX-1 expression in the β-cells may underlie the pathogenesis of β-cell failure and the onset of T2DM[26]. Therefore, the regeneration of islet β-cells requires the activation and expression of PDX-1. PDX-1 expression is the basis of regeneration of the pancreas.
Goto-Kakizaki (GK) rats are used in a genetically non-overweight type 2 diabetes model, which has the features of slightly elevated blood glucose, impaired secretion of glucose-stimulated insulin and reduced mass of pancreatic β-cells[1]. It is similar to those in T2DM patients. Therefore, GK rats have been widely used in an experimental animal model for T2DM studies. The aim of this study was to investigate the effects of RYGB surgery on the expression of PDX-1 as well as on the regeneration of pancreatic β-cells in non-obese diabetic GK rats, and the possible mechanisms.
Sixty male GK rats weighing 200-230 g, at age of 10-12 wk, were provided by Shanghai Laboratory Animal Center, Chinese Academy of Sciences, and kept in the animal facility at the Henan Key Lab of Biological Psychiatry, China. The rats were randomly divided into 3 groups with 20 rats in each group: RYGB group, sham-RYGB group and sham-operation (sham-op) group.
For experiments, GK rats in the RYGB group were fasted overnight and anesthetized with 4% chloral hydrate (Wako Co., Japan). Upper abdomen was cut open with midline incision and then the stomach was sutured bisecting it to produce two separate gastric cavities, distal one and a proximal one. The jejunum was cut off about 8 cm from the Tretiz ligaments. The proximal part of the stomach was opened and anastomosed with the distal jejunum. The proximal jejunum was anastomosed with the side of distal jejunum at the site about 10 cm away from the above stomach-jejunum anastomosis. The abdominal incisions were closed with absorbable sutures. For controls, the stomachs of rats were transected across the pylorus followed by discontinuously suturing back the two gastric stumps in the sham-RYGB group, and in the sham-op group there was only an anterior abdominal wall incision which was immediately sutured without disturbing the internal organs. After surgery, all rats were fasted and only fed with water on the same day of the operation (day 0). They were fed with food starting from day one. Animals were administered with the antibiotics, cefradine powder (Bright Future Pharmaceuticals Factory, Hong Kong), at a dose of 100-150 mg/kg body weight, once every 24 h, for a total of 3 d to prevent infections. Animals were euthanized on the 1st, 2nd, 4th and 12th wk after operation, with five rats from each group at each time-point, separately.
On the sacrifice day, each rat was first intraperitoneally injected with 5-bromo-2-deoxyuridine (Brdu, from Sigma, USA) at 50 mg/kg and two hours later, the rats were euthanized with overdose anesthesia. The pancreatic tissues were isolated and rinsed with 0.01 mol/L phosphate buffered saline (PBS). Eighty mg of pancreatic tissues from each rat was homogenized using Trizol reagent (Sigma, USA), and the total RNA was extracted according to the manufacturers’ protocol. The optical density of RNA was read with the spectrophotometry at the wavelength of 260 nm (A260). The cDNAs were synthesized by adding 1-2 μg DNA-free RNA to the RT mixture. Glyceraldehyde phosphate dehydrogenase (GAPDH) was used as an internal control. PCR was performed with the following primers: GAPDH (forward: 5′-ACCACAGTCCATGCCATCAC-3′; reverse: 5′-TCCACCACCCTGTTGCTGTA-3′), PDX-1 (forward: 5′-CCAAAACCGTCGCATGAAGTG-3′, reverse: 5′-TCTGGGTCCCAGACCCG-3′). The combination of the DNA primers produced single PCR products of 550 and 208 bp in length, respectively. PCR was carried out in a DNA thermal cycler (Biometra, Goettingen, Germany) after denaturation at 95°C for 5 min. The number of total PCR cycles was 35 for PDX-1 and 30 for GAPDH, and each cycle consisted of denaturation at 94°C for 15 s, annealing at 65°C (GAPDH at 62°C) for 30 s, and extension at 72°C for 90 s. For semiquantitative analysis, 25 μL of each PCR product was examined by electrophoresis in 1.8% agarose gel containing 0.5 μg/mL of ethidium bromide. DM 1000 (LEICA, Germany) was used as a molecular marker. OD readings of PDX-1 mRNA were normalized to those of GAPDH mRNA, and the ratios were analyzed.
The pancreatic tissues obtained from the above rats were also immediately fixed with 4% paraformaldehyde at 4°C overnight. The tissues were then dehydrated in graded ethanol, cleared in xylene and finally embedded in paraffin. The tissues were sectioned to produce 4 μm-thick sections, which were mounted onto slides for further staining.
In brief, the pancreatic sections were deparaffined in xylene and rehydrated in graded ethanol. The sections were blocked in H2O2 and sequentially incubated with goat anti-PDX-1 polyclonal primary antibody (1:50 dilution; Santa Cruz, USA) overnight and anti-goat secondary antibody (1:100 dilution; Santa Cruz, USA) for 2 h. For negative control, the primary antibody was replaced with 0.01 mol/L PBS.
Total protein was obtained from the pancreatic tissues, that were lysed in the buffer containing 50 mmol/L Tris, pH 7.5, 0.3 mol/L NaCl, 0.5% Triton X-100 and 0.1% sodium azide, and protease inhibitor cocktail CLAP (chymostatin, leupeptin, aprotinin and pepstatin, 100 μmol of each, Sigma Aldrich, USA). Then protein samples (50 μg) were separated in 10% sodium dodecyl sulphate polyamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane (Amersham, UK). The membranes were then blocked in 0.01 mol/L PBS containing 5% dry milk and 0.3% Tween 20 for 1 h. The membranes were then incubated with goat anti-PDX-1 antibody (1:10 000 dilution) at room temperature for 2 h and horseradish peroxidase-labelled secondary antibody (1:5000 dilution; Amersham, Japan) at room temperature for 1 h, respectively. Finally, the proteins were visualized on enhanced chemiluminescence hyperfilm (Amersham, Japan) by application of Amershem’s Enhanced Chemiluminescence Western blotting Detection System. The band intensity was quantitated with GelAnalysis software (GreyStone-Iconix).
Briefly, the pancreatic sections were sequentially incubated with guinea pig anti-insulin polyclonal antibody (1:50 dilution; Santa Cruz, USA), biotinylated rabbit anti-guinea pig antibody (1:50 dilution; Santa Cruz, USA), and streptavidin-alkaline phosphatase complex (Santa Cruz, USA), for a period of 45 min each. The alkaline phosphatase activity was identified using new fuchsin under light microscope. The sections were counterstained with Harris hematoxylin. To detect the second antigen Brdu, the sections were placed in 2 N hydrochloric acid and kept at 70°C for 15 min for antigen retrieval. The reaction was stopped by 0.1 mol/L sodium borate and left in 0.01 mol/L PBS for 20 min. Tissue sections were incubated with hydrogen peroxide for 45 min, mouse anti-Brdu monoclonal antibody for 3 h, biotinylated goat anti-mouse antibody for 45 min, and streptavidin-peroxidase complex for 45 min, respectively. Finally, the peroxidase activity was visualized with diaminobenzidine under light microscope, and the reaction was stopped by rinsing with PBS. Avidin and biotin were then applied to the sections for 45 min each.
The images of the above double-stained sections were captured at 100 × magnifications. In each rat, a total of 1000 nuclei from five sections were counted, and the sections were randomly selected from tail (3 sections), body (1 section) and head (1 section). The index of Brdu-labeling was calculated as follows: The index of Brdu labeling = (The number of double-stained cells/1000) × 100%.
All data were presented as mean ± SD (n = 5). The data were analyzed using SPSS13.0 statistical package. The differences were analyzed by factorial design of variance. The comparison of variance between two groups was analyzed by LSD method. Missing variance was analyzed using Dunnett T3. Non-normal data were analyzed non-parametrically using a number of independent samples and tested by Kruskal-Wallis method. P < 0.05 was considered to indicate statistical significance of the test results.
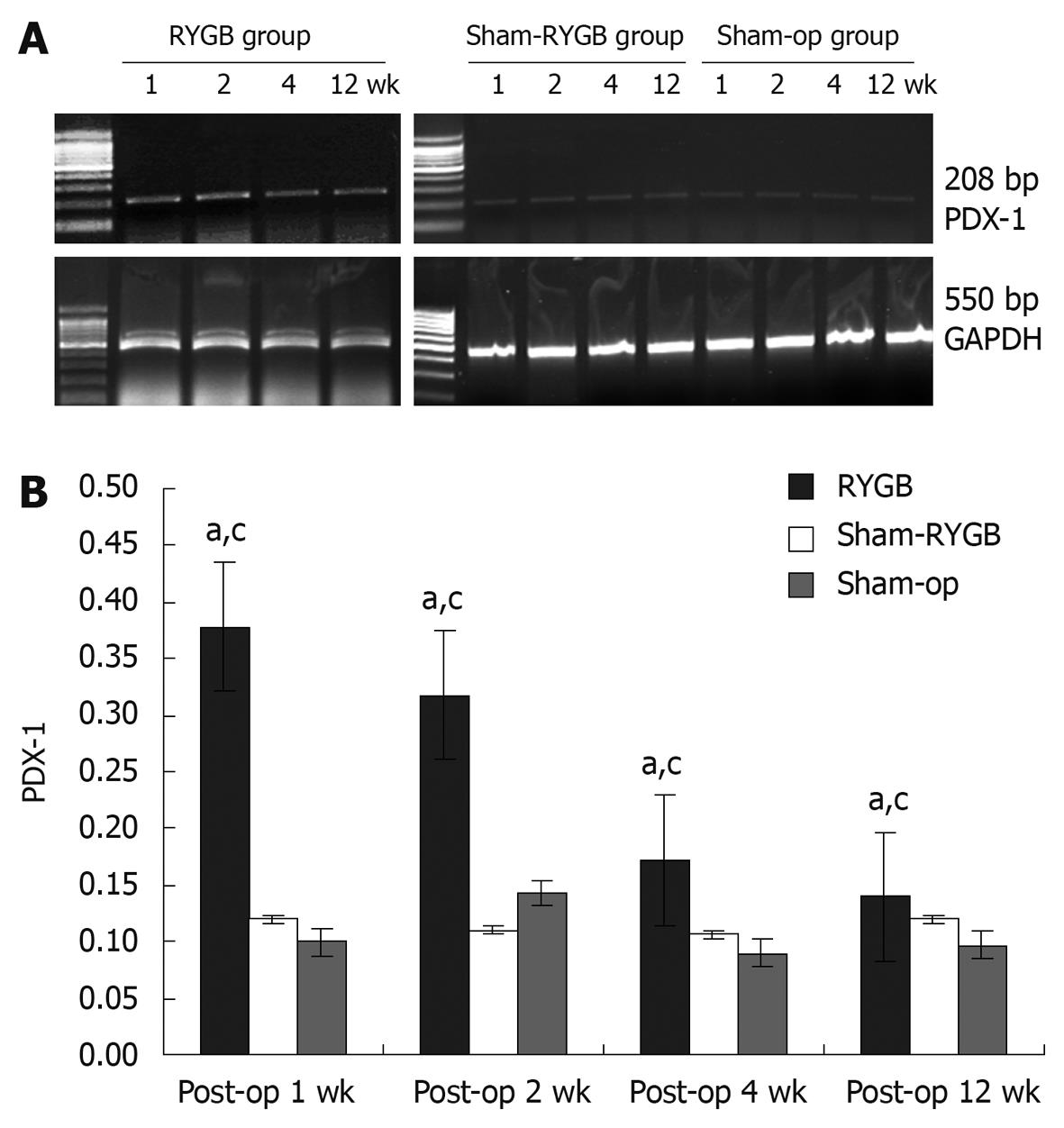
Compared with internal control, GAPDH, the data of relative mRNA expression of PDX-1 at weeks 1, 2, 4 and 12 after surgery were 0.378 ± 0.013, 0.318 ± 0.013, 0.172 ± 0.011 and 0.140 ± 0.007 in the rat pancreas of RYGB group, 0.120 ± 0.010, 0.110 ± 0.010, 0.107 ± 0.012 and 0.120 ± 0.010 in sham-RYGB group, and 0.100 ± 0.010, 0.110 ± 0.010, 0.090 ± 0010 and 0.097 ± 0.015 in sham-op group, respectively. The above data were plotted as shown in Figure 1B. The F values of the relative expression level of PDX-1 mRNA in the rat pancreas at 1, 2, 4 and 12 wk after surgery in the three groups were 727.717, 301.509, 64.297 and 16.392, respectively, all P < 0.05. There was statistically significance at each time point. In addition, the relative mRNA expression level of PDX-1 in the RYGB group showed statistical significance (F = 512.105, P < 0.05), being higher at 1 and 2 wk after surgery. In contrast, there was no significant difference at different time points between sham-RYGB and sham-op groups (F = 0.750, both P > 0.05).
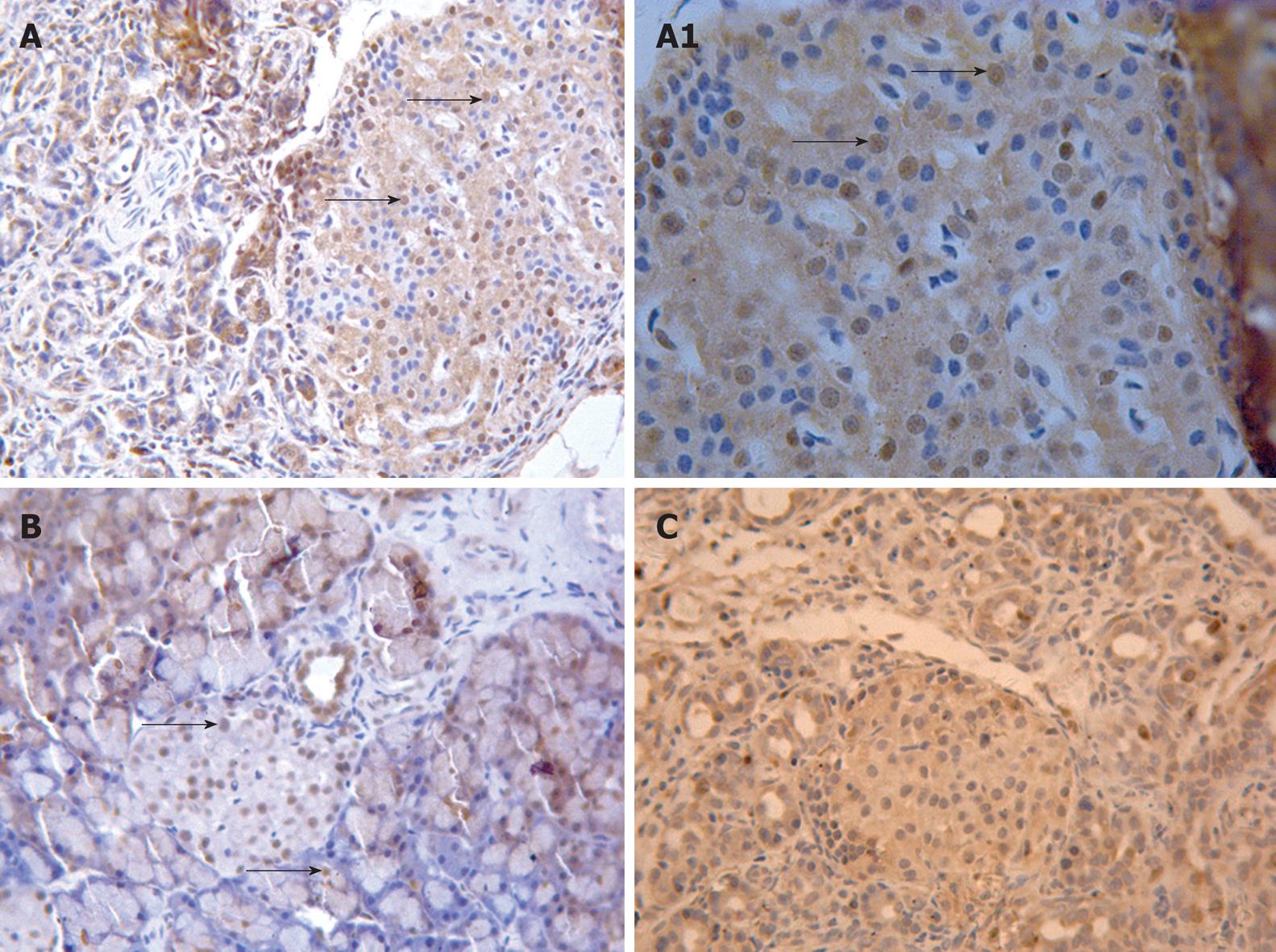
Anti-PDX-1 immunohistochemical (IHC) staining showed that there were many PDX-1 positive cells with brown nuclei in the rat pancreas in the RYGB group. These cells were mainly located in the islets. A few PDX-1 positive cells could be observed in the ducts and acini (Figure 2A and B). In contrast, few PDX-1 positive cells were found in the islets of rat pancreas at each time point in both sham-RYGB and sham-op groups (Figure 2C).
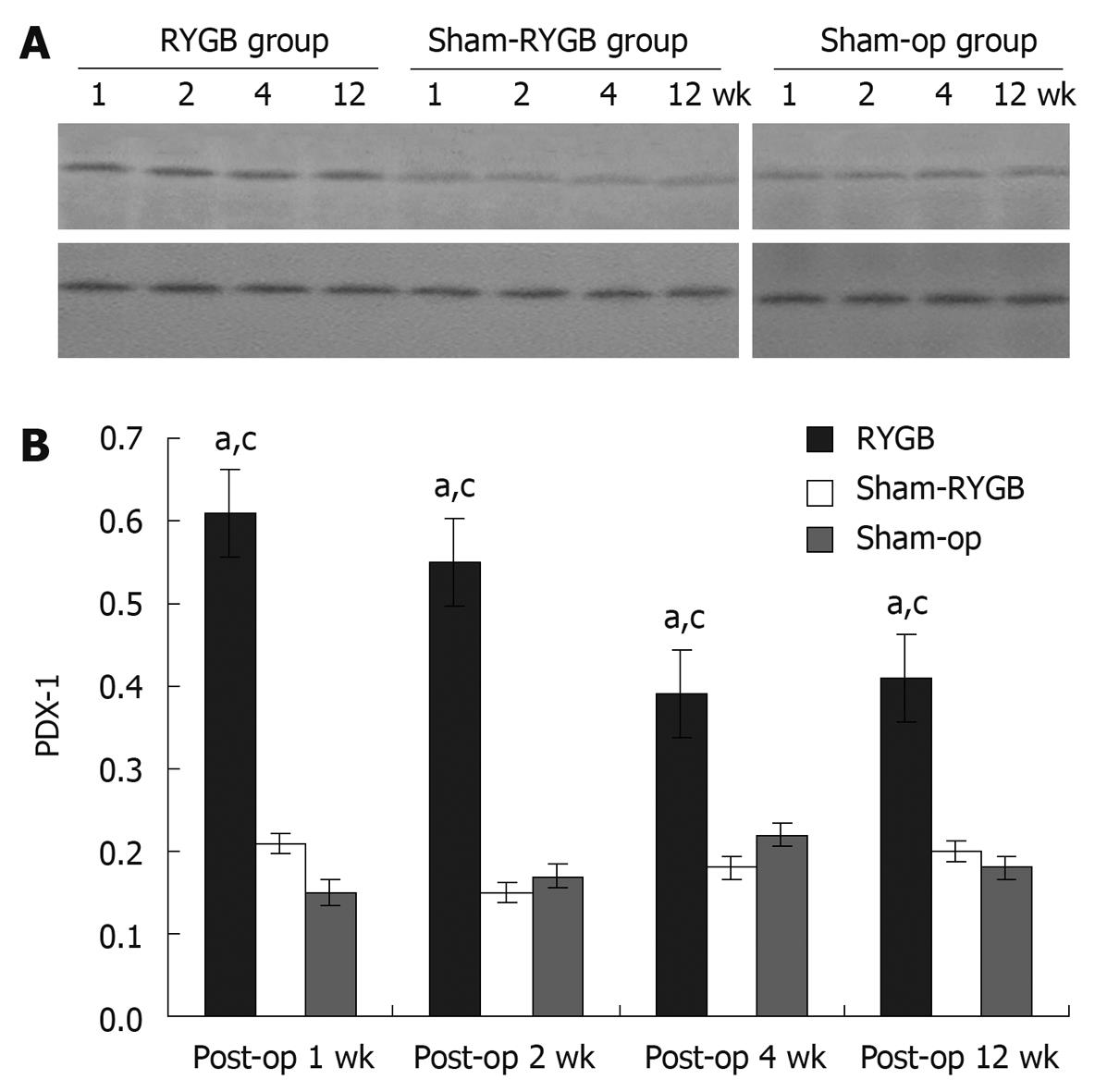
The data of the expressions of PDX-1 protein in the rat pancreas are plotted in Figure 3A. Compared with the internal control, GAPDH, the relative expressions of PDX-1 protein in the rat pancreas are shown in Figure 3B. The F values of the relative expression of PDX-1 protein in the rat pancreas at 1, 2, 4 and 12 wk after surgery in the three groups were 3031.127, 3426.455, 882.909 and 515.833, respectively (all P < 0.05). In addition, there was statistical significance at each time point among the three groups. The relative expression levels of PDX-1 protein in the rat pancreas of RYGB group showed statistical significance (F = 676.157, P < 0.05), being highest at 1 and 2 wk after surgery.
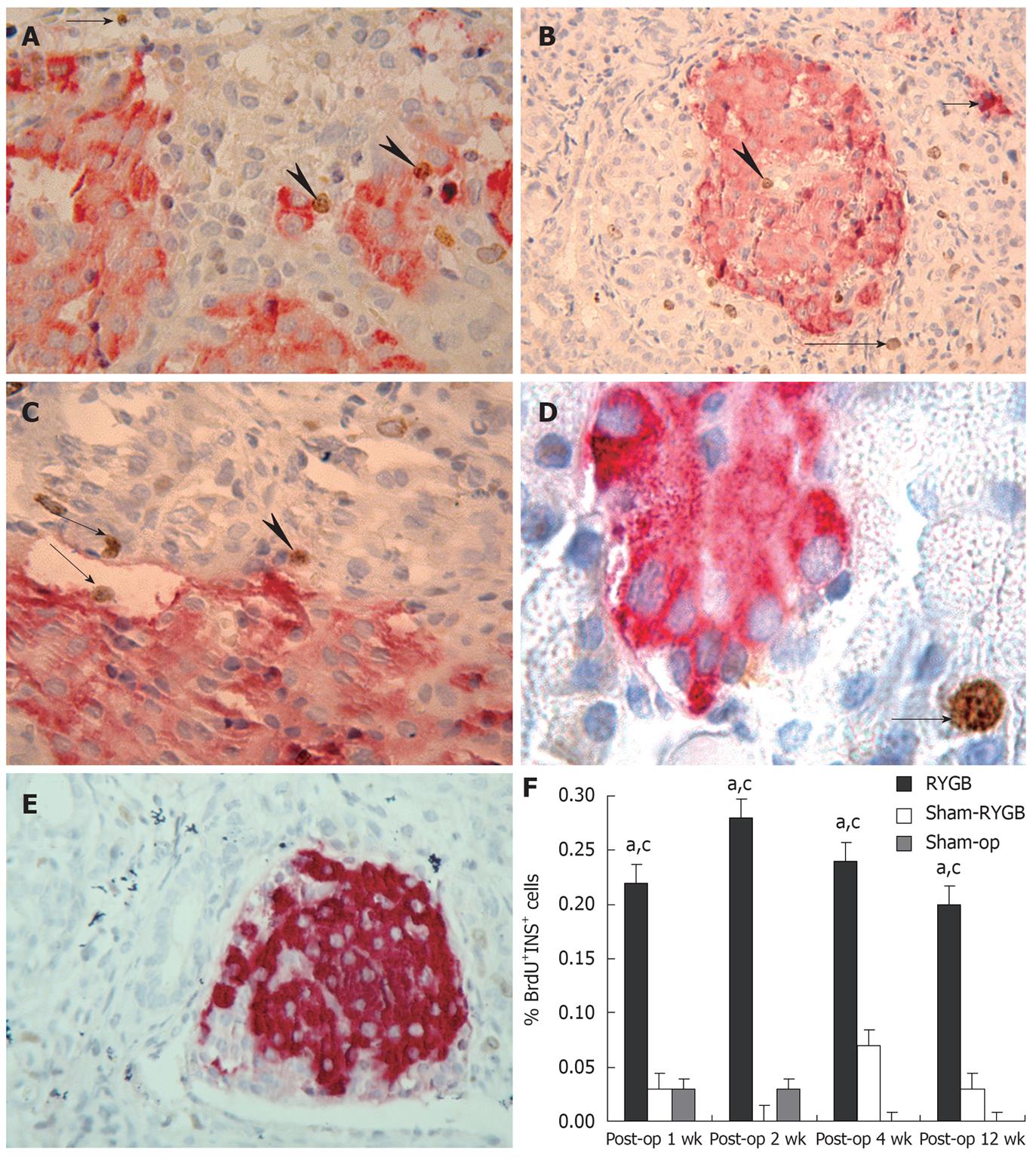
Anti-Brdu and anti-insulin double stained cells could be found in the rat pancreas in the RYGB group at each time point (Figure 4A-C). The Brdu and insulin double positive cells were found primarily in the islets and occasionally in the ducts (Figure 4C). Single insulin positive cells could be observed in the acini (Figure 4B) and there were many single Brdu positive cells in the acini of the pancreas (Figure 4A-D). However, few Brdu and insulin double positive cells could be found in the rat pancreas in both sham-RYGB and sham-op groups at each time point after surgery. The percentage of both Brdu and insulin positive cells showed a significant increase at 1, 2, 4 and 12 wk after surgery as compared with the other two groups (Figure 4E). The χ2 values were 6.197, 8.485, 7.808 and 8.166, respectively; the corresponding P values were 0.045, 0.014, 0.020 and 0.017. The rank of RYGB group was the highest at each time point. In addition, there were more single Brdu positive cells in RYGB group than in the other two groups.
In this study, the expression levels of PDX-1 mRNA in the RYGB group were significantly higher than those in the sham-RYGB and sham-op groups at all time points of 1, 2, 4 and 12 wk after surgery. It suggests that RYGB surgery may result in an increase in the expression of pancreatic PDX-1 mRNA in GK rats. In other words, RYGB surgery may promote the expression of PDX-1 mRNA, which may last at least 12 wk. The expression of PDX-1 mRNA in RYGB group peaked at the first two weeks and decreased afterwards, which may suggest that RYGB surgery played a role in promoting the expression of PDX-1 mRNA primarily at the early stage after the surgery.
Similarly, the protein expressions of PDX-1 in RYGB group were also significantly higher than those in the other two groups at all time points. The protein expression level of pancreatic PDX-1 in RYGB group also peaked at the first two weeks after surgery, and decreased afterward. Furthermore, the results of anti-PDX-1 IHC staining also showed that PDX-1 positive cells in the pancreas of GK rats increased significantly in RYGB group when compared with those in both sham-RYGB and sham-op groups. The PDX-1 positive cells were mainly located in the islets, but not in the acinus and ducts.
It is important to increase the number of β-cells in the pancreatic islets in the treatment of diabetes. The regenerated β-cells in the islets may be double labeled by anti-Brdu and anti-insulin double IHC staining. In this experiment, Brdu and insulin double positive cells were visible in the pancreas in all GK rats at all time points. In addition, the percentages of Brdu and insulin double positive cells in RYGB group were significantly higher than those in both sham-RYGB and sham-op groups. The results also suggest that RYGB surgery may promote the regeneration of islet β-cells in GK rats, which is likely another mechanism why RYGB surgery may decrease the blood glucose in diabetes patients.
The results in this study have shown that RYGB surgery may result in an increase in expressions of PDX-1 mRNA and PDX-1 protein, the number of PDX-1 positive cells and the percentage of Brdu and insulin double positive cells in GK rats. All these suggest a regeneration of islet β-cells, which may be related to persistent elevations of serum GLP-1 after RYGB surgery. It is reported that GLP-1 and its long-acting analog exendin-4 (Ex-4) enhance the expression of pancreatic PDX-1 and regeneration of islet β-cells, and inhibit the apoptosis of β-cells[27,28]. GLP-1 can stimulate the differentiation and proliferation of β cells in GK rats[29]. GLP-1 increases the number of β cells by inducing the differentiation and neogenesis of ductal progenitor cells into islet endocrine cells. Koizumi et al[27], have found that Ex-4 can significantly stimulate β-cell proliferation in PDX-1+/+ mice, but not obviously influence the apoptosis of β-cells. However, in PDX-1-/- mice, the proliferation of β-cells showed no significant change, whereas the apoptosis of β-cells increased significantly in number. Therefore, functions of GLP-1 may rely on the PDX-1 gene activation and expression[24,30]. Ex-4 has been approved by the Food and Drug Administration for the clinical treatment of diabetes[31]. GLP-1 is decomposed in vivo by enzyme dipeptidyl peptidase-IV (DPP-IV)[31], whose inhibitor, vildagliptin, has been applied in the treatment of diabetes and is at the stage of advanced clinical trials[30]. This compound may increase the mass of β-cells, and slow or even reverse the progressive islet-cell deterioration in diabetes. It is possible that the effect of GLP-1 on increasing β-cells is partly mediated by an increase in the circulating insulin, which may act as a growth factor. But, long-term over-stimulation of GLP-1 signaling may cause pathologic hypertrophy and hyperactivity of β-cells, resulting in hypoglycemia.
New pancreatic β-cells are formed in two ways: proliferation of preexisting β-cells, which is called regeneration, and transdifferentiation of non-β-cells from either duct or acinar cells to new β-cells, which is called neogenesis. Dor et al[32], have found that the removal of most of the pancreas in mice led to hyperplasia of the pancreas, and suggested that the new β-cells came from the proliferation of pre-existing β cells in the adult pancreas. Injury in the mouse model caused by pancreatic duct ligation has shown that the progenitors of β-cells exist in adult mouse pancreas, which could be activated and then produced new functional β-cells by differentiation and proliferation[33]. It has been reported that new β-cells in adult pancreas were transdifferentiated from epithelial cells in pancreatic ducts whose characteristics as duct cells were lost either in normal or injured pancreas[27,33-37]. Stem cells also existed in adult human pancreatic ducts, which not only expressed nestin and PDX-1, but also exhibited the markers of mesenchymal stem cells[38]. These data suggest that the new β-cells in pancreas may be formed through a variety of channels and derived from different cells.
Similarly, in vitro cultured stem cells from adult bone marrows[35] and adipose-derived mesenchymal stem cells[36] can be induced to differentiate into insulin-like cells which produce insulin. This indicates that the stem cells from the mesoderm may cross over the embryonic layer and differentiate into insulin-like cells in the endoderm. In other words, the mesenchymal stem or progenitor cells have a strong reserving function in the body. Yuan et al[39] and Kodama et al[40] have also proposed similar hypothesis, in which progenitor cells exist in the adult pancreas and may transform to endocrine cells under pathological conditions. In Streptozotocin-treated mice, regeneration of β-cells seemed to occur mainly from intra-islet stem/progenitor cells[40].
In this study, the regeneration of β-cells was mainly located within the islets, which is consistent with the experimental results of Kodama et al[40]. In addition, in this experiment, Brdu and insulin double positive cells were mainly found in the pancreatic duct and single insulin positive cells were mainly in the acini. These results suggest that the duct epithelial cells and acinar cells can be transdifferentiated into insulin-like cells, which are consistent with the experimental results of De Haro-Hernández et al[41]. Single Brdu positive cells were found within the pancreatic acini in RYGB rats, which suggests that RYGB promotes not only regeneration/neogenesis of pancreatic β-cells, but also regeneration/neogenesis of other cells. However, the emergence of these new cells depends on the PDX-1 activation and expression.
Roux-en-Y gastric bypass (RYGB) is a commonly used surgical treatment for patients with morbid obesity. It significantly and persistently decreases the levels of blood glucose and glycosylated hemoglobin in 80%-100% of type 2 diabetes mellitus (T2DM) patients. Efforts have been made to understand the mechanism of RYGB treatment in T2DM patients, however, the exact mechanism is still elusive.
Some patients who underwent RYGB surgery developed nesidioblastosis, a serious life-threatening postprandial hyperinsulinism with hypoglycemia, which is rarely found in the normal population. The article suggests that RYGB may be associated with the regeneration and neogenesis of the pancreatic islets. However, this hypothesis has not been supported by any animal experiment.
This study showed that RYGB could increase the expression of pancreatic PDX-1, which is the first and most important transcription factor found during the embryonic development of the pancreas and pancreas regeneration, and induce the regeneration of β-cells in GK rats used in a model of genetic non-overweight type 2 diabetes. The regeneration of islet cells is a possible mechanism how RYGB can treat T2DM.
T2DM is one of the most common chronic metabolic diseases that is characterized by insulin resistance and progressive deterioration of islet cell functions. Findings of this study explain the possible mechanism of treating T2DM with RYGB surgery. This study has provided a new basis for surgery to treat T2DM and explained why the post-surgical nesidioblastosis occurs in RYGB patients.
This is a very important paper since it is dealing with an issue of high relevance, nevertheless not properly addressed in most of the publications at present. Moreover, an extensive literature review is reported and examined.
Peer reviewers: Gianlorenzo Dionigi, MD, FACS, ESES, ETA, Associate Professor of Surgery, Department of Surgical Sciences, University of Insubria, Ospedate di Circolo, v. guicciardini, 21100 Varese, Italy; Dr. Kalpesh Jani, Consultant GI & Laparoscopic Surgeon, SIGMA Surgery, Baroda, Gujarat, India
S- Editor Wang YR L- Editor Ma JY E- Editor Ma WH
| 1. | Portha B, Lacraz G, Kergoat M, Homo-Delarche F, Giroix MH, Bailbé D, Gangnerau MN, Dolz M, Tourrel-Cuzin C, Movassat J. The GK rat beta-cell: a prototype for the diseased human beta-cell in type 2 diabetes? Mol Cell Endocrinol. 2009;297:73-85. |
| 2. | White S, Brooks E, Jurikova L, Stubbs RS. Long-term outcomes after gastric bypass. Obes Surg. 2005;15:155-163. |
| 3. | Moo TA, Rubino F. Gastrointestinal surgery as treatment for type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2008;15:153-158. |
| 4. | Rubino F, Zizzari P, Tomasetto C, Bluet-Pajot MT, Forgione A, Vix M, Grouselle D, Marescaux J. The role of the small bowel in the regulation of circulating ghrelin levels and food intake in the obese Zucker rat. Endocrinology. 2005;146:1745-1751. |
| 5. | Laferrère B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, Kovack B, Bawa B, Koshy N, Lee H. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:2479-2485. |
| 6. | Rubino F, Forgione A, Cummings DE, Vix M, Gnuli D, Mingrone G, Castagneto M, Marescaux J. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741-749. |
| 7. | Buchwald H, Estok R, Fahrbach K, Banel D, Sledge I. Trends in mortality in bariatric surgery: a systematic review and meta-analysis. Surgery. 2007;142:621-632; discussion 632-635. |
| 8. | DePaula AL, Macedo AL, Rassi N, Machado CA, Schraibman V, Silva LQ, Halpern A. Laparoscopic treatment of type 2 diabetes mellitus for patients with a body mass index less than 35. Surg Endosc. 2008;22:706-716. |
| 9. | Alvarez GC, Faria EN, Beck M, Girardon DT, Machado AC. Laparoscopic spleen-preserving distal pancreatectomy as treatment for nesidioblastosis after gastric bypass surgery. Obes Surg. 2007;17:550-552. |
| 10. | Abellán P, Cámara R, Merino-Torres JF, Pérez-Lazaro A, del Olmo MI, Ponce JL, Rayón JM, Piñón F. Severe hypoglycemia after gastric bypass surgery for morbid obesity. Diabetes Res Clin Pract. 2008;79:e7-e9. |
| 11. | Rumilla KM, Erickson LA, Service FJ, Vella A, Thompson GB, Grant CS, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis: histologic features and growth factor expression. Mod Pathol. 2009;22:239-245. |
| 13. | Klöppel G, Anlauf M, Raffel A, Perren A, Knoefel WT. Adult diffuse nesidioblastosis: genetically or environmentally induced? Hum Pathol. 2008;39:3-8. |
| 14. | Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447-2457. |
| 15. | Ohlsson H, Karlsson K, Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J. 1993;12:4251-4259. |
| 16. | Melloul D. Transcription factors in islet development and physiology: role of PDX-1 in beta-cell function. Ann N Y Acad Sci. 2004;1014:28-37. |
| 17. | Ahlgren U, Jonsson J, Edlund H. The morphogenesis of the pancreatic mesenchyme is uncoupled from that of the pancreatic epithelium in IPF1/PDX1-deficient mice. Development. 1996;122:1409-1416. |
| 18. | Gittes GK. Developmental biology of the pancreas: a comprehensive review. Dev Biol. 2009;326:4-35. |
| 19. | Hui H, Wright C, Perfetti R. Glucagon-like peptide 1 induces differentiation of islet duodenal homeobox-1-positive pancreatic ductal cells into insulin-secreting cells. Diabetes. 2001;50:785-796. |
| 20. | Liu T, Wang CY, Gou SM, Wu HS, Xiong JX, Zhou J. PDX-1 expression and proliferation of duct epithelial cells after partial pancreatectomy in rats. Hepatobiliary Pancreat Dis Int. 2007;6:424-429. |
| 21. | Guz Y, Nasir I, Teitelman G. Regeneration of pancreatic beta cells from intra-islet precursor cells in an experimental model of diabetes. Endocrinology. 2001;142:4956-4968. |
| 22. | Joglekar MV, Parekh VS, Mehta S, Bhonde RR, Hardikar AA. MicroRNA profiling of developing and regenerating pancreas reveal post-transcriptional regulation of neurogenin3. Dev Biol. 2007;311:603-612. |
| 23. | Xu X, D’Hoker J, Stangé G, Bonné S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197-207. |
| 24. | Sharma A, Zangen DH, Reitz P, Taneja M, Lissauer ME, Miller CP, Weir GC, Habener JF, Bonner-Weir S. The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes. 1999;48:507-513. |
| 25. | Laybutt DR, Glandt M, Xu G, Ahn YB, Trivedi N, Bonner-Weir S, Weir GC. Critical reduction in beta-cell mass results in two distinct outcomes over time. Adaptation with impaired glucose tolerance or decompensated diabetes. J Biol Chem. 2003;278:2997-3005. |
| 26. | Shao S, Fang Z, Yu X, Zhang M. Transcription factors involved in glucose-stimulated insulin secretion of pancreatic beta cells. Biochem Biophys Res Commun. 2009;384:401-404. |
| 27. | Koizumi M, Doi R, Fujimoto K, Ito D, Toyoda E, Mori T, Kami K, Kawaguchi Y, Gittes GK, Imamura M. Pancreatic epithelial cells can be converted into insulin-producing cells by GLP-1 in conjunction with virus-mediated gene transfer of pdx-1. Surgery. 2005;138:125-133. |
| 28. | Green BD, Flatt PR. Incretin hormone mimetics and analogues in diabetes therapeutics. Best Pract Res Clin Endocrinol Metab. 2007;21:497-516. |
| 29. | Tourrel C, Bailbe D, Lacorne M, Meile MJ, Kergoat M, Portha B. Persistent improvement of type 2 diabetes in the Goto-Kakizaki rat model by expansion of the beta-cell mass during the prediabetic period with glucagon-like peptide-1 or exendin-4. Diabetes. 2002;51:1443-1452. |
| 30. | Flock G, Baggio LL, Longuet C, Drucker DJ. Incretin receptors for glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide are essential for the sustained metabolic actions of vildagliptin in mice. Diabetes. 2007;56:3006-3013. |
| 31. | Chang-Chen KJ, Mullur R, Bernal-Mizrachi E. Beta-cell failure as a complication of diabetes. Rev Endocr Metab Disord. 2008;9:329-343. |
| 32. | Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41-46. |
| 33. | Miyatsuka T, Kaneto H, Shiraiwa T, Matsuoka TA, Yamamoto K, Kato K, Nakamura Y, Akira S, Takeda K, Kajimoto Y. Persistent expression of PDX-1 in the pancreas causes acinar-to-ductal metaplasia through Stat3 activation. Genes Dev. 2006;20:1435-1440. |
| 34. | Yatoh S, Dodge R, Akashi T, Omer A, Sharma A, Weir GC, Bonner-Weir S. Differentiation of affinity-purified human pancreatic duct cells to beta-cells. Diabetes. 2007;56:1802-1809. |
| 35. | Moriscot C, de Fraipont F, Richard MJ, Marchand M, Savatier P, Bosco D, Favrot M, Benhamou PY. Human bone marrow mesenchymal stem cells can express insulin and key transcription factors of the endocrine pancreas developmental pathway upon genetic and/or microenvironmental manipulation in vitro. Stem Cells. 2005;23:594-603. |
| 36. | Timper K, Seboek D, Eberhardt M, Linscheid P, Christ-Crain M, Keller U, Müller B, Zulewski H. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochem Biophys Res Commun. 2006;341:1135-1140. |
| 37. | Bonner-Weir S, Inada A, Yatoh S, Li WC, Aye T, Toschi E, Sharma A. Transdifferentiation of pancreatic ductal cells to endocrine beta-cells. Biochem Soc Trans. 2008;36:353-356. |
| 38. | Lin HT, Chiou SH, Kao CL, Shyr YM, Hsu CJ, Tarng YW, Ho LL, Kwok CF, Ku HH. Characterization of pancreatic stem cells derived from adult human pancreas ducts by fluorescence activated cell sorting. World J Gastroenterol. 2006;12:4529-4535. |
| 39. | Yuan L, Wang J, Wang CL, Shen BL, Dai JX. Fasciology: a new theory on the human self-supervision and control system. Science & Technology Review. 2006;24:85-89. |
| 40. | Kodama S, Toyonaga T, Kondo T, Matsumoto K, Tsuruzoe K, Kawashima J, Goto H, Kume K, Kume S, Sakakida M. Enhanced expression of PDX-1 and Ngn3 by exendin-4 during beta cell regeneration in STZ-treated mice. Biochem Biophys Res Commun. 2005;327:1170-1178. |
| 41. | De Haro-Hernández R, Cabrera-Muñoz L, Méndez JD. Regeneration of beta-cells and neogenesis from small ducts or acinar cells promote recovery of endocrine pancreatic function in alloxan-treated rats. Arch Med Res. 2004;35:114-120. |