Published online May 7, 2010. doi: 10.3748/wjg.v16.i17.2151
Revised: August 13, 2009
Accepted: August 20, 2009
Published online: May 7, 2010
AIM: To assess the impact of bolus volume on the characteristics of small intestinal (SI) impedance signals.
METHODS: Concurrent SI manometry-impedance measurements were performed on 12 healthy volunteers to assess the pattern of proximal jejunal fluid bolus movement over a 14 cm-segment. Each subject was given 34 boluses of normal saline (volume from 1 to 30 mL) via the feeding tube placed immediately above the proximal margin of the studied segment. A bolus-induced impedance event occurred if there was > 12% impedance drop from baseline, over ≥ 3 consecutive segments within 10 s of bolus injection. A minor or major impedance event was defined as a duration of impedance drop < 60 s or ≥ 60 s, respectively.
RESULTS: The minimum volume required for a detectable SI impedance event was 2 mL. A direct linear relationship between the SI bolus volume and the occurrence of impedance events was noted until SI bolus volume reached 10 mL, a volume which always produced an impedance flow event. There was a moderate correlation between the bolus volume and the duration of impedance drop (r = 0.63, P < 0.0001) and the number of propagated channels (r = 0.50, P < 0.0001). High volume boluses were associated with more major impedance events (≥ 10 mL boluses = 63%, 3 mL boluses = 17%, and < 3 mL boluses = 0%, P = 0.02).
CONCLUSION: Bolus volume had an impact on the type and length of propagation of SI impedance events and a threshold of 2 mL is required to produce an event.
- Citation: Nguyen NQ, Bryant LK, Burgstad CM, Fraser RJ, Sifrim D, Holloway RH. Impact of bolus volume on small intestinal intra-luminal impedance in healthy subjects. World J Gastroenterol 2010; 16(17): 2151-2157
- URL: https://www.wjgnet.com/1007-9327/full/v16/i17/2151.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i17.2151
Absorption of nutrients is the main function of the small intestine and requires not only normal mucosal integrity but also normal motor function[1-4]. Adequate mixing and proper transit of chyme are important in ensuring optimal absorption[3,4]. Accurate assessment of small bowel motor function is important for understanding the physiology of the gastrointestinal tract, as well as assessment of clinical disorders of the intestine[1,5-7]. Currently, several tests are available for the assessment of intestinal motility and transit. Although intra-luminal manometry detects contractile patterns, it provides only indirect data regarding flow[5]. Similarly, although intestinal transit can be measured by conventional scintigraphy and more recently by breath tests, these methods detect only total transit time and not patterns of flow; transit can be affected by gastric emptying rate and conditions such as bacterial overgrowth[5]. Fluoroscopic studies, on the other hand, are able to assess overall intestinal motility and bolus transit, but are greatly limited by the exposure of patients to radiation[5].
In combination with manometry, multiple intra-luminal impedance (MII) has been introduced as a technique to assess motility and bolus transit concurrently[8-10]. Although this combined technique has been validated and applied extensively in the study of oesophageal physiology and disease[11-14], few data are available regarding its use in the evaluation of small bowel motor function[15-17]. Recently, detailed analysis from a study using combined video-fluoroscopy, manometry and MII showed that an impedance drop of > 12% from the baseline which propagated over 5 cm is associated with a flow event as seen on fluoroscopy[17]. Furthermore, various patterns of impedance flow events during fasting and in the post-prandial state have been reported in the proximal intestine of healthy subjects[16]. This study, however, did not have concurrent manometric assessment and it is unclear whether the variation in impedance patterns of flow was influenced by intestinal motor activity or volume of the transported bolus or chyme. Knowledge on the relationship between bolus volume and impedance changes in the oesophagus[18] allows investigators to use intra-luminal impedance to assess oesophageal flow and clearance, without the need for radiological assessment. Corresponding data for the small intestine, however, are lacking. The aim of the current study was to assess the impact of bolus volume on the characteristics of small intestinal (SI) impedance signals in healthy humans.
Studies were performed in 12 healthy subjects (6 males; age: 53 ± 6 years; body mass index: 24.5 ± 1.1 kg/m2) at the Department of Gastroenterology and Hepatology, Royal Adelaide Hospital. Exclusion criteria included previous or current gastrointestinal symptoms or surgery, evidence of acute or chronic illness and medications known to influence gastrointestinal motor function. The protocol was approved by the Research Ethics Committee of the Royal Adelaide Hospital and all subjects gave written informed consent.
SI motility and intraluminal electrical impedance were recorded concurrently using (1) a 110-cm perfused multi-lumen manometric assembly (DentSleeve Pty Ltd., Wayville, Australia); and (2) an impedance catheter (Sandhill Scientific, Highland Ranch, CO, USA).
Intestinal motility was recorded using an assembly comprising 11 pressure recording side holes: eight spaced at 2 cm intervals and the following three at 10 cm intervals, from the catheter tip. All manometric lumina were perfused with degassed distilled water at a rate of 0.15 mL/min, by a pneumo-hydraulic capillary perfusion pump (Dentsleeve).
SI luminal flow was recorded using an electrode with eight impedance rings (4 mm in length) spaced 2 cm apart, enabling 7 consecutive recording segments over a distance of 14 cm (Z1 to Z7; proximal to distal, respectively). Each segment straddled a corresponding manometric side hole and was activated by a high frequency (1 kHz) low amplitude (< 6 μA) alternating current.
Manometric and impedance signals were recorded simultaneously using a specialised computer system (Insight Acquisition, Sandhill Scientific) and displayed and stored on a personal computer for subsequent display and analysis.
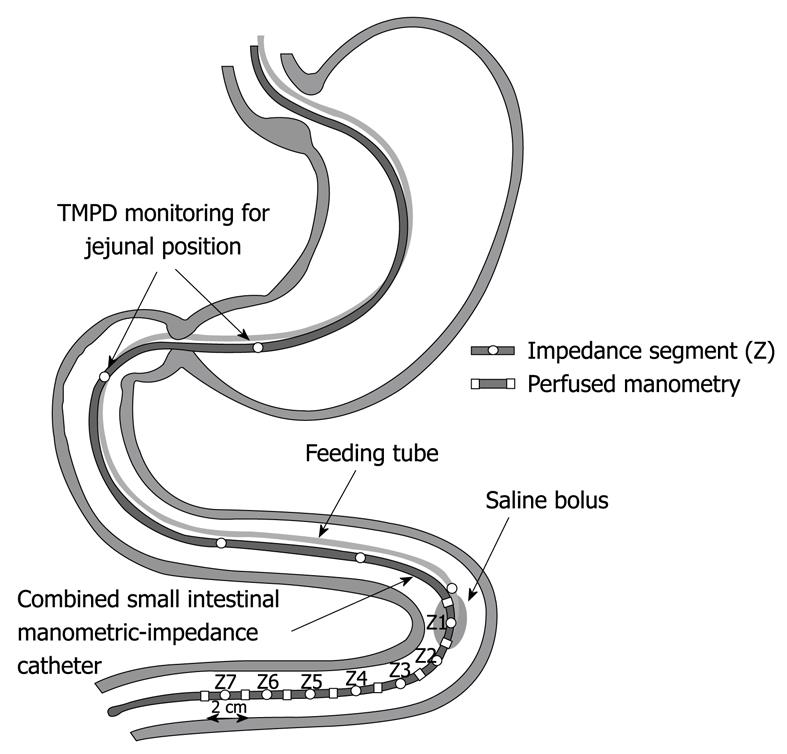
An 18F feeding tube was used to deliver the liquid boluses. The tube was attached to the manometry-impedance assembly and was positioned so that its tip was 1-cm above the most proximal impedance recording segment, Z1. The assembly configuration is outlined in Figure 1.
All subjects were studied after an overnight fast. The assembly was passed into the stomach to a distance of 65 cm, through an anaesthetized nostril. The assembly was then allowed to migrate naturally into the duodenum via peristalsis, which was monitored continuously by measurements of the antro-duodenal trans-mucosal potential difference (TMPD). The final catheter position was achieved when the most proximal manometric side-hole was located in the duodenum, confirmed by a TMPD reading ≥ -15 mV[19]. In this position, the combined manometry-impedance recording segment would be expected to lie in the proximal jejunum and at least 20 cm distal to the pylorus. Radiological confirmation of the catheter position was not performed due to the concern of unnecessary radiation exposure to healthy subjects.
Once the assembly was positioned in the proximal jejunum, subjects were positioned in a 30 degree head-up, supine position. During phase I of the interdigestive migrating motor complex (MMC), a total of 34 boluses of saline (0.9%) were given to each subject via the feeding tube. Five boluses for each volume of 1, 2 and 3 mL were given at 2-min intervals. Five boluses for each volume of 5, 10 and 15 mL were given at 5-min intervals. Two boluses for each volume of 20 and 30 mL were given at 10-min interval. Each bolus was infused at the maximal rate allowed by the assembly lumen.
Manometry: Phase I of MMC was defined as a quiescent period without any intestinal motor activity, and phase II as a period of irregular intestinal motor activity[5,19]. A SI pressure wave was defined as a pressure rise ≥ 6 mmHg from baseline and lasting between 0.8 and 7 s[19]. Pressure waves in adjacent channels were regarded as temporally related if their onsets were within 3 s, and “propagated” if there were ≥ 3 temporally-related sequential pressure waves[19].
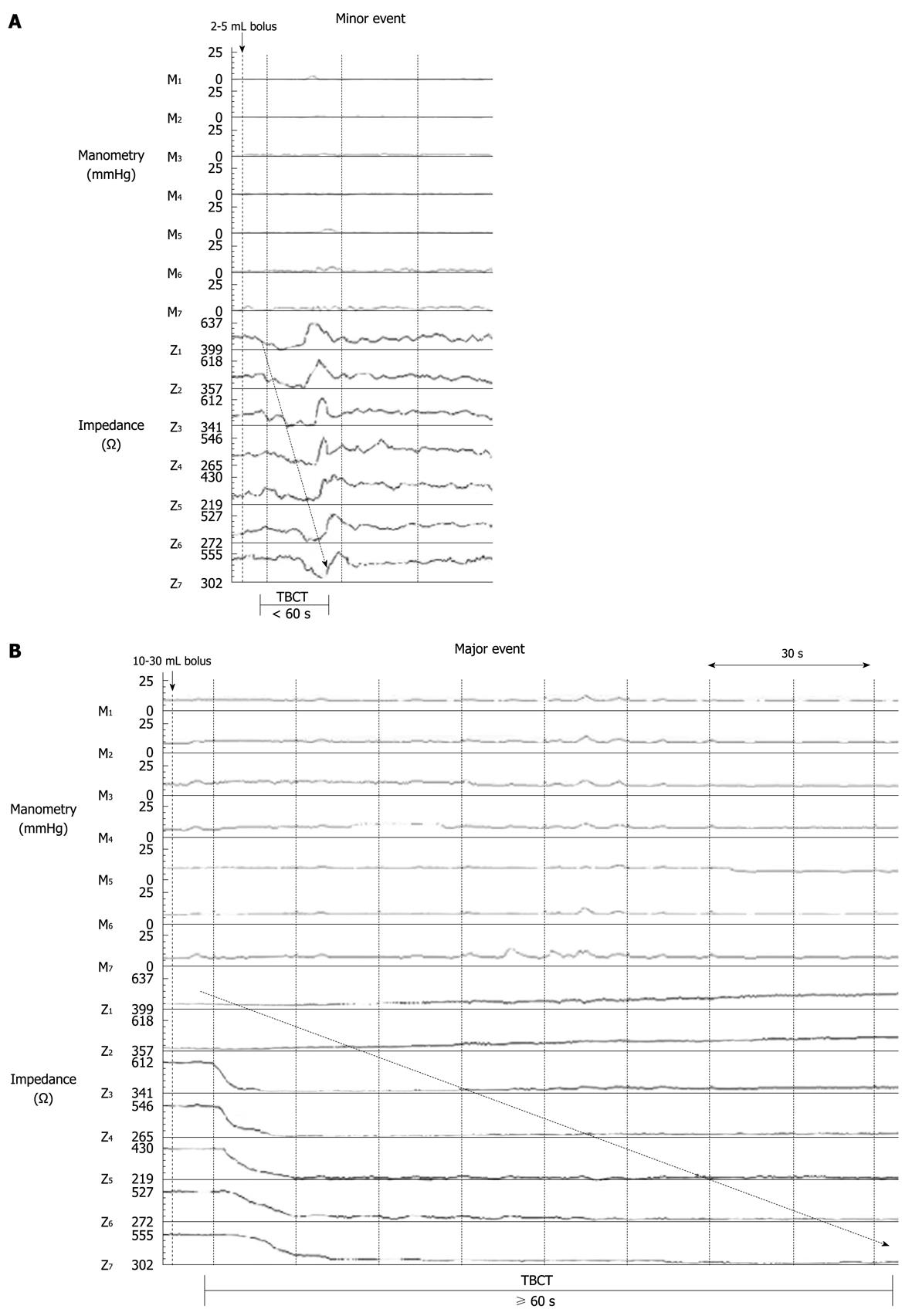
Impedance: The recordings were analysed manually using the impedance analysis software (Bioview Analysis, Sandhill Scientific). An impedance event judged to be associated with flow was defined as having an impedance drop of > 12% below the baseline and propagated over 3 or more impedance segments[17]. The impedance baseline for which the percentage drop was based on was the average impedance value over the 5 s immediately before the impedance drop. For each impedance event, the following variables were characterized: (1) the baseline impedance value (5 s) before injection of the bolus; (2) the magnitude of impedance drop, including minimum, average and maximum values; (3) the number of propagated channels; and (4) total bolus clearance time (TBCT), which was defined as the time taken (in seconds) for the bolus to traverse the whole recording segment in the intestine. TBCT was measured from the time the bolus entered the most proximal intestinal recording segment (Z1) until it cleared the most distal recording segment (Z7). Impedance events with TBCT greater than 60 s were classified as ‘major’ events (Figure 2). For each tested bolus, an impedance event was deemed to be associated with the tested bolus if the event occurred within 10 s from the administration of the bolus and an absence of corresponding motility. Boluses that had the associated impedance signal interfered by movement artefacts, or that coincided with intestinal contractions at the time of bolus administration were excluded from the final analysis as it was not possible to confidently determine whether the impedance changes in these events were related to the bolus administration, the movement artefact or coincident intestinal contractions.
Data are presented as mean ± SE. Categorical data were compared by chi-square test with Yates’ correction and continuous data by Student’s t-test, using GraphPad Prism 4 (v 4.02, San Diego, CA, USA) statistical software. The relationships between bolus volume and impedance flow events, magnitude of impedance drop, number of propagated channels and TBCT were determined by repeated measures ANOVA and Pearson’s linear regression analysis. Significance was accepted at a P value < 0.05.
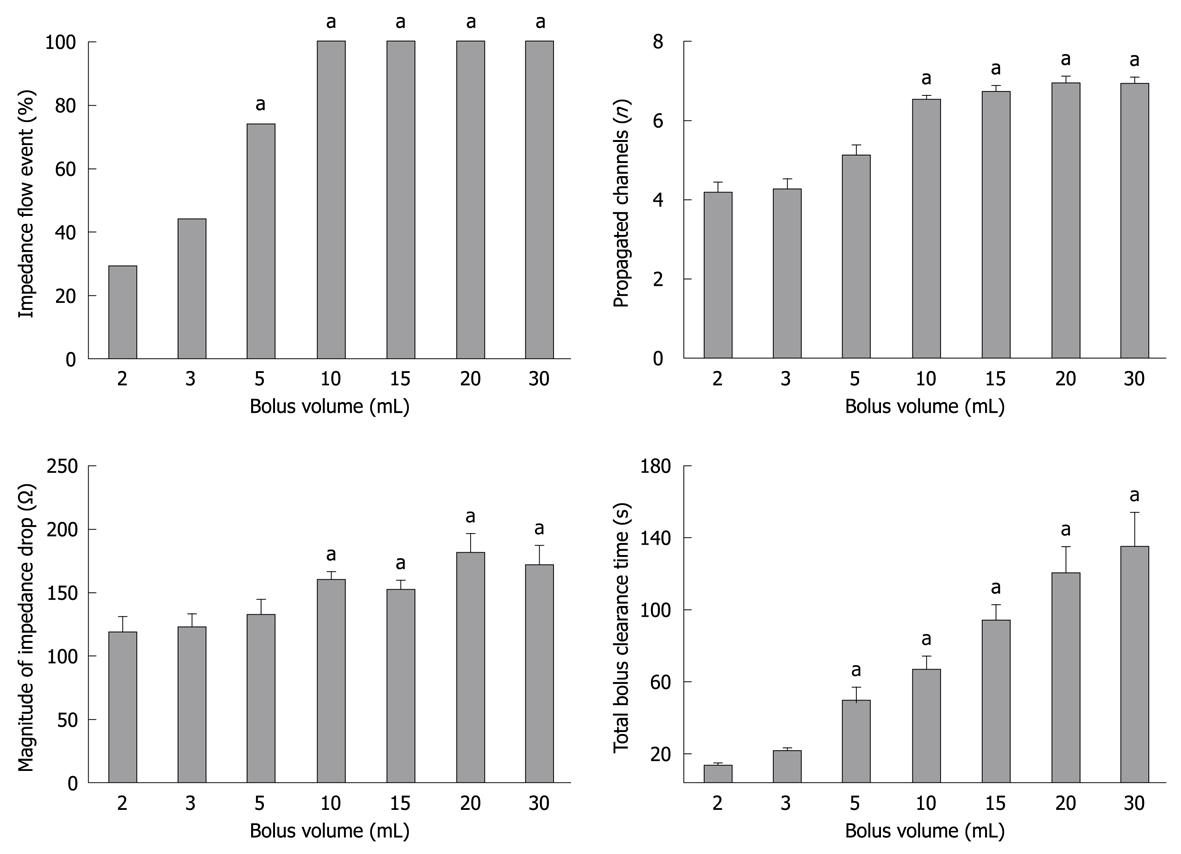
Injection of a saline bolus at the proximal end of the recording segment induced a prompt impedance drop that propagated distally. A minimum volume of 2 mL was required to induce a recognizable intestinal impedance flow event (Figure 3). As the bolus volume increased from 2 to 10 mL, the proportion of detectable intestinal impedance events increased in a linear relationship. A bolus volume of ≥ 10 mL always produced an impedance event.
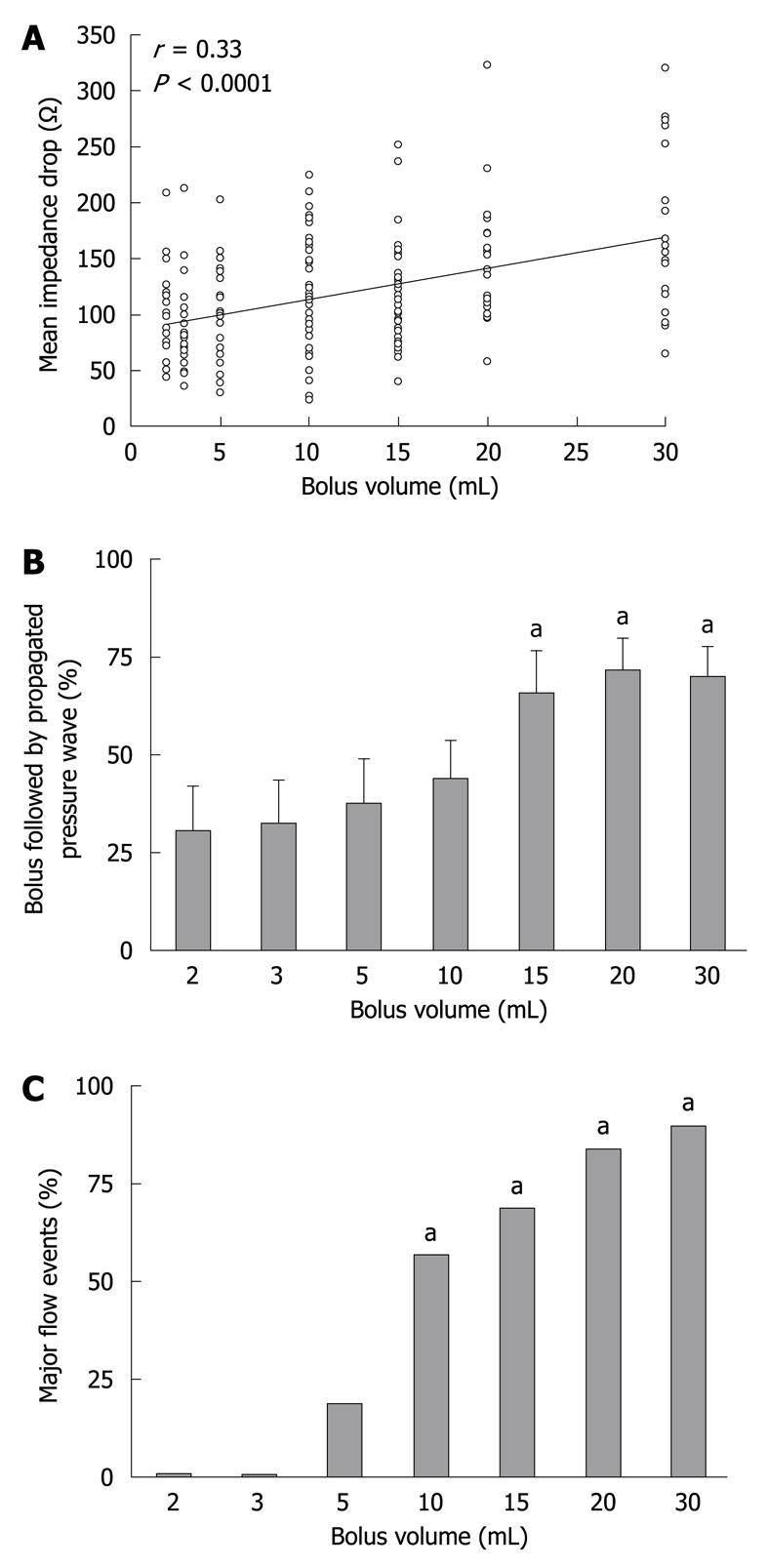
The mean baseline impedance value was 389 ± 58 ohms. There were no significant differences in the baseline impedance values among the different bolus volumes. The mean impedance drop for a detectable flow event was 151 ± 4 ohms. The median impedance drop from baseline associated with a detectable flow event was 27% (IQR: 12%-53%). There was a weak relationship between the bolus volumes and the magnitude of impedance drop (mean: r = 0.33, P < 0.0001; minimum: r = 0.28, P < 0.000; maximum: r = 0.30, P < 0.01) (Figure 4A).
Boluses with a volume of ≥ 5 mL propagated over a longer distance than those with a volume < 5 mL. Once the bolus volume was ≥ 10 mL, an impedance drop always propagated over at least 7 channels (i.e. 14 cm) (Figure 3). Up to a bolus volume of 10 mL, there was a strong correlation between the bolus volume and the number of channels in which an impedance drop occurred (r = 0.71, P < 0.00001).
Of the administered test boluses, 48% ± 5% were followed by an intestinal pressure wave that propagated over 6.0 ± 0.1 channels. The duration between the bolus administration and the initiation of the propagated pressure wave was 20 ± 2 s. There was a positive correlation between the bolus volume and proportion of boluses followed by a propagated pressure wave (r = 0.54, P < 0.0001), which was significantly greater for bolus volumes ≥ 15 mL (66% ± 12% vs 30% ± 11%, P = 0.04; vs 2 mL, respectively, Figure 4B). The bolus volume was also positively correlated with the duration between the bolus administration and the initiation of the propagated pressure wave (r = 0.53, P < 0.001).
The baseline impedance level was recovered after all bolus-induced drops, indicating bolus clearance from the impedance segment. For the majority of boluses ≤ 5 mL, the impedance drop recovered spontaneously without an intestinal clearance pressure wave, with a TBCT of < 60 s (Figure 3). In contrast, for the majority of boluses ≥ 10 mL, the recovery of impedance signal was more likely to require the assistance of an intestinal clearance pressure wave. Overall, there was a direct relationship between bolus volume and TBCT (r = 0.63, P < 0.0001). With increasing bolus volume, the proportion of boluses with a TBCT > 60 s also significantly increased (P < 0.01, Figure 4C).
This is the first methodological study to examine the relationship between bolus volume and impedance changes in the proximal small intestine. In particular, the study examined the limit of detection, and thus sensitivity, of impedance changes for identifying bolus volume. These data will enable the application of impedance as a technique to assess intestinal flow, without radiology. The main findings were (1) a bolus volume of at least 2 mL is required to generate an impedance event; (2) there is a linear relationship between bolus volume (between 2 and 10 mL) and impedance signal; (3) volumes of 10 mL or greater always generate an impedance event; and (4) bolus volume positively correlates with the magnitude of impedance drop, distance of propagation and clearance time. This modest relationship between bolus volume and intestinal impedance signals may explain, at least in part, the various patterns of flow events associated with chyme transport in the small intestine.
The threshold volume for bolus detection by impedance appears to be higher in the small intestine compared with that previously reported in the oesophagus[18]. However, the relationship between volume of liquid boluses and impedance signal observed in the proximal intestine does not exist in the oesophagus[18]. These differences may reflect structural and functional differences between the two organs[1-4,17]. The absence of mucosal secretion and possibly a more complete clearance from stronger contraction amplitudes of the oesophagus are likely to explain the higher baseline level of intra-luminal impedance in the oesophagus (approximately 1000 Ω), compared to the intestine (approximately 400 Ω)[16-18]. With a higher baseline signal, liquid boluses of 1 mL or less can induce significant and readily identifiable impedance drops in the oesophagus[18]. In contrast, with a lower baseline impedance signal, possibly caused by a relatively ‘wet’ lumen from constant intestinal secretions, impedance drops detected in the small intestine are proportionately smaller, and larger volumes are required to induce an identifiable impedance signal. This is supported by the current study, in which a volume threshold of 2 mL was required to induce an intestinal impedance drop. Even with this volume, impedance flow events were only observed in approximately one third of boluses, and were not observed consistently until the volume was at least 10 mL.
The characteristics of impedance changes associated with liquid transit in the small intestine were also influenced by the bolus volume. In the current study, transit of liquid boluses with a volume between 2 and 5 mL was typically associated with a shorter clearance time (less than 60 s) and propagation distance (less than 10 cm). This pattern of flow has been reported to occur during the post-prandial period and possibly phase 2 of the MMC[16,17]. In contrast, transit of larger volumes (> 20 mL) were predominantly associated with clearance times greater than 60 s and propagating over 14 cm. The signals of these ‘major’ impedance events did not spontaneously recover to baseline level until the bolus was cleared by intestinal contraction(s). This pattern of luminal flow is typically seen at the end of phase 3 activity, which may be consistent with the clearance function of the MMC[16,17]. Characteristics of impedance events induced by liquid boluses between 10 and 20 mL varied considerably, particularly in clearance duration. This appears to depend on the timing of intestinal contraction(s) which occur after the bolus is delivered. However, there was also a direct linear relationship between the clearance time and bolus volume. The understanding of the relationship and characteristics between bolus volume and impedance changes will aid the interpretation of various patterns of luminal flow events in the small intestine.
In the current study, impedance changes associated with bolus transit were assessed in the quiescent phase of the MMC cycle, in order to avoid interference from intestinal contraction(s)[17]. Consequently, this relationship is applicable only to bolus transit within a relatively inactive small intestine. However, under normal physiological conditions of digestion, frequent intestinal contractions would be expected and may shorten the clearance time, but not propagation distance.
Furthermore, the current study used saline as a test medium, rather than chyme, which may not reflect the true transit of intestinal contents during normal digestion in humans. Nevertheless, although there are no human data that have evaluated spontaneous flow events in the small intestine, the bolus volumes used in the present study are similar to those reported during the pulsatile flow of gastric emptying in pigs[20]. However, chyme or digested food in the small intestine is typically more viscous than saline, and it is well known that viscosity significantly influences the characteristics of impedance flow events in the oesophagus[21]. Further investigation is required to assess the flow volume of chyme and its relationship with impedance signals. Due to the small diameter of the feeding tube, evaluation of a viscous medium was not possible in the current study.
A potential limitation of this study is the lack of fluoroscopic confirmation of bolus flow. However, fluoroscopy would have exposed the volunteers to excessive radiation exposure due to the prolonged recording period. More importantly, the criteria used in this study to define an impedance “flow event” in the small intestine has already been established and validated against fluoroscopy[17]. Flow could thus be reasonably inferred from the pattern of impedance changes with evidence of propagated clearance from successive impedance segments.
In conclusion, there is a strong relationship between bolus volume and changes in impedance signals within the small intestine. Bolus volume has an impact on both the type and length of propagation of flow events and a threshold volume of 2 mL is required to produce a flow event.
Fluoroscopy and scintigraphy are the currently available techniques to assess intestinal motility and bolus transit, but their utilities are greatly limited by radiation exposure. Although combined intraluminal manometry and impedance has been recently used to assess flow in the small intestine, the relationship between bolus volume and impedance changes has not been assessed.
The ability to use a combined intraluminal manometry-impedance technique to assess bolus transit in the small intestine will not only improve the safety of the procedure, but also its utilization in clinical practice.
This is the first methodological study to demonstrate a linear relationship between bolus volume and impedance changes in the proximal small intestine. Bolus volumes of at least 2 mL are required to generate an impedance signal that can be recognised as a flow event. Volumes of 10 mL or greater will always generate an impedance drop. These findings may explain the various patterns of flow that are associated with chyme transport in the small intestine.
These findings assist the interpretation of various patterns of bolus transit or flow of chyme in the small intestine assessed by a manometry-impedance technique, without the need for fluoroscopy or scintigraphy.
Intra-luminal impedance is a new technique designed to detect intraluminal flow without the use of radiation. The impedance technique measures changes in resistance (in Ohms) to alternating electrical current when a bolus passes a pair of metallic rings mounted on a catheter. In an empty tubular organ (i.e. small intestine), the electrical current is conducted by the few ions present in and on the intestinal mucosa. Liquid boluses with an increased number of ions have a higher conductivity and when entering the measurement segment will lower the impedance to a nadir value. Measurement of flow at multiple sites allows for determination of bolus direction based upon temporal differences in bolus entry and exit.
This is a high quality manuscript, presenting a series of experimental results which demonstrates, for the first time, the relationship between bolus volume and impedance changes in the proximal small intestine.
Peer reviewer: Ming Li, Associate Professor, Tulane University Health Sciences Center, 1430 Tulane Ave Sl-83, New Orleans, LA 70112, United States
S- Editor Tian L L- Editor Webster JR E- Editor Zheng XM
| 1. | Husebye E. The patterns of small bowel motility: physiology and implications in organic disease and functional disorders. Neurogastroenterol Motil. 1999;11:141-161. |
| 2. | Kellow JE, Borody TJ, Phillips SF, Tucker RL, Haddad AC. Human interdigestive motility: variations in patterns from esophagus to colon. Gastroenterology. 1986;91:386-395. |
| 3. | Sarr MG, Kelly KA, Phillips SF. Canine jejunal absorption and transit during interdigestive and digestive motor states. Am J Physiol. 1980;239:G167-G172. |
| 4. | Schwartz MP, Samsom M, Renooij W, van Steenderen LW, Benninga MA, van Geenen EJ, van Herwaarden MA, de Smet MB, Smout AJ. Small bowel motility affects glucose absorption in a healthy man. Diabetes Care. 2002;25:1857-1861. |
| 5. | Camilleri M, Hasler WL, Parkman HP, Quigley EM, Soffer E. Measurement of gastrointestinal motility in the GI laboratory. Gastroenterology. 1998;115:747-762. |
| 6. | Nguyen HN, Silny J, Wüller S, Marschall HU, Rau G, Matern S. Abnormal postprandial duodenal chyme transport in patients with long standing insulin dependent diabetes mellitus. Gut. 1997;41:624-631. |
| 7. | Benson MJ, Roberts JP, Wingate DL, Rogers J, Deeks JJ, Castillo FD, Williams NS. Small bowel motility following major intra-abdominal surgery: the effects of opiates and rectal cisapride. Gastroenterology. 1994;106:924-936. |
| 8. | Silny J. Intraluminal multiple electric impedance procedure for measurement of gastrointestinal motility. J Gastrointest Motil. 1991;3:151-162. |
| 9. | Silny J, Knigge K, Fass J, Rau G, Matern S, Schumpelick V. Verification of the intraluminal multiple electrical impedance measurement for the recording of gastrointestinal motility. J Gastrointest Motil. 1993;5:107-122. |
| 10. | Nguyen HN, Silny J, Matern S. Multiple intraluminal electrical impedancometry for recording of upper gastrointestinal motility: current results and further implications. Am J Gastroenterol. 1999;94:306-317. |
| 11. | Shay SS, Bomeli S, Richter J. Multichannel intraluminal impedance accurately detects fasting, recumbent reflux events and their clearing. Am J Physiol Gastrointest Liver Physiol. 2002;283:G376-G383. |
| 12. | Nguyen NQ, Rigda R, Tippett M, Conchillo J, Smout AJ, Holloway RH. Assessment of oesophageal motor function using combined perfusion manometry and multi-channel intra-luminal impedance measurement in normal subjects. Neurogastroenterol Motil. 2005;17:458-465. |
| 13. | Sifrim D, Holloway R, Silny J, Xin Z, Tack J, Lerut A, Janssens J. Acid, nonacid, and gas reflux in patients with gastroesophageal reflux disease during ambulatory 24-hour pH-impedance recordings. Gastroenterology. 2001;120:1588-1598. |
| 14. | Conchillo JM, Nguyen NQ, Samsom M, Holloway RH, Smout AJ. Multichannel intraluminal impedance monitoring in the evaluation of patients with non-obstructive Dysphagia. Am J Gastroenterol. 2005;100:2624-2632. |
| 15. | Nguyen HN, Silny J, Albers D, Roeb E, Gartung C, Rau G, Matern S. Dynamics of esophageal bolus transport in healthy subjects studied using multiple intraluminal impedancometry. Am J Physiol. 1997;273:G958-G964. |
| 16. | Nguyen HN, Silny J, Wüller S, Marschall HU, Rau G, Matern S. Chyme transport patterns in human duodenum, determined by multiple intraluminal impedancometry. Am J Physiol. 1995;268:G700-G708. |
| 17. | Imam H, Sanmiguel C, Larive B, Bhat Y, Soffer E. Study of intestinal flow by combined videofluoroscopy, manometry, and multiple intraluminal impedance. Am J Physiol Gastrointest Liver Physiol. 2004;286:G263-G270. |
| 18. | Srinivasan R, Vela MF, Katz PO, Tutuian R, Castell JA, Castell DO. Esophageal function testing using multichannel intraluminal impedance. Am J Physiol Gastrointest Liver Physiol. 2001;280:G457-G462. |
| 19. | Heddle R, Collins PJ, Dent J, Horowitz M, Read NW, Chatterton B, Houghton LA. Motor mechanisms associated with slowing of the gastric emptying of a solid meal by an intraduodenal lipid infusion. J Gastroenterol Hepatol. 1989;4:437-447. |
| 20. | Anvari M, Dent J, Malbert C, Jamieson GG. Mechanics of pulsatile transpyloric flow in the pig. J Physiol. 1995;488:193-202. |
| 21. | Soffer EE, Adrian TE. Effect of meal composition and sham feeding on duodenojejunal motility in humans. Dig Dis Sci. 1992;37:1009-1014. |