Published online Apr 28, 2010. doi: 10.3748/wjg.v16.i16.1943
Revised: February 11, 2010
Accepted: February 18, 2010
Published online: April 28, 2010
Insulin resistance is one of the pathological features in patients with hepatitis C virus (HCV) infection. Generally, persistence of insulin resistance leads to an increase in the risk of life-threatening complications such as cardiovascular diseases. However, these complications are not major causes of death in patients with HCV-associated insulin resistance. Indeed, insulin resistance plays a crucial role in the development of various complications and events associated with HCV infection. Mounting evidence indicates that HCV-associated insulin resistance may cause (1) hepatic steatosis; (2) resistance to anti-viral treatment; (3) hepatic fibrosis and esophageal varices; (4) hepatocarcinogenesis and proliferation of hepatocellular carcinoma; and (5) extrahepatic manifestations. Thus, HCV-associated insulin resistance is a therapeutic target at any stage of HCV infection. Although the risk of insulin resistance in HCV-infected patients has been documented, therapeutic guidelines for preventing the distinctive complications of HCV-associated insulin resistance have not yet been established. In addition, mechanisms for the development of HCV-associated insulin resistance differ from lifestyle-associated insulin resistance. In order to ameliorate HCV-associated insulin resistance and its complications, the efficacy of the following interventions is discussed: a late evening snack, coffee consumption, dietary iron restriction, phlebotomy, and zinc supplements. Little is known regarding the effect of anti-diabetic agents on HCV infection, however, a possible association between use of exogenous insulin or a sulfonylurea agent and the development of HCC has recently been reported. On the other hand, insulin-sensitizing agents are reported to improve sustained virologic response rates. In this review, we summarize distinctive complications of, and therapeutic strategies for, HCV-associated insulin resistance. Furthermore, we discuss supplementation with branched-chain amino acids as a unique insulin-sensitizing strategy for patients with HCV-associated insulin resistance.
- Citation: Kawaguchi T, Sata M. Importance of hepatitis C virus-associated insulin resistance: Therapeutic strategies for insulin sensitization. World J Gastroenterol 2010; 16(16): 1943-1952
- URL: https://www.wjgnet.com/1007-9327/full/v16/i16/1943.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i16.1943
Insulin resistance is frequently seen in patients with hepatitis C virus (HCV) infection[1,2]. Although in the general population, lack of exercise and overeating are major causes of insulin resistance, in patients with HCV infection, hepatic inflammation, activated inflammatory cytokines, and HCV-induced impairments of insulin and lipid signaling molecules are also important factors for the development of insulin resistance[3-14]. Therefore, the prevalence of insulin resistance is higher in patients with HCV infection compared to that in the general population and patients with other hepatobiliary disorders[6,15].
Generally, insulin resistance results in the development of type 2 diabetes mellitus and increases the risk of life-threatening complications such as cardiovascular diseases, renal failure, and infections. However, these complications are not major causes of death in cirrhotic patients with insulin resistance[16]. On the other hand, the development of intrahepatic complications, including hepatocellular carcinoma (HCC), is known to be associated with insulin resistance[17-21]. Insulin resistance is also reported to be involved in the development of extrahepatic manifestations of HCV infection including gastric cancer[22-24].
Reduction of fasting blood glucose and hemoglobin A1c (HbA1c) is a well-established therapeutic strategy for prevention of complications in diabetic patients[25,26]. However, in patients with chronic liver diseases, fasting blood glucose and HbA1c are not always available for evaluation of glucose metabolism because of decreased hepatic glycogen content[27] and increased turnover of hemoglobin[28]. Furthermore, an association between the use of exogenous insulin or sulfonylurea agents and the development of HCC has recently been reported[29,30]. Although therapeutic guidelines for inhibiting the distinctive complications of HCV-associated insulin resistance are not yet available, amelioration of insulin resistance is considered to inhibit complications and improve prognosis. Here, we summarize treatments that could reduce HCV-associated insulin resistance.
In this review, we summarize distinctive complications of, and therapeutic strategies for, HCV-associated insulin resistance. In addition, we discuss the merits of branched-chain amino acid (BCAA) supplementation as a unique insulin-sensitizing strategy for patients with HCV-associated insulin resistance.
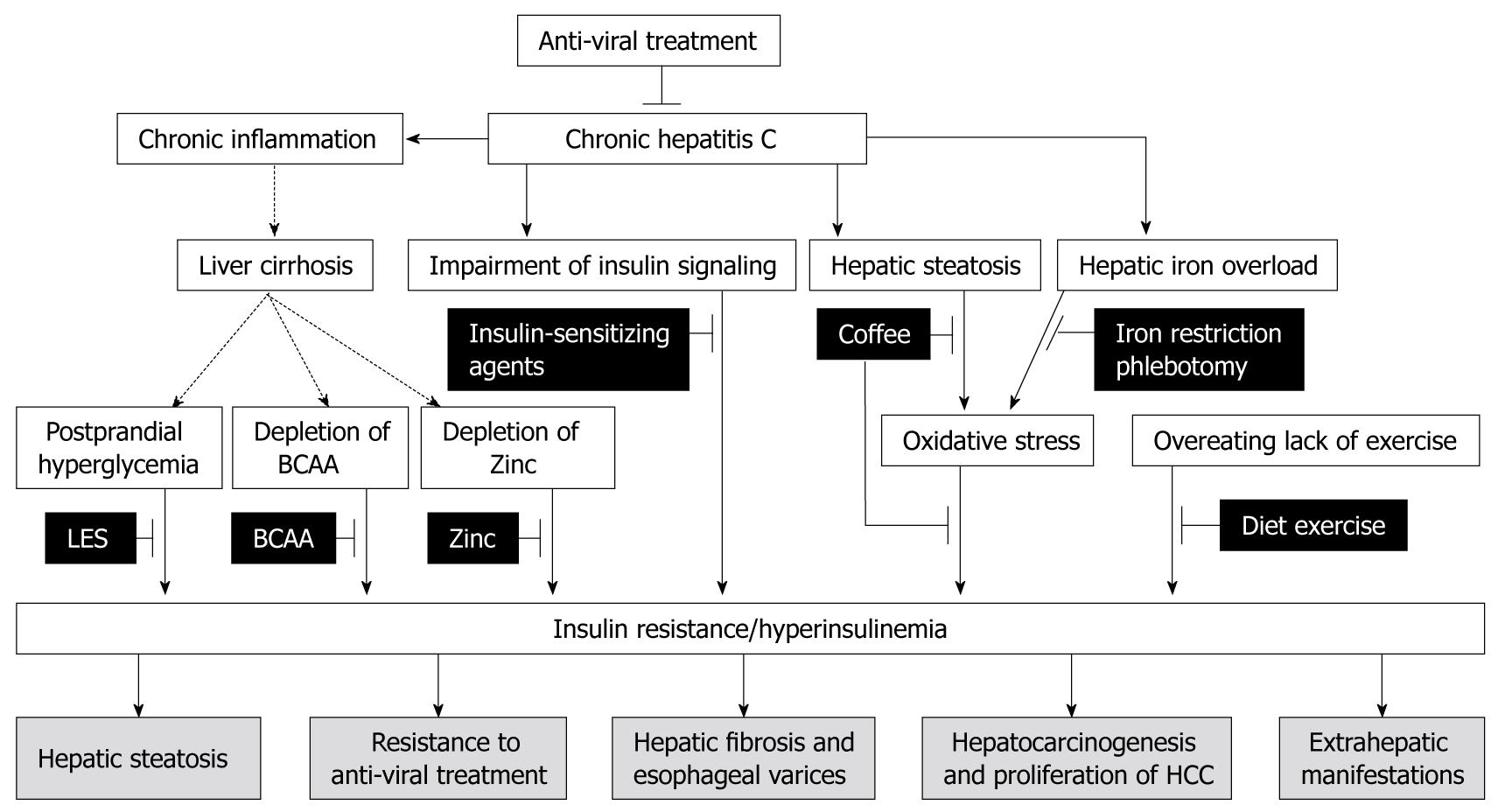
Complications of HCV-associated insulin resistance are different from those of lifestyle-associated insulin resistance[16]. Cardiovascular diseases are major causes of death in patients with lifestyle-associated insulin resistance[31]. However, these complications are not major causes of death in patients with HCV-associated insulin resistance[16]. In contrast, HCV-associated insulin resistance is involved in the development of various complications associated with HCV infection. Here, we summarize events associated with insulin resistance that are distinctive complications of HCV-associated insulin resistance (Figure 1).
Hepatic steatosis is commonly observed[32,33] and is an independent risk factor for disease progression in patients with HCV infection[34]. Various mechanisms are operative in the development of hepatic steatosis. HCV core protein induces production of reactive oxygen species and lipid peroxidation[35]. HCV core protein also regulates secretion of very low-density lipoprotein, triglycerides, and apoliprotein B through regulation of fatty acid synthase, microsomal triglyceride transport protein, peroxisome proliferator-activated receptor γ (PPARγ), and sterol regulatory element binding protein-1c[9,36-38]. Thus, HCV itself is directly involved in the development of hepatic steatosis. In addition, insulin is an anabolic hormone and promotes hepatic lipogenesis through activation of hydroxymethylglutaryl-CoA reductase and acetyl-CoA carboxylase[39]. In addition, insulin inhibits lipolysis through regulation of phosphodiesterase type 3B[19]. In HCV core gene transgenic mice, the development of insulin resistance precedes the development of hepatic steatosis, suggesting that insulin resistance may induce hepatic steatosis[8,40]. However, hepatic steatosis could also cause insulin resistance[41,42], and therefore, the initial step in HCV-related metabolic disorders remains unclear in patients with HCV infection.
Insulin resistance is associated with a poor response to anti-viral treatment in patients with HCV genotype 1, 2, and 3 infections[10,43-46]. Although the reason for an association between insulin resistance and resistance to anti-viral treatment is largely unknown, the following are possibilities. Insulin resistance is known to increase hepatic lipid synthesis[47]. Since the lipid droplet is an important organelle for HCV replication[48], accumulation of hepatic lipid droplets may increase HCV replication and result in poor responses to anti-viral treatment, even in patients with HCV genotype 2 and 3[45].
Alternatively, HCV core protein is reported to upregulate suppressor of cytokine signaling (SOCS) 3[6,49-52], which acts as an adaptor to facilitate the ubiquitination of signaling proteins, leading to subsequent proteasomal degradation of SOCS3[19]. HCV core protein-induced SOCS3 upregulation promotes proteasomal degradation of insulin receptor substrate (IRS) 1 and IRS2, resulting in the development of insulin resistance in patients with HCV infection[6,19,44]. Simultaneously, SOCS3 is also known to inhibit interferon-alpha-induced expression of the anti-viral proteins 2',5'-oligoadenylate synthetase and myxovirus resistance A through inactivation of Janus kinase, a signal transducer and activator of the transcription pathway[49]. Thus, SOCS3 seems to be a key molecule for a cross-talk between insulin resistance and resistance in patients with HCV infection. In fact, hepatic expression of SOCS3 has predictive value for the outcome of anti-viral therapy in patients with HCV infection[53,54].
Insulin resistance is closely associated with progression of hepatic fibrosis in patients with HCV infection[6,11,55]. The hepatocyte is known to degrade circulating insulin, and, therefore, hepatic fibrosis may reduce insulin clearance, resulting in increased serum insulin levels regardless of the presence of insulin resistance[56]. However, insulin resistance is seen in early stages of chronic hepatitis C[6]. Furthermore, even in patients that have received a liver transplantation for HCV-related liver cirrhosis, insulin resistance is a risk factor for rapid progression of hepatic fibrosis[57]. These findings suggest that insulin resistance promotes hepatic fibrosis. Insulin resistance may directly affect hepatic stellate cells and increase connective tissue growth factor (CTGF), which causes production of extracellular matrix[58]. Alternatively, insulin resistance-induced hepatic lipid accumulation may increase oxidative stress, resulting in progression of hepatic fibrosis[32].
Insulin resistance is also a risk factor for esophageal varices in cirrhotic patients with HCV infection[59]. As the hepatic fibrosis is correlated with the development of esophageal varices, insulin resistance may be associated with the development of esophageal varices through progression of hepatic fibrosis[60]. In addition, insulin modulates the endothelial synthesis of nitric oxide and endothelin[61], regulators of sinusoidal blood flow[62]. Thus, insulin-induced hepatic fibrosis and vasoconstriction may be possible mechanisms for the development of esophageal varices.
Liver cirrhosis, aging, and being a male are well-known risk factors for the development of HCC in patients with HCV infection[18,63]. In addition, insulin resistance is now recognized as an independent risk factor for the development of HCC worldwide[18,63]. Diabetes is reported as the only independent risk factor for HCC in patients with chronic hepatitis C[21]. Moreover, development of diabetes-related HCC is reported to be independent of viral hepatitis and alcoholism[64]. These findings suggest that insulin resistance has direct effects on hepatocarcinogenesis. Although precise mechanisms for this effect remain unclear, the following explanations may be put forward. Insulin resistance causes lipid accumulation[19]. Visceral adiposity results in changes in serum adipocytokine levels, including reduction of adiponectin, which suppresses effects for hepatocarcinogenesis[65]. Hepatic lipid accumulation also increases oxidative stress, which may be responsible for the development of HCC[18,63]. Besides these possibilities, insulin has a mitogenic effect[19,30], suggesting that insulin may be directly linked to hepatocarcinogenesis[19].
Insulin resistance may be associated not only with hepatocarcinogenesis, but also with proliferation of HCC. We have examined the significance of insulin resistance on the prognosis in patients with HCV-associated HCC and found that insulin resistance is an independent risk factor for poor prognosis[20]. As no significant difference was seen in disease-free survival between patients with and without insulin resistance, these findings indicate that insulin resistance accelerates the proliferation of HCC[20]. In good accordance with our results, Saito et al[66] reported that reduction of serum insulin levels by continuous infusion of octreotide significantly suppressed proliferation of HCC. Although the mechanisms for insulin-induced proliferation of HCC remain obscure, insulin exerts growth-promoting activity through activation of a mitogen-activated protein kinase pathway[19]. In addition, overexpression of transducing molecules for insulin signaling, IRS1[67] and IRS2[68], and downregulation of suppressing molecules for insulin signaling, phosphatase and tensin homologue[69], and SH2 domain-containing inositol phosphatase- 2[20] occur in HCC. Thus, HCC may be sensitive to insulin stimulation.
HCV causes extrahepatic manifestations including mixed cryoglobulinemia, Sjögren’s syndrome, and non-Hodgkin lymphoma, oral lichen planus, oral squamous cell carcinoma, and malignancies other than HCC[22-24,70-73]. In patients with extrahepatic manifestations of HCV, fasting insulin levels and homeostasis model assessment for insulin resistance are significantly higher than for patients without extrahepatic manifestations[22]. Among various extrahepatic manifestations, insulin resistance is associated with oral lichen planus[23], oral squamous cell carcinoma[24], and multiple primary cancers including gastric cancer[24]. Although reasons for this association remain unclear, a high prevalence of precancerous lesions and cancers are seen in patients with type 2 diabetes mellitus[74,75], suggesting that insulin resistance or hyperinsulinemia may enhance carcinogenic activities.
Despite awareness of the increased risk of insulin resistance, therapeutic guidelines to inhibit distinctive complications of HCV-associated insulin resistance have not yet been established. HCV itself has a significant impact on the development of insulin resistance, and eradication of HCV improves insulin resistance[44,46,76]. Thus, anti-viral therapy is a fundamental therapeutic strategy for patients with HCV infection. In addition, amelioration of insulin resistance is considered to inhibit complications and improve prognosis. Here, we summarize treatments which could improve HCV-associated insulin resistance as therapeutic strategies (Figure 1).
Proper diet and exercise are fundamental for patients with lifestyle-associated insulin resistance as well as patients with HCV-associated insulin resistance[77-80]. As a nutritional treatment for liver cirrhosis, divided energy intake (4 to 6 meals/d) has been recommended[77,79]. As postprandial hyperglycemia is characteristic of HCV-associated insulin resistance[77-80], a decrease in energy intake per meal reduces postprandial hyperglycemia and hyperinsulinemia. In particular, a late evening snack is reported not only to improve glucose intolerance[81-84], but also to suppress hepatocarcinogenesis in cirrhotic patients[85].
Coffee consumption reduces the risk of elevated serum alanine aminotransferase activity[86], hepatic fibrosis[87], and disease progression in chronic hepatitis C[88]. Coffee consumption also reduces the risk of HCC independent of HCC etiology[89]. Caffeine is metabolized by hepatic cytochrome P450 1A2 into 3 metabolites, the dimethylxanthines paraxanthine, theobromine, and theophylline. Of these metabolites, theophylline inhibits transforming growth factor-β-stimulated CTGF expression through PPARγ and Smad 2/3-dependent pathways. Since CTGF and transforming growth factor-β are important factors associated with progression of hepatic fibrosis and hepatocarcinogenesis, a metabolite of caffeine, theophylline, may have an inhibitory effect on the development of complications associated with HCV infection. In addition, coffee has significant effects on glucose metabolism[90]. In an animal experiment, the insulin-sensitizing effects of coffee have been demonstrated[91]. Similarly, in a human study, coffee consumption reduced fasting glucose and insulin levels[90,92]. Although the mechanisms for the coffee-induced insulin-sensitizing effect remain unclear, some possibilities exist. Chlorogenic acids, a constituent of coffee, inhibits hepatic glucose-6-phosphate translocation[90,93], limits glucose absorption from the gut by inhibiting Na+-dependent transport[94], and increases the secretion of glucose regulating hormone, glucagon-like peptide (GLP)-1, from the gut[90,95,96]. These findings suggest that a constituent of coffee, chlorogenic acid, directly ameliorates HCV-associated insulin resistance. Furthermore, coffee modulates lipid metabolism[97,98] and lowers body weight[90], indicating that coffee may suppress the lipid-induced increase in oxidative stress and ameliorates HCV-associated insulin resistance.
Hepatic iron overload produces oxidative stress and is a factor responsible for the development of HCV-associated insulin resistance[4,99-101]. Although the pathogenesis of hepatic iron overload remains unclear, recent studies showed that iron-regulating molecules are modulated by HCV infection. Hepcidin is a negative regulator of duodenal iron absorption and macrophage iron release[100] and decreased hepatic expression of hepcidin is seen in both HCV polyprotein transgenic mice[102] and patients with HCV infection[103-105]. In addition, upregulation of hepatic expression of transferrin receptor 2, a mediator of iron uptake, is responsible for hepatic iron overload[106].
In order to reduce hepatic iron deposition, dietary iron restriction and phlebotomy are effective. Dietary iron restriction (less than 7 mg/d) decreases serum alanine aminotransferase levels in patients with HCV infection[107]. Phlebotomy reduces oxidative stress as well as insulin resistance in patients with HCV infection[101,108,109]. A long-term combination treatment with phlebotomy and dietary iron restriction reduces the risk of development of HCC in patients with HCV infection[110].
Zinc plays a crucial role in the metabolism of protein, carbohydrate, lipid, nucleic acid, and ammonia[111-113]. In fact, zinc supplementation improves glucose disposal in patients with cirrhosis[114]. Zinc also inhibits hepatic inflammation[115] and hepatic fibrosis[116]. More recently, zinc supplementation was shown to lower the cumulative incidence of HCC in patients with HCV infection[117]. It is unclear whether these inhibitory effects of zinc on progression of liver disease are mediated by amelioration of insulin resistance. However, zinc participates in the synthesis, storage and secretion of insulin[118] and regulates the binding ability of insulin to bind to its receptor[113]. As the serum zinc level is decreased in patients with HCV infection[115,117], supplementation of zinc could be a therapeutic option.
Exogenous insulin and sulfonylurea agents: Anti-diabetic agents are effective for decreasing plasma glucose and HbA1c levels, leading to prevention of diabetes mellitus-associated complications including cardiovascular diseases[119,120]. However, it has never been determined whether anti-diabetic agents prevent complications or improve prognosis in patients with HCV infection. Use of exogenous insulin or sulfonylurea agents may worsen hyperinsulinemia. In fact, we, along with others, recently reported an association between exogenous insulin or sulphonylurea treatment and the development of HCC in patients with HCV infection[29,30,121]. Use of exogenous insulin is also reported to be associated with the development of colon cancer[122] and other malignancies[123]. Although a causal relationship between exogenous insulin and the development of HCC remains controversial[124], the reduction of serum insulin levels is a first line therapeutic strategy for insulin resistance[125-128].
Insulin-sensitizing agents: Insulin resistance is associated with a poor response to anti-viral treatment in patients with HCV infection[10,43-46]. Amelioration of insulin resistance may improve the response to anti-viral treatment. However, the impact of insulin-sensitizing agents, biguanides and thiazolidinediones, on sustained virologic response (SVR) rates has not yet been established. Recently, metformin, a biguanide agent, has been reported to ameliorate HCV-associated insulin resistance, and increase the SVR rate in HCV genotype 1 infected patients with normalization of homeostasis model assessment for insulin resistance at week 24 of therapy[129]. Pioglitazone, a thiazolidinedione agent, has also been reported to ameliorate insulin resistance and increase SVR rates in patients with HCV genotype 4 infection[130]. Although the insulin-sensitizing mechanisms of metformin and pioglitazone are different, both agents are known to upregulate IRS[131,132], which is the molecule responsible for HCV-associated insulin resistance[3,6,50], and to improve HCV-associated insulin resistance. Because both agents have severe adverse effects, neither is recommended for patients with liver cirrhosis. Biguanides predispose cirrhotic patients to lactic acidosis[133]. Thiazolidinediones cause overproduction of hydrogen peroxide leading to severe hepatotoxicity[134]. Thus, further validation for safety is required.
Dipeptidyl peptidase IV (DPPIV) inhibitor is a new therapeutic agent[135] and its clinical efficacy in type 2 diabetes has been shown[136]. Although no study has examined the effect of DPPIV inhibitor on HCV-associated insulin resistance, we found that activation of DPPIV is a factor responsible for HCV-associated insulin resistance[27]. Thus, a DPPIV inhibitor may be suited for ameliorating HCV-associated insulin resistance.
BCAA are constituents of proteins and are required for protein synthesis[19,78,137,138]. In addition, BCAA are reported to modulate glucose metabolism. Leucine and isoleucine induce glucose transporter 1 and 4 translocation to the plasma membrane of muscle cells and improve glucose metabolism in a carbon tetrachloride-induced cirrhotic rat model[139]. In addition, leucine enhances the insulin-induced activation of the Akt/mammalian target of the rapamycin pathway in adipocytes of db/db mice[140]. Moreover, isoleucine increases hepatic phosphatidylinositol 3-kinase activity and improves insulin resistance in Zucker fa/fa rats, a model of severe insulin resistance[141]. Recently, knockout of the mitochondrial BCAA aminotransferase gene in mice, in which results in elevated plasma BCAA levels, was found to ameliorate insulin resistance[142]. Thus, BCAA improve insulin signaling in various animal models via several pathways. In good agreement with these results in animals, in human studies, we have recently shown that BCAA-enriched supplementation reduces insulin resistance in patients with HCV infection[143,144]. In a multicenter, randomized, controlled trial, BCAA supplementation led to a reduction in the risk of HCC in cirrhotic patients[145]. This suppressive effect on hepatocarcinogenesis was more evident in obese patients with HCV infection[145]. Both obesity and HCV induce the development of insulin resistance. Thus, BCAA may improve insulin resistance and subsequently inhibit insulin resistance-induced hepatocarcinogenesis[19,145].
In this review, we summarize the distinctive complications of, and therapeutic strategies for, HCV-associated insulin resistance. Although cardiovascular diseases, renal failure, and infections are well-known complications of lifestyle-associated insulin resistance, these complications are not major causes of death in cirrhotic patients with insulin resistance. HCV-associated insulin resistance rather causes (1) hepatic steatosis, (2) resistance to anti-viral treatment, (3) hepatic fibrosis and esophageal varices, (4) hepatocarcinogenesis and proliferation of HCC, and (5) extrahepatic manifestations. These complications are life-threatening, and therapeutic strategies for HCV-associated insulin resistance have to be considered on the basis of its pathogenic mechanisms.
Pathogenic mechanisms for HCV-associated insulin resistance differ from those for lifestyle-associated insulin resistance. Postprandial hyperglycemia, lipid-induced oxidative stress, hepatic iron overload, and depletion of zinc are responsible for the development of HCV-associated insulin resistance. Therefore, a late evening snack, coffee consumption, dietary iron restriction, phlebotomy, and supplementation of zinc are recommended therapeutic strategies. No clinical guidelines for the use of anti-diabetic agents are available for patients with HCV-associated insulin resistance. However, use of exogenous insulin or sulphonylurea may increase the risk for HCC. On the other hand, insulin-sensitizing agents may improve the SVR rate of anti-viral treatment. In addition, BCAA supplementation has an insulin-sensitizing effect as well as a suppressive effect on hepatocarcinogenesis. Thus, in order to ameliorate HCV-associated insulin resistance, various therapeutic approaches are required.
Peer reviewer: Atsushi Tanaka, MD, PhD, Associate Professor, Department of Medicine, Teikyo University School of Medicine, 2-11-1, Kaga, Itabashi-ku, Tokyo 173-8605, Japan
S- Editor Wang YR L- Editor Cant MR E- Editor Ma WH
| 1. | Allison ME, Wreghitt T, Palmer CR, Alexander GJ. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J Hepatol. 1994;21:1135-1139. |
| 2. | Caronia S, Taylor K, Pagliaro L, Carr C, Palazzo U, Petrik J, O'Rahilly S, Shore S, Tom BD, Alexander GJ. Further evidence for an association between non-insulin-dependent diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;30:1059-1063. |
| 3. | Aytug S, Reich D, Sapiro LE, Bernstein D, Begum N. Impaired IRS-1/PI3-kinase signaling in patients with HCV: a mechanism for increased prevalence of type 2 diabetes. Hepatology. 2003;38:1384-1392. |
| 4. | Furutani M, Nakashima T, Sumida Y, Hirohama A, Yoh T, Kakisaka Y, Mitsuyoshi H, Senmaru H, Okanoue T. Insulin resistance/beta-cell function and serum ferritin level in non-diabetic patients with hepatitis C virus infection. Liver Int. 2003;23:294-299. |
| 5. | Maeno T, Okumura A, Ishikawa T, Kato K, Sakakibara F, Sato K, Ayada M, Hotta N, Tagaya T, Fukuzawa Y. Mechanisms of increased insulin resistance in non-cirrhotic patients with chronic hepatitis C virus infection. J Gastroenterol Hepatol. 2003;18:1358-1363. |
| 6. | Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, Taniguchi E, Kumemura H, Hanada S, Maeyama M. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165:1499-1508. |
| 7. | Narita R, Abe S, Kihara Y, Akiyama T, Tabaru A, Otsuki M. Insulin resistance and insulin secretion in chronic hepatitis C virus infection. J Hepatol. 2004;41:132-138. |
| 8. | Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840-848. |
| 9. | Negro F. Mechanisms and significance of liver steatosis in hepatitis C virus infection. World J Gastroenterol. 2006;12:6756-6765. |
| 10. | Romero-Gómez M. Insulin resistance and hepatitis C. World J Gastroenterol. 2006;12:7075-7080. |
| 11. | Taura N, Ichikawa T, Hamasaki K, Nakao K, Nishimura D, Goto T, Fukuta M, Kawashimo H, Fujimoto M, Kusumoto K. Association between liver fibrosis and insulin sensitivity in chronic hepatitis C patients. Am J Gastroenterol. 2006;101:2752-2759. |
| 12. | Tuma P, Vispo E, Barreiro P, Soriano V. [Role of tenofovir in HIV and hepatitis C virus coinfection]. Enferm Infecc Microbiol Clin. 2008;26 Suppl 8:31-37. |
| 13. | Akuta N, Suzuki F, Hirakawa M, Kawamura Y, Yatsuji H, Sezaki H, Suzuki Y, Hosaka T, Kobayashi M, Kobayashi M. Amino acid substitutions in the hepatitis C virus core region of genotype 1b are the important predictor of severe insulin resistance in patients without cirrhosis and diabetes mellitus. J Med Virol. 2009;81:1032-1039. |
| 14. | Eguchi Y, Mizuta T, Ishibashi E, Kitajima Y, Oza N, Nakashita S, Hara M, Iwane S, Takahashi H, Akiyama T. Hepatitis C virus infection enhances insulin resistance induced by visceral fat accumulation. Liver Int. 2009;29:213-220. |
| 15. | Kawaguchi T, Nagao Y, Tanaka K, Ide T, Harada M, Kumashiro R, Sata M. Causal relationship between hepatitis C virus core and the development of type 2 diabetes mellitus in a hepatitis C virus hyperendemic area: a pilot study. Int J Mol Med. 2005;16:109-114. |
| 16. | Bianchi G, Marchesini G, Zoli M, Bugianesi E, Fabbri A, Pisi E. Prognostic significance of diabetes in patients with cirrhosis. Hepatology. 1994;20:119-125. |
| 17. | Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54:533-539. |
| 18. | El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27-S34. |
| 19. | Kawaguchi T, Yamagishi S, Sata M. Branched-chain amino acids and pigment epithelium-derived factor: novel therapeutic agents for hepatitis c virus-associated insulin resistance. Curr Med Chem. 2009;16:4843-4857. |
| 20. | Sumie S, Kawaguchi T, Komuta M, Kuromatsu R, Itano S, Okuda K, Taniguchi E, Ando E, Takata A, Fukushima N. Significance of glucose intolerance and SHIP2 expression in hepatocellular carcinoma patients with HCV infection. Oncol Rep. 2007;18:545-552. |
| 21. | Tazawa J, Maeda M, Nakagawa M, Ohbayashi H, Kusano F, Yamane M, Sakai Y, Suzuki K. Diabetes mellitus may be associated with hepatocarcinogenesis in patients with chronic hepatitis C. Dig Dis Sci. 2002;47:710-715. |
| 22. | Nagao Y, Kawaguchi T, Tanaka K, Kumashiro R, Sata M. Extrahepatic manifestations and insulin resistance in an HCV hyperendemic area. Int J Mol Med. 2005;16:291-296. |
| 23. | Nagao Y, Kawasaki K, Sata M. Insulin resistance and lichen planus in patients with HCV-infectious liver diseases. J Gastroenterol Hepatol. 2008;23:580-585. |
| 24. | Nagao Y, Sata M. High incidence of multiple primary carcinomas in HCV-infected patients with oral squamous cell carcinoma. Med Sci Monit. 2009;15:CR453-CR459. |
| 25. | Franz MJ, Bantle JP, Beebe CA, Brunzell JD, Chiasson JL, Garg A, Holzmeister LA, Hoogwerf B, Mayer-Davis E, Mooradian AD. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care. 2002;25:148-198. |
| 26. | Global Guideline for Type 2 Diabetes: recommendations for standard, comprehensive, and minimal care. Diabet Med. 2006;23:579-593. |
| 27. | Itou M, Kawaguchi T, Taniguchi E, Sumie S, Oriishi T, Mitsuyama K, Tsuruta O, Ueno T, Sata M. Altered expression of glucagon-like peptide-1 and dipeptidyl peptidase IV in patients with HCV-related glucose intolerance. J Gastroenterol Hepatol. 2008;23:244-251. |
| 28. | Qamar AA, Grace ND, Groszmann RJ, Garcia-Tsao G, Bosch J, Burroughs AK, Ripoll C, Maurer R, Planas R, Escorsell A. Incidence, prevalence, and clinical significance of abnormal hematologic indices in compensated cirrhosis. Clin Gastroenterol Hepatol. 2009;7:689-695. |
| 29. | Donadon V, Balbi M, Ghersetti M, Grazioli S, Perciaccante A, Della Valentina G, Gardenal R, Dal Mas M, Casarin P, Zanette G. Antidiabetic therapy and increased risk of hepatocellular carcinoma in chronic liver disease. World J Gastroenterol. 2009;15:2506-2511. |
| 30. | Kawaguchi T, Taniguchi E, Morita Y, Shirachi M, Tateishi I, Nagata E, Sata M. Association of exogenous insulin or sulphonylurea treatment with an increased incidence of hepatoma in patients with hepatitis C virus infection. Liver Int. 2009;Epub ahead of print. |
| 31. | Jansson SP, Andersson DK, Svärdsudd K. Mortality trends in subjects with and without diabetes during 33 years of follow-up. Diabetes Care. 2010;33:551-556. |
| 32. | Negro F, Sanyal AJ. Hepatitis C virus, steatosis and lipid abnormalities: clinical and pathogenic data. Liver Int. 2009;29 Suppl 2:26-37. |
| 33. | Ong JP, Younossi ZM, Speer C, Olano A, Gramlich T, Boparai N. Chronic hepatitis C and superimposed nonalcoholic fatty liver disease. Liver. 2001;21:266-271. |
| 34. | Leandro G, Mangia A, Hui J, Fabris P, Rubbia-Brandt L, Colloredo G, Adinolfi LE, Asselah T, Jonsson JR, Smedile A. Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology. 2006;130:1636-1642. |
| 35. | Korenaga M, Wang T, Li Y, Showalter LA, Chan T, Sun J, Weinman SA. Hepatitis C virus core protein inhibits mitochondrial electron transport and increases reactive oxygen species (ROS) production. J Biol Chem. 2005;280:37481-37488. |
| 36. | McPherson S, Jonsson JR, Barrie HD, O'Rourke P, Clouston AD, Powell EE. Investigation of the role of SREBP-1c in the pathogenesis of HCV-related steatosis. J Hepatol. 2008;49:1046-1054. |
| 37. | Negro F. Peroxisome proliferator-activated receptors and hepatitis C virus-induced insulin resistance. PPAR Res. 2009;2009:483485. |
| 38. | Perlemuter G, Sabile A, Letteron P, Vona G, Topilco A, Chrétien Y, Koike K, Pessayre D, Chapman J, Barba G. Hepatitis C virus core protein inhibits microsomal triglyceride transfer protein activity and very low density lipoprotein secretion: a model of viral-related steatosis. FASEB J. 2002;16:185-194. |
| 39. | Geelen MJ, Harris RA, Beynen AC, McCune SA. Short-term hormonal control of hepatic lipogenesis. Diabetes. 1980;29:1006-1022. |
| 40. | Koike K. Hepatitis C as a metabolic disease: Implication for the pathogenesis of NASH. Hepatol Res. 2005;33:145-150. |
| 41. | Kawaguchi T, Osatomi K, Yamashita H, Kabashima T, Uyeda K. Mechanism for fatty acid "sparing" effect on glucose-induced transcription: regulation of carbohydrate-responsive element-binding protein by AMP-activated protein kinase. J Biol Chem. 2002;277:3829-3835. |
| 42. | Kawaguchi T, Takenoshita M, Kabashima T, Uyeda K. Glucose and cAMP regulate the L-type pyruvate kinase gene by phosphorylation/dephosphorylation of the carbohydrate response element binding protein. Proc Natl Acad Sci USA. 2001;98:13710-13715. |
| 43. | Cammà C, Bruno S, Di Marco V, Di Bona D, Rumi M, Vinci M, Rebucci C, Cividini A, Pizzolanti G, Minola E. Insulin resistance is associated with steatosis in nondiabetic patients with genotype 1 chronic hepatitis C. Hepatology. 2006;43:64-71. |
| 44. | Kawaguchi T, Ide T, Taniguchi E, Hirano E, Itou M, Sumie S, Nagao Y, Yanagimoto C, Hanada S, Koga H. Clearance of HCV improves insulin resistance, beta-cell function, and hepatic expression of insulin receptor substrate 1 and 2. Am J Gastroenterol. 2007;102:570-576. |
| 45. | Poustchi H, Negro F, Hui J, Cua IH, Brandt LR, Kench JG, George J. Insulin resistance and response to therapy in patients infected with chronic hepatitis C virus genotypes 2 and 3. J Hepatol. 2008;48:28-34. |
| 46. | Romero-Gómez M, Del Mar Viloria M, Andrade RJ, Salmerón J, Diago M, Fernández-Rodríguez CM, Corpas R, Cruz M, Grande L, Vázquez L. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology. 2005;128:636-641. |
| 47. | Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Goldstein JL. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell. 2000;6:77-86. |
| 48. | Miyanari Y, Atsuzawa K, Usuda N, Watashi K, Hishiki T, Zayas M, Bartenschlager R, Wakita T, Hijikata M, Shimotohno K. The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol. 2007;9:1089-1097. |
| 49. | Vlotides G, Sörensen AS, Kopp F, Zitzmann K, Cengic N, Brand S, Zachoval R, Auernhammer CJ. SOCS-1 and SOCS-3 inhibit IFN-alpha-induced expression of the antiviral proteins 2,5-OAS and MxA. Biochem Biophys Res Commun. 2004;320:1007-1014. |
| 50. | Pazienza V, Clément S, Pugnale P, Conzelman S, Foti M, Mangia A, Negro F. The hepatitis C virus core protein of genotypes 3a and 1b downregulates insulin receptor substrate 1 through genotype-specific mechanisms. Hepatology. 2007;45:1164-1171. |
| 51. | Persico M, Capasso M, Persico E, Svelto M, Russo R, Spano D, Crocè L, La Mura V, Moschella F, Masutti F. Suppressor of cytokine signaling 3 (SOCS3) expression and hepatitis C virus-related chronic hepatitis: Insulin resistance and response to antiviral therapy. Hepatology. 2007;46:1009-1015. |
| 52. | Del Campo JA, Romero-Gómez M. Steatosis and insulin resistance in hepatitis C: a way out for the virus? World J Gastroenterol. 2009;15:5014-5019. |
| 53. | Miyaaki H, Ichikawa T, Nakao K, Matsuzaki T, Muraoka T, Honda T, Takeshita S, Shibata H, Ozawa E, Akiyama M. Predictive value of suppressor of cytokine signal 3 (SOCS3) in the outcome of interferon therapy in chronic hepatitis C. Hepatol Res. 2009;39:850-855. |
| 54. | Walsh MJ, Jonsson JR, Richardson MM, Lipka GM, Purdie DM, Clouston AD, Powell EE. Non-response to antiviral therapy is associated with obesity and increased hepatic expression of suppressor of cytokine signalling 3 (SOCS-3) in patients with chronic hepatitis C, viral genotype 1. Gut. 2006;55:529-535. |
| 55. | Muzzi A, Leandro G, Rubbia-Brandt L, James R, Keiser O, Malinverni R, Dufour JF, Helbling B, Hadengue A, Gonvers JJ. Insulin resistance is associated with liver fibrosis in non-diabetic chronic hepatitis C patients. J Hepatol. 2005;42:41-46. |
| 56. | Petrides AS, Vogt C, Schulze-Berge D, Matthews D, Strohmeyer G. Pathogenesis of glucose intolerance and diabetes mellitus in cirrhosis. Hepatology. 1994;19:616-627. |
| 57. | Veldt BJ, Poterucha JJ, Watt KD, Wiesner RH, Hay JE, Rosen CB, Heimbach JK, Janssen HL, Charlton MR. Insulin resistance, serum adipokines and risk of fibrosis progression in patients transplanted for hepatitis C. Am J Transplant. 2009;9:1406-1413. |
| 58. | Paradis V, Perlemuter G, Bonvoust F, Dargere D, Parfait B, Vidaud M, Conti M, Huet S, Ba N, Buffet C. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001;34:738-744. |
| 59. | Cammà C, Petta S, Di Marco V, Bronte F, Ciminnisi S, Licata G, Peralta S, Simone F, Marchesini G, Craxì A. Insulin resistance is a risk factor for esophageal varices in hepatitis C virus cirrhosis. Hepatology. 2009;49:195-203. |
| 60. | Svegliati-Baroni G, De Minicis S, Marzioni M. Hepatic fibrogenesis in response to chronic liver injury: novel insights on the role of cell-to-cell interaction and transition. Liver Int. 2008;28:1052-1064. |
| 61. | Vincent MA, Montagnani M, Quon MJ. Molecular and physiologic actions of insulin related to production of nitric oxide in vascular endothelium. Curr Diab Rep. 2003;3:279-288. |
| 62. | Iwakiri Y, Groszmann RJ. Vascular endothelial dysfunction in cirrhosis. J Hepatol. 2007;46:927-934. |
| 63. | El-Serag HB. Epidemiology of hepatocellular carcinoma in USA. Hepatol Res. 2007;37 Suppl 2:S88-S94. |
| 64. | El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460-468. |
| 65. | Marra F, Bertolani C. Adipokines in liver diseases. Hepatology. 2009;50:957-969. |
| 66. | Saito K, Inoue S, Saito T, Kiso S, Ito N, Tamura S, Watanabe H, Takeda H, Misawa H, Togashi H. Augmentation effect of postprandial hyperinsulinaemia on growth of human hepatocellular carcinoma. Gut. 2002;51:100-104. |
| 67. | Nehrbass D, Klimek F, Bannasch P. Overexpression of insulin receptor substrate-1 emerges early in hepatocarcinogenesis and elicits preneoplastic hepatic glycogenosis. Am J Pathol. 1998;152:341-345. |
| 68. | Boissan M, Beurel E, Wendum D, Rey C, Lécluse Y, Housset C, Lacombe ML, Desbois-Mouthon C. Overexpression of insulin receptor substrate-2 in human and murine hepatocellular carcinoma. Am J Pathol. 2005;167:869-877. |
| 69. | Yao YJ, Ping XL, Zhang H, Chen FF, Lee PK, Ahsan H, Chen CJ, Lee PH, Peacocke M, Santella RM. PTEN/MMAC1 mutations in hepatocellular carcinomas. Oncogene. 1999;18:3181-3185. |
| 70. | Nagao Y, Sata M, Noguchi S, Tajiri N, Ono N, Fukuda T, Kameyama T, Ueno T. Various extrahepatic manifestations caused by hepatitis C virus infection. Int J Mol Med. 1999;4:621-625. |
| 71. | Nagao Y, Sata M, Tanikawa K, Itoh K, Kameyama T. Lichen planus and hepatitis C virus in the northern Kyushu region of Japan. Eur J Clin Invest. 1995;25:910-914. |
| 72. | Nagao Y, Tanaka J, Nakanishi T, Moriya T, Katayama K, Kumagai J, Komiya Y, Itoh Y, Myoken Y, Fujihara M. High incidence of extrahepatic manifestations in an HCV hyperendemic area. Hepatol Res. 2002;22:27-36. |
| 73. | Ohtsubo K, Sata M, Kawaguchi T, Morishige S, Takata Y, Oku E, Imamura R, Seki R, Hashiguchi M, Osaki K. Characterization of the light chain-restricted clonal B cells in peripheral blood of HCV-positive patients. Int J Hematol. 2009;89:452-459. |
| 74. | Husseini A, Abu-Rmeileh NM, Mikki N, Ramahi TM, Ghosh HA, Barghuthi N, Khalili M, Bjertness E, Holmboe-Ottesen G, Jervell J. Cardiovascular diseases, diabetes mellitus, and cancer in the occupied Palestinian territory. Lancet. 2009;373:1041-1049. |
| 75. | Ship JA. Diabetes and oral health: an overview. J Am Dent Assoc. 2003;134 Spec No:4S-10S. |
| 76. | Kawaguchi Y, Mizuta T, Oza N, Takahashi H, Ario K, Yoshimura T, Eguchi Y, Ozaki I, Hisatomi A, Fujimoto K. Eradication of hepatitis C virus by interferon improves whole-body insulin resistance and hyperinsulinaemia in patients with chronic hepatitis C. Liver Int. 2009;29:871-877. |
| 77. | ASPEN Board of Directors and the Clinical Guidelines Task Force. Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. JPEN J Parenter Enteral Nutr. 2002;26:1SA-138SA. |
| 78. | Kato A, Suzuki K. How to select BCAA preparations. Hepatol Res. 2004;30S:30-35. |
| 79. | Plauth M, Cabré E, Riggio O, Assis-Camilo M, Pirlich M, Kondrup J, Ferenci P, Holm E, Vom Dahl S, Müller MJ. ESPEN Guidelines on Enteral Nutrition: Liver disease. Clin Nutr. 2006;25:285-294. |
| 80. | Tajika M, Kato M, Mohri H, Miwa Y, Kato T, Ohnishi H, Moriwaki H. Prognostic value of energy metabolism in patients with viral liver cirrhosis. Nutrition. 2002;18:229-234. |
| 81. | Korenaga K, Korenaga M, Uchida K, Yamasaki T, Sakaida I. Effects of a late evening snack combined with alpha-glucosidase inhibitor on liver cirrhosis. Hepatol Res. 2008;38:1087-1097. |
| 82. | Okamoto M, Sakaida I, Tsuchiya M, Suzuki C, Okita K. Effect of a late evening snack on the blood glucose level and energy metabolism in patients with liver cirrhosis. Hepatol Res. 2003;27:45-50. |
| 83. | Sakaida I, Tsuchiya M, Okamoto M, Okita K. Late evening snack and the change of blood glucose level in patients with liver cirrhosis. Hepatol Res. 2004;30S:67-72. |
| 84. | Tsuchiya M, Sakaida I, Okamoto M, Okita K. The effect of a late evening snack in patients with liver cirrhosis. Hepatol Res. 2005;31:95-103. |
| 85. | Ohfuji S, Fukushima W, Tanaka T, Habu D, Takeda T, Tamori A, Sakaguchi H, Seki S, Kawada N, Nishiguchi S. Does a late evening meal reduce the risk of hepatocellular carcinoma among patients with chronic hepatitis C? Hepatol Res. 2008;38:860-868. |
| 86. | Ruhl CE, Everhart JE. Coffee and caffeine consumption reduce the risk of elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2005;128:24-32. |
| 87. | Modi AA, Feld JJ, Park Y, Kleiner DE, Everhart JE, Liang TJ, Hoofnagle JH. Increased caffeine consumption is associated with reduced hepatic fibrosis. Hepatology. 2010;51:201-209. |
| 88. | Freedman ND, Everhart JE, Lindsay KL, Ghany MG, Curto TM, Shiffman ML, Lee WM, Lok AS, Di Bisceglie AM, Bonkovsky HL. Coffee intake is associated with lower rates of liver disease progression in chronic hepatitis C. Hepatology. 2009;50:1360-1369. |
| 89. | Gelatti U, Covolo L, Franceschini M, Pirali F, Tagger A, Ribero ML, Trevisi P, Martelli C, Nardi G, Donato F. Coffee consumption reduces the risk of hepatocellular carcinoma independently of its aetiology: a case-control study. J Hepatol. 2005;42:528-534. |
| 90. | Tunnicliffe JM, Shearer J. Coffee, glucose homeostasis, and insulin resistance: physiological mechanisms and mediators. Appl Physiol Nutr Metab. 2008;33:1290-1300. |
| 91. | Shearer J, Farah A, de Paulis T, Bracy DP, Pencek RR, Graham TE, Wasserman DH. Quinides of roasted coffee enhance insulin action in conscious rats. J Nutr. 2003;133:3529-3532. |
| 92. | Naismith DJ, Akinyanju PA, Szanto S, Yudkin J. The effect, in volunteers, of coffee and decaffeinated coffee on blood glucose, insulin, plasma lipids and some factors involved in blood clotting. Nutr Metab. 1970;12:144-151. |
| 93. | Gerin I, Van Schaftingen E. Evidence for glucose-6-phosphate transport in rat liver microsomes. FEBS Lett. 2002;517:257-260. |
| 94. | Welsch CA, Lachance PA, Wasserman BP. Dietary phenolic compounds: inhibition of Na+-dependent D-glucose uptake in rat intestinal brush border membrane vesicles. J Nutr. 1989;119:1698-1704. |
| 95. | Johnston KL, Clifford MN, Morgan LM. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: glycemic effects of chlorogenic acid and caffeine. Am J Clin Nutr. 2003;78:728-733. |
| 96. | McCarty MF. A chlorogenic acid-induced increase in GLP-1 production may mediate the impact of heavy coffee consumption on diabetes risk. Med Hypotheses. 2005;64:848-853. |
| 97. | Heyden S. Does coffee influence the lipid metabolism? Z Ernahrungswiss. 1969;9:388-396. |
| 99. | Garrido Serrano A, Guerrero Igea FJ, Lepe Jiménez JA, Palomo Gil S, Grilo Reina A. Hepatitis C virus infection, increased serum ferritin and hyperinsulinemia. Rev Esp Enferm Dig. 2001;93:639-648. |
| 100. | Lecube A, Hernández C, Simó R. Glucose abnormalities in non-alcoholic fatty liver disease and chronic hepatitis C virus infection: the role of iron overload. Diabetes Metab Res Rev. 2009;25:403-410. |
| 101. | Mitsuyoshi H, Itoh Y, Sumida Y, Minami M, Yasui K, Nakashima T, Okanoue T. Evidence of oxidative stress as a cofactor in the development of insulin resistance in patients with chronic hepatitis C. Hepatol Res. 2008;38:348-353. |
| 102. | Nishina S, Hino K, Korenaga M, Vecchi C, Pietrangelo A, Mizukami Y, Furutani T, Sakai A, Okuda M, Hidaka I. Hepatitis C virus-induced reactive oxygen species raise hepatic iron level in mice by reducing hepcidin transcription. Gastroenterology. 2008;134:226-238. |
| 103. | Fujita N, Sugimoto R, Takeo M, Urawa N, Mifuji R, Tanaka H, Kobayashi Y, Iwasa M, Watanabe S, Adachi Y. Hepcidin expression in the liver: relatively low level in patients with chronic hepatitis C. Mol Med. 2007;13:97-104. |
| 104. | Nagashima M, Kudo M, Chung H, Ishikawa E, Hagiwara S, Nakatani T, Dote K. Regulatory failure of serum prohepcidin levels in patients with hepatitis C. Hepatol Res. 2006;36:288-293. |
| 105. | Piperno A, Mariani R, Trombini P, Girelli D. Hepcidin modulation in human diseases: from research to clinic. World J Gastroenterol. 2009;15:538-551. |
| 106. | Takeo M, Kobayashi Y, Fujita N, Urawa N, Iwasa M, Horiike S, Tanaka H, Kaito M, Adachi Y. Upregulation of transferrin receptor 2 and ferroportin 1 mRNA in the liver of patients with chronic hepatitis C. J Gastroenterol Hepatol. 2005;20:562-569. |
| 107. | Iwasa M, Iwata K, Kaito M, Ikoma J, Yamamoto M, Takeo M, Kuroda M, Fujita N, Kobayashi Y, Adachi Y. Efficacy of long-term dietary restriction of total calories, fat, iron, and protein in patients with chronic hepatitis C virus. Nutrition. 2004;20:368-371. |
| 108. | Fujita N, Miyachi H, Tanaka H, Takeo M, Nakagawa N, Kobayashi Y, Iwasa M, Watanabe S, Takei Y. Iron overload is associated with hepatic oxidative damage to DNA in nonalcoholic steatohepatitis. Cancer Epidemiol Biomarkers Prev. 2009;18:424-432. |
| 109. | Kaito M, Iwasa M, Kobayashi Y, Fujita N, Tanaka H, Gabazza EC, Adachi Y, Kojima Y, Nakagawa N, Watanabe S. Iron reduction therapy by phlebotomy reduces lipid peroxidation and oxidative stress in patients with chronic hepatitis C. J Gastroenterol. 2006;41:921-922. |
| 110. | Kato J, Miyanishi K, Kobune M, Nakamura T, Takada K, Takimoto R, Kawano Y, Takahashi S, Takahashi M, Sato Y. Long-term phlebotomy with low-iron diet therapy lowers risk of development of hepatocellular carcinoma from chronic hepatitis C. J Gastroenterol. 2007;42:830-836. |
| 111. | Hayashi M, Ikezawa K, Ono A, Okabayashi S, Hayashi Y, Shimizu S, Mizuno T, Maeda K, Akasaka T, Naito M. Evaluation of the effects of combination therapy with branched-chain amino acid and zinc supplements on nitrogen metabolism in liver cirrhosis. Hepatol Res. 2007;37:615-619. |
| 112. | Katayama K. Ammonia metabolism and hepatic encephalopathy. Hepatol Res. 2004;30S:73-80. |
| 113. | Marreiro DN, Geloneze B, Tambascia MA, Lerário AC, Halpern A, Cozzolino SM. [Role of zinc in insulin resistance]. Arq Bras Endocrinol Metabol. 2004;48:234-239. |
| 114. | Marchesini G, Bugianesi E, Ronchi M, Flamia R, Thomaseth K, Pacini G. Zinc supplementation improves glucose disposal in patients with cirrhosis. Metabolism. 1998;47:792-798. |
| 115. | Himoto T, Hosomi N, Nakai S, Deguchi A, Kinekawa F, Matsuki M, Yachida M, Masaki T, Kurokochi K, Watanabe S. Efficacy of zinc administration in patients with hepatitis C virus-related chronic liver disease. Scand J Gastroenterol. 2007;42:1078-1087. |
| 116. | Takahashi M, Saito H, Higashimoto M, Hibi T. Possible inhibitory effect of oral zinc supplementation on hepatic fibrosis through downregulation of TIMP-1: A pilot study. Hepatol Res. 2007;37:405-409. |
| 117. | Matsuoka S, Matsumura H, Nakamura H, Oshiro S, Arakawa Y, Hayashi J, Sekine N, Nirei K, Yamagami H, Ogawa M. Zinc supplementation improves the outcome of chronic hepatitis C and liver cirrhosis. J Clin Biochem Nutr. 2009;45:292-303. |
| 118. | Chausmer AB. Zinc, insulin and diabetes. J Am Coll Nutr. 1998;17:109-115. |
| 119. | Ajjan RA, Grant PJ. Cardiovascular disease prevention in patients with type 2 diabetes: The role of oral anti-diabetic agents. Diab Vasc Dis Res. 2006;3:147-158. |
| 120. | Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560-2572. |
| 121. | Komura T, Mizukoshi E, Kita Y, Sakurai M, Takata Y, Arai K, Yamashita T, Ohta T, Shimizu K, Nakamoto Y. Impact of diabetes on recurrence of hepatocellular carcinoma after surgical treatment in patients with viral hepatitis. Am J Gastroenterol. 2007;102:1939-1946. |
| 122. | Yang YX, Hennessy S, Lewis JD. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients. Gastroenterology. 2004;127:1044-1050. |
| 123. | Colhoun HM. Use of insulin glargine and cancer incidence in Scotland: a study from the Scottish Diabetes Research Network Epidemiology Group. Diabetologia. 2009;52:1755-1765. |
| 124. | Kath R, Schiel R, Müller UA, Höffken K. Malignancies in patients with insulin-treated diabetes mellitus. J Cancer Res Clin Oncol. 2000;126:412-417. |
| 125. | Fogli-Cawley JJ, Dwyer JT, Saltzman E, McCullough ML, Troy LM, Meigs JB, Jacques PF. The 2005 Dietary Guidelines for Americans and insulin resistance in the Framingham Offspring Cohort. Diabetes Care. 2007;30:817-822. |
| 126. | Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, Dallongeville J, De Backer G, Ebrahim S, Gjelsvik B. European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Eur J Cardiovasc Prev Rehabil. 2007;14 Suppl 2:E1-E40. |
| 127. | Haupt A, Häring HU, Matthaei S. [National practice guidelines. New aspects in therapy of type 2 diabetes mellitus]. MMW Fortschr Med. 2003;145:41-46. |
| 128. | Melkersson KI, Dahl ML, Hulting AL. Guidelines for prevention and treatment of adverse effects of antipsychotic drugs on glucose-insulin homeostasis and lipid metabolism. Psychopharmacology (Berl). 2004;175:1-6. |
| 129. | Romero-Gómez M, Diago M, Andrade RJ, Calleja JL, Salmerón J, Fernández-Rodríguez CM, Solà R, García-Samaniego J, Herrerías JM, De la Mata M. Treatment of insulin resistance with metformin in naïve genotype 1 chronic hepatitis C patients receiving peginterferon alfa-2a plus ribavirin. Hepatology. 2009;50:1702-1708. |
| 130. | Khattab M, Emad M, Abdelaleem A, Eslam M, Atef R, Shaker Y, Hamdy L. Pioglitazone improves virological response to peginterferon alpha-2b/ribavirin combination therapy in hepatitis C genotype 4 patients with insulin resistance. Liver Int. 2009;Epub ahead of print. |
| 131. | Smith U, Gogg S, Johansson A, Olausson T, Rotter V, Svalstedt B. Thiazolidinediones (PPARgamma agonists) but not PPARalpha agonists increase IRS-2 gene expression in 3T3-L1 and human adipocytes. FASEB J. 2001;15:215-220. |
| 132. | Yuan L, Ziegler R, Hamann A. Metformin modulates insulin post-receptor signaling transduction in chronically insulin-treated Hep G2 cells. Acta Pharmacol Sin. 2003;24:55-60. |
| 133. | Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334:574-579. |
| 134. | Shishido S, Koga H, Harada M, Kumemura H, Hanada S, Taniguchi E, Kumashiro R, Ohira H, Sato Y, Namba M. Hydrogen peroxide overproduction in megamitochondria of troglitazone-treated human hepatocytes. Hepatology. 2003;37:136-147. |
| 135. | Deacon CF, Holst JJ. Dipeptidyl peptidase IV inhibitors: a promising new therapeutic approach for the management of type 2 diabetes. Int J Biochem Cell Biol. 2006;38:831-844. |
| 136. | DeFronzo RA, Fleck PR, Wilson CA, Mekki Q. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin in patients with type 2 diabetes and inadequate glycemic control: a randomized, double-blind, placebo-controlled study. Diabetes Care. 2008;31:2315-2317. |
| 137. | Suzuki K, Suzuki K, Koizumi K, Ichimura H, Oka S, Takada H, Kuwayama H. Measurement of serum branched-chain amino acids to tyrosine ratio level is useful in a prediction of a change of serum albumin level in chronic liver disease. Hepatol Res. 2008;38:267-272. |
| 138. | Moriwaki H, Miwa Y, Tajika M, Kato M, Fukushima H, Shiraki M. Branched-chain amino acids as a protein- and energy-source in liver cirrhosis. Biochem Biophys Res Commun. 2004;313:405-409. |
| 139. | Nishitani S, Takehana K, Fujitani S, Sonaka I. Branched-chain amino acids improve glucose metabolism in rats with liver cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1292-G1300. |
| 140. | Hinault C, Mothe-Satney I, Gautier N, Lawrence JC Jr, Van Obberghen E. Amino acids and leucine allow insulin activation of the PKB/mTOR pathway in normal adipocytes treated with wortmannin and in adipocytes from db/db mice. FASEB J. 2004;18:1894-1896. |
| 141. | Broca C, Breil V, Cruciani-Guglielmacci C, Manteghetti M, Rouault C, Derouet M, Rizkalla S, Pau B, Petit P, Ribes G. Insulinotropic agent ID-1101 (4-hydroxyisoleucine) activates insulin signaling in rat. Am J Physiol Endocrinol Metab. 2004;287:E463-E471. |
| 142. | She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, Hutson SM. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab. 2007;6:181-194. |
| 143. | Kawaguchi T, Nagao Y, Matsuoka H, Ide T, Sata M. Branched-chain amino acid-enriched supplementation improves insulin resistance in patients with chronic liver disease. Int J Mol Med. 2008;22:105-112. |
| 144. | Kawaguchi T, Taniguchi E, Itou M, Sumie S, Oriishi T, Matsuoka H, Nagao Y, Sata M. Branched-chain amino acids improve insulin resistance in patients with hepatitis C virus-related liver disease: report of two cases. Liver Int. 2007;27:1287-1292. |
| 145. | Muto Y, Sato S, Watanabe A, Moriwaki H, Suzuki K, Kato A, Kato M, Nakamura T, Higuchi K, Nishiguchi S. Overweight and obesity increase the risk for liver cancer in patients with liver cirrhosis and long-term oral supplementation with branched-chain amino acid granules inhibits liver carcinogenesis in heavier patients with liver cirrhosis. Hepatol Res. 2006;35:204-214. |