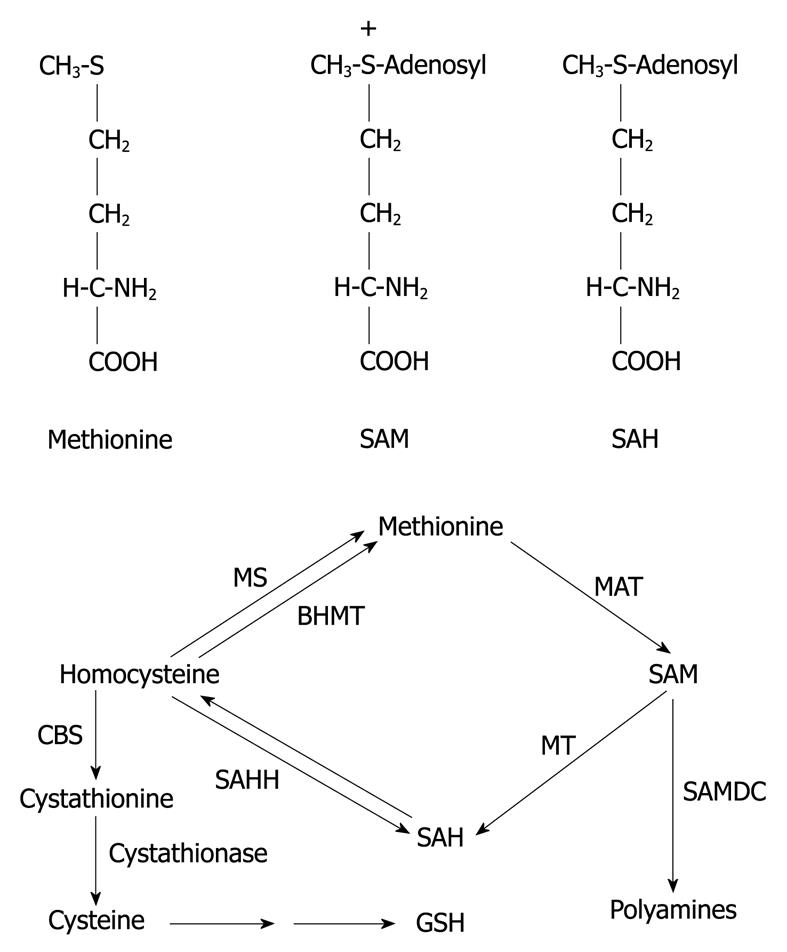
INTRODUCTION
S-adenosyl-L-methionine (SAM) is the principal biological methyl donor, the precursor of aminopropyl groups utilized in polyamine biosynthesis and, in the liver, SAM is also a precursor of glutathione (GSH) through its conversion to cysteine via the transsulfuration pathway[1,2]. SAM is important for the metabolism and regulation of nucleic acids and for the structure and function of membranes and many other cellular constituents[3,4]. SAM is particularly important for opposing the toxicity of free radicals generated by various toxins, including alcohol[1,2]. Abnormalities in SAM metabolism have been well recognized in liver diseases and in various neurological disorders. SAM is synthesized in the cytosol of every cell, but the liver plays a central role in the homeostasis of SAM as the major site of its synthesis and degradation[3-5]. Methionine adenosyltransferase (MAT) is the enzyme responsible for the synthesis of SAM using methionine and ATP. SAM can transfer its methyl group to a large variety of acceptor substrates. After methyl transfer, SAM is converted to S-adenosylhomocysteine (SAH). SAH is a potent competitive inhibitor of transmethylation reactions, and is removed by hydrolysis to homocysteine plus adenosine. In the liver, there are 3 pathways that metabolize homocysteine. One is the transsulfuration pathway, which converts homocysteine to cysteine. Cysteine is often rate-limiting for GSH synthesis hence methionine metabolism via SAM and transsulfuration is very important in regulating GSH levels in the liver. The other 2 pathways that metabolize homocysteine resynthesize methionine from homocysteine, methionine synthase and betaine-homocysteine methyltransferase. This cycle is shown in Figure 1.
Figure 1 Structure of S-adenosyl-L-methionine (SAM), S-adenosylhomocysteine (SAH), and methionine and hepatic methionine metabolism.
BHMT: Betaine homocysteine methyltransferase; CBS: Cystathionine β-synthase; GSH: Glutathione; MAT: Methionine adenosyltransferase; MS: Methionine synthase; MT: Methyltransferase; SAHH: SAH hydrolase; SAMDC: SAM decarboxylase.
In mammals, 2 different genes, MAT1A and MAT2A, encode for 2 homologous MAT catalytic subunits, α1 and α2[6-8]. MAT1A is expressed only in the liver while MAT2A encodes for a catalytic subunit (α2) found in a native MAT isozyme (MAT II), which is widely distributed[4]. MAT2A predominates in the fetal liver and is progressively replaced by MAT1A during development[9]. Expression of MAT2A is associated with rapid growth of the liver. A switch in the gene expression from MAT1A to MAT2A in liver cancer occurs[10-12]. MAT isozymes differ in kinetic parameters and in their regulatory properties so that a switch in MAT expression is likely to affect the steady state SAM level and methylation. Apart from the presence of MAT1A and 2A in liver parenchymal cells, both are also present in hepatic macrophages and endothelial cells, whereas stellate cells only express MAT2A[13].
Rodents fed diets deficient in lipotropes, such as choline and methionine, develop steatosis, which can proceed to non-alcoholic steatohepatitis (NASH) and to fibrosis and cirrhosis[14]. A decrease in SAM synthesis in the liver results in a decrease in hepatic GSH levels[15]. MAT1A knockout mice develop steatosis and hyperplasia[16]. Liver injury causes a decrease in SAM concentration largely because of decreased MAT1A activity[17,18]. Prooxidant conditions decrease liver MAT1A activity as a critical thiol residue (cysteine 121) becomes oxidized by reactive oxygen species (ROS) or nitrosylated by nitric oxide[19,20]. Impairment of SAM synthesis is believed to play an important role in hepatic injury induced by various agents, and indeed there is a considerable literature, which shows that exogenous administration of SAM can protect against injury induced by CCl4, acetaminophen, galactosamine, cytokines, thioacetamide, and ischemia-reperfusion[21-28]. The decrease in liver GSH produced by various hepatotoxins was prevented by SAM; conversely, MAT and SAM synthesis is regulated by GSH e.g. treatment with l-buthionine sulfoximine (BSO), which lowers GSH, decreases MAT1A activity in vivo or in hepatocytes, probably because of oxidation of cysteine 121[29,30]. Interestingly, MAT1A mRNA and protein declined during 12 h of rat hepatocyte culture, whereas MAT2A mRNA levels increased.
The effects of ethanol on SAM concentrations are somewhat variable. Baboons fed ethanol chronically had decreased hepatic levels of SAM and GSH, and administration of SAM elevated these and protected against liver injury[31]. Rats fed the Lieber-DeCarli diet showed no or a small decrease in SAM after 4 wk but a more substantial decrease occurred after 8 wk on the diet[32,33]. Mini pigs fed ethanol for 1 year had no change in SAM levels[34]. Rats fed ethanol in the intragastric infusion model for 9 wk had an increase in MAT1A and MAT2A mRNA but only MAT2A protein was elevated[35]; these changes were associated with a 40% fall in SAM levels. Depletion of mitochondrial GSH appears to be an important sensitizing factor for susceptibility to tumor necrosis factor-α (TNF-α) toxicity after chronic ethanol feeding[36]. This depletion results from a decrease in transport of GSH into the mitochondria and can be corrected by administration of SAM[37]. A carrier transport system for SAM entry into the mitochondria has been characterized[38]. The correction by SAM appeared to reflect an increase in fluidization of the mitochondrial membrane[37]. In isolated hepatocytes, SAM prevented the decrease in GSH caused by ethanol[39] and in perfused rat liver studies, SAM prevented the decline in GSH and oxygen consumption and liver damage produced by ethanol[40].
Cytochrome P4502E1 (CYP2E1), an ethanol-inducible form of P450, is of interest because of its ability to metabolize and activate many important toxicological substrates including ethanol, carbon tetrachloride, acetaminophen, and N-nitrosodimethylamine to more toxic products[41-43]. Whereas most ethanol is oxidized by alcohol dehydrogenase, CYP2E1 assumes a more important role in ethanol oxidation at elevated concentrations of ethanol and after chronic consumption of ethanol[44,45]. The major interest in CYP2E1 reflects the ability of this enzyme to oxidize ethanol, to generate reactive products from ethanol oxidation, e.g. acetaldehyde and the 1-hydroxyethyl radical, to activate various agents (CCl4, acetaminophen, benzene, halothane, halogenated alkanes, alcohols) to reactive products, to generate ROS, and to be “induced” by ethanol[41-48].
CYP2E1 from rat and rabbit liver exhibits enhanced NADPH oxidase activity as it appears to be poorly coupled with NADPH-cytochrome P450 reductase[49,50]. Microsomes from ethanol-treated rats, in which CYP2E1 is predominantly induced, displayed elevated rates of production of superoxide and hydrogen peroxide[49,51-54]. Increases in formation of ROS after ethanol treatment are prevented by anti-CYP2E1 IgG thus linking them to induction of CYP2E1[55]. There is considerable interest in the role of oxidative stress and ethanol generation of ROS in the mechanisms by which ethanol is hepatotoxic[56,57]. A major advance has been the development of the intragastric model of ethanol feeding in which prominent induction of CYP2E1 occurs and in which significant alcohol liver injury occurs[58-60]. In these models, the ethanol-induced liver pathology has been shown to correlate with CYP2E1 levels and elevated lipid peroxidation. Chlormethiazole (CMZ), an inhibitor of CYP2E1, prevented the elevation of lipid peroxidation and partially blocked the ethanol-induced liver pathology[61].
Understanding the biochemical and toxicological properties of CYP2E1 is important for many reasons, even apart from its role in contributing to alcohol-induced liver injury, since CYP2E1 is induced under a variety of pathophysiological conditions such as fasting, diabetes, obesity, and a high-fat diet[62-66]. Besides ethanol, CYP2E1 can be induced by drugs such as isoniazid, hydrocarbons such as trichloroethylene, benzene, chloroform, and solvents such as DMSO, acetone, and pyridine[41-45]. NASH causes steatosis, liver cell injury, inflammation, and variable necrosis. NASH is associated with obesity, type 2 diabetes, and hyperlipidemia, conditions in which CYP2E1 is induced. Increased CYP2E1 protein, activity, and mRNA levels were observed in a rat nutritional model of NASH[67]. CYP2E1 was also increased in patients with NASH[68]. It was concluded that induction of CYP2E1 is involved in the pathogenic mechanisms for NASH[67,68]. SAM was recently shown to attenuate NASH produced in rats fed with high fat diets by decreasing CYP2E1 mRNA and protein levels[69]. These decreases in CYP2E1 were accompanied by a lowering of oxidative stress[69]. In view of the induction of CYP2E1 under a variety of conditions and by many chemicals, further understanding of how levels of CYP2E1 can be downregulated within the cell and how CYP2E1-dependent toxicity can be prevented is important. The goal of this report is to evaluate the effects of SAM on CYP2E1 metabolism and toxicity.
ANTIOXIDANT EFFECTS OF SAM IN VITRO
Our laboratory has investigated the possible direct antioxidant effects of SAM in in vitro systems[70]. This study focused on the effect of SAM on the reactions of Fe2+ with dioxygen and Fe2+ with H2O2, as these are considered to be the most important routes of initiation of biological free radical oxidations. Aerobic HEPES-buffered solutions of Fe2+ spontaneously oxidize and consume O2 with concomitant production of ROS and oxidation of substrates to radical products, e.g. ethanol to hydroxyethyl radical. SAM inhibited this oxidation of ethanol and inhibited aerobic Fe2+ oxidation and consumption of O2. SAM did not regenerate Fe2+ from Fe3+ and was not consumed after incubation with Fe2+. SAM less effectively inhibited aerobic Fe2+ oxidation in the presence of competing chelating agents such as EDTA, citrate, and ADP. The effects of SAM were mimicked by SAH, but not by methionine or methylthioadenosine. SAM did not inhibit Fe2+ oxidation by H2O2 and was a relatively poor inhibitor of the Fenton reaction. Lipid peroxidation initiated by Fe2+ in liposomes was associated with Fe2+ oxidation; these 2 processes were inhibited by SAM. However, SAM did not show significant peroxyl radical scavenging activity. SAM also inhibited the nonenzymatic lipid peroxidation initiated by Fe2+ + ascorbate in rat liver microsomes. These results suggest that SAM inhibits alcohol and lipid oxidation mainly by Fe2+ chelation and inhibition of Fe2+ autoxidation. This could represent an important mechanism by which SAM exerts cellular protective actions and reduces oxidative stress in biological systems.
IN VITRO INHIBITION OF CYP2E1 CATALYTIC ACTIVITY BY SAM
We studied the possible in vitro interactions of SAM and its metabolites SAH, 5’-deoxy-5’-(methylthio)adenosine (MTA) and methionine with cytochrome P450 enzymes, in particular CYP2E1[71]. SAM (but not SAH, MTA or methionine) produced a type II binding spectrum with liver microsomal cytochrome P450 from rats treated with acetone or isoniazid to induce CYP2E1. Binding was less effective for control microsomes. SAM did not alter the carbon monoxide binding spectrum of P450, nor denature P450 to P420, nor inhibit the activity of NADPH-P450 reductase. However, SAM inhibited the catalytic activity of CYP2E1 with typical substrates such as p-nitrophenol, ethanol, and dimethylnitrosamine, with an IC50 of around 1.5-5 mmol/L. SAM was a non-competitive inhibitor of CYP2E1 catalytic activity and its inhibitory actions could not be mimicked by methionine, SAH or MTA. However, SAM did not inhibit the oxidation of ethanol to α-hydroxyethyl radical, an assay for hydroxyl radical generation. In microsomes engineered to express individual human P450s, SAM produced a type II binding spectrum with CYP2E1-expressing, but not with CYP3A4-expressing microsomes, and SAM was a weaker inhibitor against the metabolism of a specific CYP3A4 substrate than a specific CYP2E1 substrate. SAM also inhibited CYP2E1 catalytic activity in intact HepG2 cells engineered to express CYP2E1. These results suggest that SAM interacts with cytochrome P450s, especially CYP2E1, and inhibits the catalytic activity of CYP2E1 in a reversible and non competitive manner. However, SAM is a considerably weaker inhibitor than other typical CYP2E1 inhibitors such as diethyldithiocarbamate or 4-methylpyrazole and it is unclear whether inhibition of CYP2E1 activity by SAM plays a significant role in the protection by SAM against the hepatotoxicity produced by toxins which require activation by CYP2E1 e.g. acetaminophen, thioacetamide, carbon tetrachloride.
HEPATOCYTES STUDIES
The ability of SAM in vitro to protect against CYP2E1-dependent toxicity was studied in pyrazole-treated rat hepatocytes, with high levels of CYP2E1[72]. Toxicity was initiated by the addition of arachidonic acid (AA) or by depletion of glutathione after treatment with BSO. In pyrazole hepatocytes, SAM (0.25-1 mmol/L) protected against AA but not BSO toxicity. SAM elevated GSH levels, thus preventing the decline in GSH caused by AA, and SAM prevented AA-induced lipid peroxidation. SAM analogs such as methionine or SAH which elevate GSH, also protected against AA toxicity. MTA, which cannot produce GSH, did not have a protective effect. The toxicity of BSO was not prevented by SAM and the analogs because GSH cannot be synthesized. In pyrazole hepatocytes, SAM prevented the decline in mitochondrial membrane potential produced by AA. These results suggest that the ability of SAM to prevent the decline in GSH produced by AA blunts the AA initiation of lipid peroxidation and ROS production and the subsequent loss of hepatocyte viability.
The exposure of hepatocytes to ethanol caused c-Jun amino-terminal kinase (JNK) activation, c-Jun phosphorylation, Bid fragmentation, cytochrome c release and procaspase 3 cleavage; these effects were diminished by SP600125, which caused a significant decrease in ethanol-induced apoptosis[73]. SAM exerted an antioxidant effect maintaining glutathione levels and decreasing ROS generation, without a significant effect on JNK activity, and SAM prevented cytochrome c release and procaspase 3 cleavage. The JNK signaling cascade is a key component of the proapoptotic signaling pathway induced by ethanol. JNK activation may be independent from ROS generation, since SAM, which exerted antioxidant properties, did not have a significant effect on JNK activity. JNK pathway modulator agents and SAM may be components of promising therapies for alcoholic liver disease treatment.
Kharbanda et al[74] compared the effects of betaine and SAM, on ethanol-induced changes of methionine metabolism and hepatic steatosis. Wistar rats were fed ethanol or a control Lieber-Decarli liquid diet for 4 wk, and metabolites of the methionine cycle were measured in isolated hepatocytes. Hepatocytes from ethanol-fed rats had a 50% lower intracellular SAM/SAH ratio and almost 2-fold greater homocysteine release into the media compared with controls. Supplementation with betaine or SAM in the incubation media increased the SAM/SAH ratio in hepatocytes from both control and ethanol-fed rats and attenuated the ethanol-induced increase in hepatocellular triglyceride levels by approximately 20%. On the other hand, only betaine prevented the increase in generation of homocysteine in the incubation media under basal and methionine-loaded conditions. SAM can correct only the SAM/SAH ratio and the methylation defects and may in fact be detrimental after prolonged use because of its propensity to increase homocysteine release. Both SAM and betaine are effective in increasing the SAM/SAH ratio in hepatocytes and in attenuating hepatic steatosis; however, only betaine can effectively methylate homocysteine and prevent increased homocysteine release by the liver[74].
DECLINE OF SAM BY CYCLOLEUCINE TREATMENT POTENTIATES CYP2E1 TOXICITY IN HEPATOCYTES
Cycloleucine is an inhibitor of MAT and prevents the conversion of 5’-methylthioadenosine to SAM through the methionine salvage pathway[75,76]. SAM levels were lower in hepatocytes isolated from pyrazole-treated rats than control hepatocytes. Mato et al[4] found that defective formation of SAM is associated with an impaired synthesis of GSH and that reduced synthesis of SAM and GSH can both act together in a self-perpetuating cycle, where a reduction in hepatic GSH leads to an inhibition of MAT activity via a mechanism involving ROS. A critical cysteine residue required for MAT1A activity is oxidized by ROS, with a subsequent decline in MAT1A activity and levels of SAM[19,20]. Pyrazole increases the expression of CYP2E1, which produces ROS during its catalytic cycle. We speculate that CYP2E1-derived ROS inhibit the MAT1A which results in lower SAM levels in the hepatocytes isolated from pyrazole-treated rats[77]. We therefore studied whether lowering of SAM levels in hepatocytes by treatment with cycloleucine would increase CYP2E1-dependent toxicity[77].
Treatment with 5 mmol/L cycloleucine decreased SAM levels to 50% of initial values in pyrazole or saline hepatocytes. This more modest decline in SAM levels allowed the pyrazole hepatocytes to remain viable and thus permitted evaluating the effect of an added prooxidant such as AA on cell viability when SAM levels were further lowered by cycloleucine treatment.
Pyrazole hepatocytes that express elevated CYP2E1 were more sensitive to 20 mmol/L cycloleucine or 5 mmol/L cycloleucine plus 20 μmol/L AA treatment, and exhibited significant cell death compared with that of control hepatocytes. Cell nuclear morphology, DNA ladder and caspase 3 cleavage revealed that cycloleucine or cycloleucine plus AA treatment induced apoptosis in pyrazole hepatocytes to a much greater extent than in control hepatocytes. The lactate dehydrogenase leakage assay indicated that necrosis may also be occurring and contributing to the pyrazole hepatocyte death[77]. The CYP2E1 inhibitor, CMZ, protected pyrazole hepatocytes from cycloleucine or cycloleucine plus AA cytotoxicity by decreasing the elevated CYP2E1 activity, maintaining SAM levels and decreasing ROS levels. It is important to emphasize the very effective hepatoprotective effects of SAM since toxicity is observed only when SAM levels are lowered to undetectable levels. Decreases of “only” 70% to 80% as found with non-treated pyrazole hepatocytes or cycloleucine-treated control hepatocytes did not result in hepatotoxicity even after 3 d in culture.
A likely explanation as to why cycloleucine or cycloleucine plus AA toxicity is increased in the pyrazole hepatocytes is elevated oxidant stress, as ROS is generated via the induction of CYP2E1 in the endoplasmic reticulum, and ROS detoxification is reduced as a result of the decline in SAM. The antioxidant Trolox could rescue pyrazole hepatocytes from cycloleucine or cycloleucine plus AA induced cell death. Trolox also decreased the elevated ROS and intracellular O2.- produced by cycloleucine in the pyrazole hepatocytes, indicating that enhanced ROS production appears to be central to the mechanism leading to the death of pyrazole hepatocytes[77].
Trifluoroperazine, an inhibitor of the mitochondrial membrane permeability transition, effectively protected pyrazole hepatocytes from toxicity induced by cycloleucine or cycloleucine plus AA treatment, which suggest that a decrease in mitochondrial membrane potential contributes to the potentiation of cell death caused by cycloleucine treatment in the pyrazole hepatocytes.
SAM levels were lowered in animal models of alcoholic liver injury and human alcoholic liver disease[25,26]. In these situations the CYP2E1 levels are elevated and liver injury is potentiated by diets enriched in polyunsaturated fatty acids[59,60]. A decrease in SAM (with or without addition of AA) and elevated CYP2E1 expression combine to lead to an increased oxidative stress in pyrazole hepatocytes, which plays a crucial role in execution of downstream events leading to apoptosis.
MAT1A KNOCKOUT MOUSE
The MAT1A knockout mouse has been a most valuable contribution to research[16,78,79]. Genomic profiling via microarrays revealed changes in genes involved in cell proliferation, differentiation and the acute phase response. Of significance for this review is the observation that CYP2E1 mRNA and activity was increased in the MAT1A knockout mouse. Conversely, CYP4A10 and 4A14 were decreased[79], in agreement with the observations in the CYP2E1 knockout mouse that there is reciprocal expression of CYP2E1 compared to CYP4A10[67]. Toxicity by CCl4 was more severe in the MAT1A knockout mouse perhaps because of the 70% decrease in SAM and the increase in CYP2E1; toxicity was prevented by the CYP2E1 inhibitor diallylsulfide. Importantly, the decrease in MAT activity in wild type mice produced by CCl4 was also prevented by diallylsulfide and the authors suggested that CYP2E1-derived ROS may inactivate MAT during liver injury, and this may sensitize the liver to further oxidative injury[79]. We recently found that protection by SAM against CYP2E1-dependent toxicity was associated with a decrease in CYP2E1 levels and activity (discussed below). These results suggest possible interactions/modulation between CYP2E1 and MAT1A, which are worthy of more detailed investigations.
ANTI-FIBROGENIC EFFECTS OF SAM AND PROTECTION AGAINST ALCOHOL-DEPENDENT TOXICITY IN VIVO
To study the anti-fibrogenic actions of SAM, transgenic mice harboring the -17 kb to +54 bp of the collagen α2 promoter (COL1A2) cloned upstream from the β-gal reporter gene were injected with CCl4 to induce fibrosis and coadministered with either SAM or saline[80]. Control groups received mineral oil. SAM lowered the pathology in the CCl4 livers, and decreased the elevated levels of collagen and trichrome staining. Elevated β-galactosidase activity indicated activation of the COL1A2 promoter in isolated stellate cells from the CCl4-treated mice and such activation was repressed by in vivo treatment with SAM. SAM also prevented the increase in lipid peroxidation and transforming growth factor-β (TGF-β) and the decline in GSH produced by CCl4. Incubation of primary stellate cells with SAM downregulated basal levels of collagen and TGF-β stimulation of collagen protein and smooth muscle actin. SAM repressed basal and TGF-β-induced reporter activity in stellate cells transfected with COL1A2 promoter deletion constructs. SAM blocked TGF-β induction of the -378 bp region of the COL1A2 promoter and prevented activation of ERK and binding of Sp1 to the TGF-β-responsive element. These observations reveal antifibrogenic actions of SAM[80]. In vitro, SAM was found to blunt activation of hepatic primary stellate cells in culture as shown by decreased levels of collagen and smooth muscle actin, and a decrease in DNA synthesis stimulated by platelet-derived growth factor and a decrease in stellate cell contractility[81].
Chronic ethanol consumption by baboons (50% of energy from a liquid diet) for 18 to 36 mo resulted in significant depletion of hepatic SAM concentration and levels of GSH[82]. These depletions were corrected with SAM administration. There was a significant correlation between hepatic SAM and GSH level. This attenuation by SAM of the ethanol-induced increase in plasma glutamic dehydrogenase was associated with a decrease in the number of giant mitochondria (assessed in percutaneous liver biopsy specimens), with a corresponding change in the activity of succinate dehydrogenase, a mitochondrial marker enzyme. Succinate dehydrogenase activity was increased in liver homogenates of animals fed ethanol, probably reflecting the increased mitochondrial mass. SAM decreased succinate dehydrogenase levels. SAM supplementation also significantly lessened the ethanol-induced increase of plasma aspartate aminotransferase[82].
Esfandiari et al[83] reported that feeding micropigs with ethanol at 40% of total calories with folate-deficient diets for 14 wk increased, and supplemental SAM maintained, levels of liver and plasma triglyceride. Serum adiponectin, liver transcripts of adiponectin receptor-1, and phosphorylated adenosine monophosphate kinase-β were each reduced by ethanol feeding and were sustained at normal levels by SAM supplementation of the ethanol diets. Ethanol feeding activated and SAM supplementation maintained control levels of endoplasmic reticulum stress-induced transcription factor SREBP-1c (sterol regulatory element-binding protein-1c) and its targeted transcripts of lipid synthesizing enzymes acetyl-CoA carboxylase, fatty acid synthase, and glycerol-3-phosphate acyltransferase. SAM attenuated oxidative liver injury in micropigs fed ethanol with a folate-deficient diet[84]. The elevated transcripts and protein levels of CYP2E1 and activities of NADPH oxidase and inducible nitric oxide synthase were lowered upon supplementation of the diet with SAM. Thus, SAM protected against ethanol toxicity by decreasing oxidative enzymes such as CYP2E1. Liver mRNA and protein levels of CYP2E1 correlated positively to SAH or homocysteine but negatively to the SAM/SAH ratio in minipigs fed ethanol plus a folate-deficient diet, and the authors concluded that induction of abnormal methionine metabolism is associated with activation of CYP2E1[85].
The protective effect of SAM against rat liver steatosis induced by chronic ethanol ingestion was investigated by Feo et al[86]. SAM given during ethanol treatment prevented steatosis and accelerated recovery from steatosis when given after ethanol withdrawal. It also caused a slight inhibition of blood ethanol concentration in both acutely and chronically intoxicated rats. About 30% inhibition of alcohol dehydrogenase, but not of the microsomal ethanol oxidation system, occurred in rats subjected to acute ethanol toxicity as well as in normal rats as a consequence of SAM treatment. Ethanol induced a drastic decrease of GSH liver content. SAM treatment almost completely reconstituted the liver GSH pool. It is suggested that, although SAM induced a small inhibition of ethanol metabolism in the liver, its antisteatotic effect could largely depend on its role as a modulator of the GSH liver content[86].
Bailey et al[87] found that alcohol feeding to rats for 5 wk caused a significant decrease in mitochondrial state 3 respiration and the respiratory control ratio whereas SAM administration prevented these alcohol-mediated defects and preserved hepatic SAM levels. SAM treatment prevented alcohol-associated increases in mitochondrial superoxide production, mitochondrial DNA damage, and inducible nitric oxide synthase induction, without a significant lessening of steatosis. SAM also prevented alcohol-mediated losses in cytochrome c oxidase subunits, which resulted in partial preservation of complex IV activity. SAM treatment attenuated the upregulation of the mitochondrial stress chaperone prohibitin. Although SAM supplementation did not alleviate steatosis by itself, SAM prevented several key alcohol-mediated defects to the mitochondria genome and proteome that contribute to the bioenergetic defect in the liver after alcohol consumption[87]. Supplementation of SAM to ethanol diets which were fed to rats prevented dissociation of hepatic mitochondrial ribosomes and elevated respiratory rates with glutamate/malate or with succinate as substrates[88]. Thus, SAM is protective against ethanol-induced mitochondrial dysfunction.
Male C57BL/6 mice received ethanol (5 g/kg body weight) by gavage every 12 h for a total of 3 doses. SAM (5 mg/kg body weight) was administrated ip before ethanol administration[28]. Acute ethanol administration caused prominent microvesicular steatosis with mild necrosis and an elevation of serum ALT activity. SAM treatment significantly attenuated the liver injury. In association with the hepatocyte injury, acute alcohol administration induced significant decreases in both hepatic SAM and mitochondrial GSH levels along with enhanced lipid peroxidation. SAM treatment attenuated hepatic SAM and mitochondrial GSH depletion and lipid peroxidation following acute alcohol exposure[28].
SAM PROTECTS AGAINST CYP2E1-DEPENDENT TOXICITY IN VIVO
As CYP2E1-mediated toxicity may play a role in alcoholic liver injury and toxicity of many hepatotoxins, whereas SAM can prevent alcoholic liver injury and toxicity of hepatotoxins, it is of interest to study whether and how SAM can affect CYP2E1-mediated toxicity in the liver in vivo. We recently reported that induction of CYP2E1 in mice by treatment with pyrazole increased the hepatotoxicity caused by Fas agonistic Jo2 antibody[89]. Increased hepatotoxicity in the pyrazole/Jo2-treated mice was associated with increased oxidative and nitrosative stress in association with decreased GSH and other antioxidant levels. CMZ, an inhibitor of CYP2E1, prevented the synergistic toxicity of Jo2 antibody in pyrazole-treated mice. We investigated the effect of SAM on agonistic Jo2 Fas-induced hepatotoxicity following induction of CYP2E1 by pyrazole pretreatment in vivo and explored a possible relationship among oxidative stress, hepatic apoptosis, and homeostasis of SAM metabolism[90]. Suboptimal administration of Jo2 Fas antibody combined with pyrazole pretreatment caused severe hepatotoxicity as determined by elevations in serum transaminase levels and histopathology. Exogenous administration of SAM (50 mg ip/kg body weight every 12 h for 3 d) significantly decreased serum transaminases and ameliorated morphological changes of the liver. Addition of SAM elevated hepatic SAM and total glutathione levels and inhibited CYP2E1 activity. SAM also lowered the elevated oxidative stress (lipid peroxidation, protein carbonyls, and superoxide production) and nitrosative stress (induction of inducible nitric oxide synthase and 3-nitrotyrosine adducts) and lowered increases in caspase-8 and -3 activation produced by the pyrazole plus Jo2 treatment. SAM did not prevent the increase in serum TNF-α levels or the decrease in catalase activity in this model. These results indicate that SAM can have an important hepatoprotective role as an effective reagent against Fas plus CYP2E1-induced hepatotoxicity by lowering oxidative and nitrosative stress.
A study was carried out[91] to investigate the effect of SAM on the enhanced hepatotoxicity induced by Fas agonistic Jo2 antibody plus acute ethanol administration in mice. Acute ethanol plus Fas produced toxicity under conditions in which the ethanol or the Jo2 alone had no effect. SAM attenuated this elevated hepatoxicity. Levels of SAM and activity of MAT1A were decreased by the ethanol plus Jo2 treatment but restored after administration of SAM. The ethanol plus Jo2 treatment elevated levels of CYP2E1, iNOS and TNF-α: these increases were blunted by SAM. SAM also lowered the elevated oxidative and nitrosative stress produced by ethanol plus Jo2. Calcium-induced mitochondrial swelling was elevated by ethanol plus Jo2 and lowered by SAM. The activation of JNK by the ethanol plus Jo2 combined treatment was also prevented by SAM. It was suggested that SAM protects against the acute ethanol plus Jo2 toxicity by restoring MAT activity and levels of SAM, preventing the increases in CYP2E1, iNOS, JNK and TNF-α, which thereby lowers oxidative/nitrosative stress and protects against loss of mitochondrial function[91].
Obese mice have been used extensively as animal models to study human obesity. We have recently observed that pyrazole-induced CYP2E1 promotes liver injury in ob/ob mice, compared with saline-treated ob/ob mice or with lean controls treated with pyrazole[92]. We investigated whether SAM can prevent this CYP2E1-mediated toxicity to the obese mouse liver[93]. Pyrazole treatment of ob/ob mice for 2 d caused necrosis, steatosis, and elevated serum transaminase and triglyceride levels compared with saline-treated ob/ob mice. Administration of SAM (50 mg/kg body wt ip every 12 h for 3 d) prevented the observed pathological changes as well as the increase of apoptotic hepatocytes, caspase 3 activity, and serum TNF-α levels. SAM administration inhibited CYP2E1 activity but not CYP2E1 content. The pyrazole treatment increased lipid peroxidation, 4-hydroxynonenal and 3-nitrotyrosine protein adducts, and protein carbonyls. These increases in oxidative and nitrosative stress were prevented by SAM. Treatment of ob/ob mice with pyrazole lowered the endogenous SAM levels, and these were elevated after SAM administration. Mitochondrial GSH levels were very low after pyrazole treatment of the ob/ob mice; this was associated with elevated levels of malondialdehyde and 4-hydroxynonenal and 3-nitrotyrosine protein adducts in the mitochondria. All these changes were prevented with SAM administration. SAM protected against pyrazole-induced increase in serum transaminases, necrosis, triglyceride levels, caspase-3 activity, and lipid peroxidation even when administered 1 d after pyrazole treatment. In summary, pyrazole induced necrotic changes and apoptosis in livers of obese mice, and SAM pretreatment abrogated these pathological changes. Important mechanisms involved in the protective actions of SAM are the blunting of the increased catalytic activity of CYP2E1, possibly the decline in TNF-α levels, and the lowering of the elevated oxidative/nitrosative stress produced by SAM treatment. SAM also protected pyrazole-treated obese mice from CYP2E1-generated oxidative and nitrosative stress in the mitochondria, especially the depletion of mitochondrial GSH. Therefore, SAM is effective in protecting against pyrazole-induced oxidative and nitrosative stress and liver injury in obese mice by lowering CYP2E1-generated oxidative/nitrosative stress in the liver and in the mitochondrial compartment[93].
A model of chronic alcohol-induced liver injury using Cu, Zn-superoxide dismutase deficient mice (SOD-/-) was developed[94]. Feeding the homozygous SOD-/- mice, but not the wild type mice, with ethanol resulted in liver injury characterized by extensive centrilobular necrosis and inflammation, and increased transaminase levels. Liver injury in the SOD-/- mice was associated with induction of CYP2E1, and increased protein carbonyls, lipid peroxidation aldehydic products and 3-nitrotyrosine protein adducts, indicative of enhanced oxidative and nitrosative stress. SAM protected SOD-/- mice from alcohol-induced liver injury. SAM lowered the serum ALT and AST levels, decreased steatosis, necrosis and inflammation as compared to alcohol alone in treated SOD1 knockout mice (unpublished data).
MITOCHONDRIAL SAM
Normal intra-mitochondrial SAM concentrations play a pivotal role in mitochondrial functions because methylation reactions are required for the methylation of RNA and proteins, and they function as intermediates in the biosynthesis of lipoic acid, ubiquinone and biotin[95]. Since mitochondria have a relatively large pool of SAM[96], and the enzyme required for SAM synthesis (MAT) is present only in the cytosol and not in the mitochondria[97] a specific SAM transporter is needed to maintain normal mitochondrial SAM levels. The human mitochondrial SAM carrier exhibited a high transport affinity for SAM and was found to be expressed in all human tissues examined and was localized to the mitochondria. The physiological role of the SAM carrier is probably to exchange cytosolic SAM for mitochondrial SAH[98]. It has been reported that increased cytosolic SAH caused a decrease in SAM concentration in the mitochondria in rat hepatocytes[38]. Furthermore, studies by Colell et al[99] demonstrated that exogenous SAM supplementation prevented the depletion of the mitochondrial GSH pool induced by alcohol consumption and protected hepatocytes from alcohol-consuming rats from TNF-α-induced hepatotoxicity. Bailey et al[87] showed that SAM treatment prevented mitochondrial dysfunction induced by chronic alcohol consumption in rats. Song et al[100] examined the effects of chronic alcohol consumption on hepatic SAM and SAH levels in both the cytosol and mitochondria, and found that mitochondrial SAM was significantly decreased in a mouse model of alcohol liver disease. Supplementation of SAM to rats fed alcohol chronically has been shown to replenish the mitochondrial GSH levels because of normalization of the microviscosity of the mitochondrial inner membrane. Because of the instrumental role of GSH in mitochondria in hepatocyte survival against inflammatory cytokines, its repletion by SAM feeding may underlie the potential therapeutic use of this hepatoprotective agent in the treatment of alcohol-induced liver injury[36,37]. Mitochondrial GSH plays an important role in maintaining a functionally competent organelle. SAM administration resulted in a significant increase in the basal cytosol and mitochondrial GSH in both periportal and perivenous cells from both pair-fed and ethanol-fed groups. When hepatocytes isolated from ethanol-fed rats which were supplemented with SAM were incubated with methionine plus serine or N-acetylcysteine, mitochondrial GSH increased in parallel with cytosolic GSH, an effect not observed in cells from ethanol-fed rats not treated with SAM. Feeding equimolar N-acetylcysteine raised cytosolic GSH but did not prevent the mitochondrial GSH defect. In addition, SAM feeding resulted in significant preservation of cellular ATP levels, mitochondrial membrane potential and the uncoupler control ratio of respiration for mitochondria. Thus, these effects of SAM suggest that it may be a useful agent to preserve the disturbed mitochondrial integrity in liver disease caused by alcoholism through maintenance of mitochondrial GSH transport[37,99].
CONCLUSION
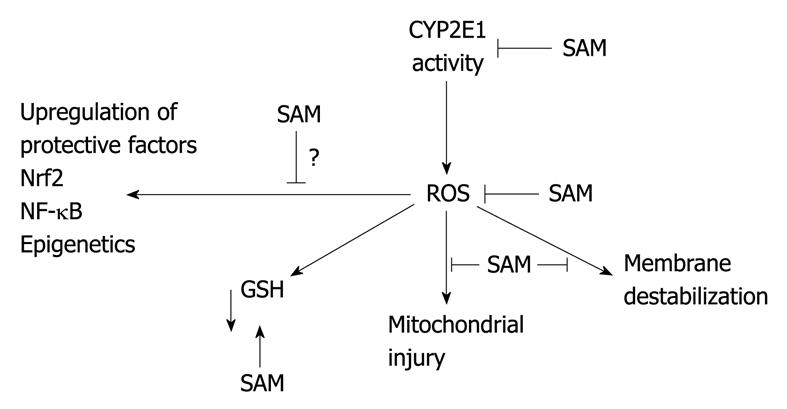
A scheme by which SAM is envisioned to protect against CYP2E1 hepatotoxicity is shown in Figure 2. SAM, at high concentrations, inhibits CYP2E1 catalytic activity, lowering formation of ROS. SAM has antioxidant actions, especially against iron potentiation of ROS production. ROS lower GSH levels, whereas SAM increases GSH levels by providing cysteine via the transsulfuration pathway. ROS cause mitochondrial dysfunction and membrane destabilization, events blunted by SAM. Mild or transient oxidant stress produced by CYP2E1 can upregulate cellular protective factors such as GSH, glutathione transferase, heme oxygenase-1, γ glutamyl cysteine ligase via activation of Nrf-2[46-48]. The effect of SAM on this upregulation would be important to evaluate especially in view of methylation reactions as epigenetic regulators.
Figure 2 Proposed mechanism by which SAM protects against CYP2e1-hepatotoxicity.
ROS: Reactive oxygen species; CYP: Cytochrome P450; NF-κB: Nuclear factor κB; Nrf2: Nuclear factor erythroid 2-related factor 2.
SAM was first described in 1952 and has been available in the United States as an over the counter supplement since 1999; in Europe, it is a prescription medicine[101]. SAM has shown protective effects against alcohol and CYP2E1 induced cytotoxicity in vitro or in animal models. Although studies in experimental animal models of liver injury support a therapeutic role for SAM, Rambaldi et al[102] identified 9 randomized clinical trials including a heterogeneous sample of 434 patients with alcoholic liver diseases and could not find evidence supporting or refuting the use of SAM in patients with alcoholic liver diseases. Therefore, more long-term, high-quality randomized trials on administration of SAM to these patients are needed before SAM may be recommended for clinical use. The mechanisms of abnormal SAM metabolism leading to liver injury and the mechanisms of pharmacologic actions of SAM deserve more research.
Peer reviewers: Maurizio Parola, Professor, Department of Experimental Medicine and Oncology, University of Turin, Corso Raffaello 30, 10125 Torino, Italy; Katsutoshi Yoshizato, PhD, Academic Advisor, Phoenix Bio Co., Ltd., 3-4-1 Kagamiyama, Higashihiroshima 739-0046, Japan
S- Editor Tian L L- Editor Cant MR E- Editor Zheng XM