Published online Mar 14, 2010. doi: 10.3748/wjg.v16.i10.1293
Revised: January 17, 2010
Accepted: January 24, 2010
Published online: March 14, 2010
Ectopic pancreas (EP) is the relatively uncommon presence of pancreatic tissue outside the normal location of the pancreas. This condition is usually asymptomatic and rarely complicated by pancreatitis and malignant transformation. A few cases of neoplastic phenomena that developed from EP into the duodenal wall are described in the literature. Herein we report a case of gastric outlet obstruction due to adenocarcinoma arising from EP of the duodenal wall. The patient underwent a Whipple’s procedure and had an uneventful post-operative recovery. Traditional imaging studies are often inconclusive in the definitive diagnosis, whilst endoscopic ultrasonography and guided biopsy may aid accurate pre-operative diagnosis. Diagnostic uncertainty warrants surgical exploration and if necessary extended resection is indicated.
- Citation: Bini R, Voghera P, Tapparo A, Nunziata R, Demarchi A, Capocefalo M, Leli R. Malignant transformation of ectopic pancreatic cells in the duodenal wall. World J Gastroenterol 2010; 16(10): 1293-1295
- URL: https://www.wjgnet.com/1007-9327/full/v16/i10/1293.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i10.1293
The presence of ectopic pancreas (EP) is relatively uncommon and generally asymptomatic. EP is defined as pancreatic tissue abnormally situated, without connection to the normal pancreas but provided with its own vascular and ductal systems[1]. Other terms such as heterotopic and aberrant pancreas have also been described in the literature. EP has been found in both abdominal and extra abdominal locations, but is most frequently encountered in the duodenum (25%-35%)[2], stomach (25%-60%)[3], and rarely in the mesocolon[4,5] and Meckel diverticulum[6]. The origin of EP is unknown, it is possible that during rotation of the foregut and fusion of the ventral and dorsal parts of the pancreas in early foetal life, small pieces of tissue become detached from the forming organ leading to entrapment in different locations[7]. The following is a case of upper gastrointestinal obstruction caused by malignant transformation of ectopic pancreatic tissue located into the duodenal wall.
A 56-year-old man was admitted to the authors’ institution with a one month history of recurrent vomiting after meals. The patient’s medical history consisted of type 1 diabetes and polydistrectual vasculopathy with no previous surgical history. The physical examination on admission did not reveal palpable abdominal masses. The vital signs were normal. Serum tumour markers such as CA 19-9, CA 125, AFP (α fetoprotein), CEA (carcinoembryonic antigen) and amylase were all within the normal range as was the basic metabolic screen.
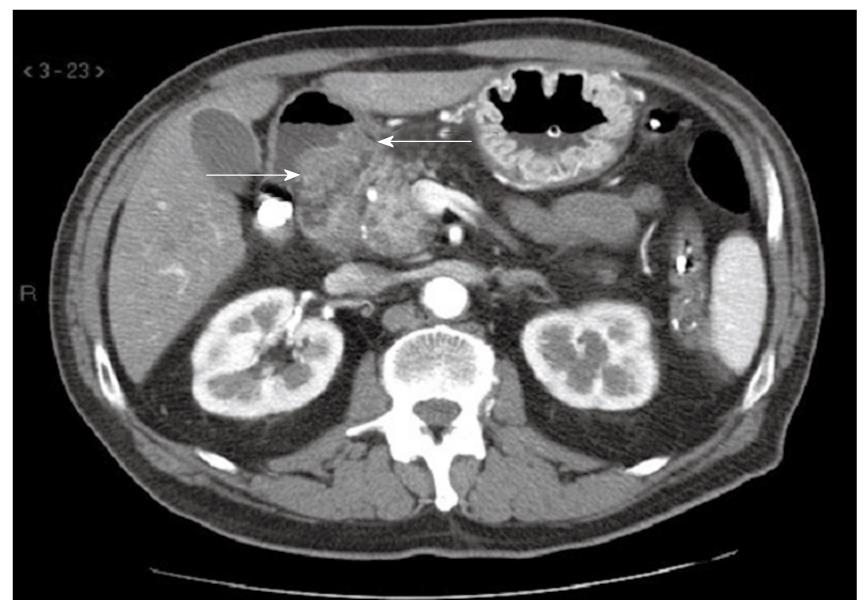
The patient underwent a sequence of radiologic investigations. A double contrast barium meal of the upper gastrointestinal tract demonstrated reduced gastric emptying together with a degree of gastric distension. Contrast-enhanced CT demonstrated significant gastric distension and increased wall thickness in the first portion of the duodenum. The radiologic appearance of the pancreas was normal (Figure 1). We actually perform EGD (esophagogastroduodenoscopy) before CT in case of upper gastrointestinal obstruction.
EGD was used to localize an area of narrowed lumen in the first portion of the duodenum. EUS (endoscopic ultrasonography) showed heterogeneous hypoechoic areas in the submucosal tissue in relation to the narrowed first part of the duodenum, where we performed multiple biopsies. Cytology of biopsy specimens revealed the presence of an adenocarcinoma of uncertain origin. A multi-disciplinary discussion led to surgical resection. The patient subsequently underwent a pancreaticoduodenectomy. A classic Whipple’s operation was performed in the distal part of the stomach (antrectomy), the distal portion of the common bile duct (choledochectomy), head of the pancreas, and the entire duodenum and proximal jejunum were resected en-block along with the regional lymph nodes. Gastrointestinal reconstruction was performed with pancreaticogastrostomy, Roux en Y choledochojejunostomy and gastrojejunostomy. Two abdominal drains were inserted and short term antibiotics as well as thrombo-prophylaxis were given according to the institutional protocol.
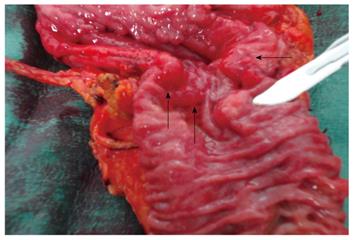
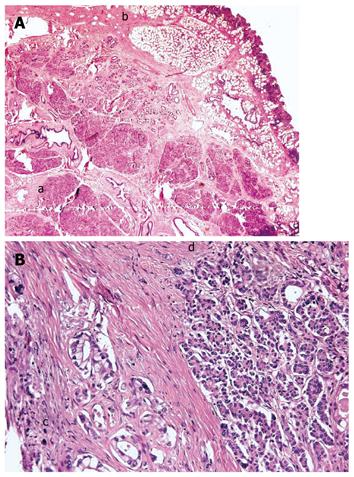
Figure 2 demonstrates the resected specimen consisting of gastric antrum, duodenum, common bile duct and proximal pancreas. The margins of the resection were negative for tumour and there was no nodal involvement. The conclusive histological report showed an infiltration of the duodenum by adenocarcinoma arising from ductal structures of heterotopic islets of pancreatic tissue into the duodenal wall, and pT4 grading according to WHO G2 (Figure 3A). The neoplasm involved the duodenal serosa with focal invasion of the orthotopic pancreas. The uninvolved EP had a microscopic (Figure 3B) appearance consistent with Heinrich’s Class 1, where the EP was characterized by cystically dilated ducts, islets and acini[8]. The post-operative recovery was uneventful. A nasogastric tube was maintained in aspiration until the sixth post-operative day and total parenteral nutrition was continued for 10 d. Progressive oral feeding was introduced from the sixth post-operative day. Upon discharge the patient was referred to the oncology department for adjuvant chemotherapy.
EP rarely gives rise to symptoms and its discovery is usually accidental[3]. EP is found in about 1/500 laparotomies carried out for upper abdominal conditions and is reported in 0.6%-13% of post-mortem examinations[7,9]. Macroscopically EP is often located in the submucosal layer, but may also be found in the muscularis mucosae, sub-serosa and serosa[10]. According to Guillou et al[11], to confirm the malignant transformation of EP, a series of criteria require to be fulfilled: (1) presence of a neoplasm located in/or in the proximity of the EP site; (2) concurrent presence and continuity of normal ectopic pancreatic tissue and adenocarcinoma; and (3) ectopic pancreatic tissue with normal histological structure. Our report met all the criteria. The simultaneous absence of primary neoplasm of orthotopic pancreas as well as the evidence of EP in the duodenal wall, along with other criteria aforementioned (Guillou et al[11]) confirmed the malignant transformation of duodenal ectopic pancreas in the case described here.
The ectopic tissue may develop the same pathological changes that occur in a normal pancreas, such as cyst formation and acute pancreatitis. Malignant transformation has been rarely reported in the literature in cases of duodenal EP. Depending on anatomical location and pathology involved, EP may also gives rise to symptoms such as abdominal pain, nausea, vomiting, gastric outlet obstruction, jaundice and gastrointestinal bleeding.
Pre-operative diagnosis of EP may be difficult with conventional imaging studies such as CT, radiographic contrast study and EGD. EUS is typically used to evaluate the submucosal lesions in the upper gastrointestinal tract[12]. EUS can in fact determine the layer of origin of the intramural lesion, which is of high importance for the diagnosis[13]. EP usually appears hypoechoic and heterogeneous with indistinct margins during EUS and most commonly originates from the third or fourth layer or a combination of these two layers of the gastrointestinal (GI) tract[14]. EUS also allows the use of targeted fine needle aspiration biopsy (FNAB), particularly helpful for the final diagnosis. However, cytological examinations are inconclusive in about 50% of cases[7].
Due to its relative rarity, there is no evidence in the scientific literature showing a greater tendency to develop neoplasms in EP as opposed to orthotopic pancreas. Most of the patients with EP are asymptomatic and require no treatment, but on discovery of EP as an accidental finding, its excision is recommended[10]. In symptomatic EP or in the case of uncertain diagnosis, surgical resection may be both diagnostic and curative. Prognosis of adenocarcinoma arising from EP is not known. In the literature, only ten cases have been reported with a survival time of between six months and ten years and all with a life expectancy longer than that in the case of orthotopic pancreas surgery[9].
In summary, we report a rare case of malignant transformation of EP of the duodenal wall in a patient with gastric outlet obstruction. Routine imaging studies provided inconclusive information on the origin of the lesion, whilst EUS was an important adjunct in locating the lesion supplemented by fine needle aspiration cytology. Although rare, EP should be considered in the differential diagnosis of a submucosal mass of the duodenal wall.
Peer reviewers: Dr. Thamara Perera, Senior Transplant Fellow, The Liver Transplant Unit, Queen Elizabeth Hospital, Edgbaston, Birmingham, B15 2TH, United Kingdom; Dr. Ashok Kumar, MD, Department of Surgical Gastroenterology, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow-226014, India
S- Editor Wang JL L- Editor Webster JR E- Editor Ma WH
| 1. | Hsu SD, Chan DC, Hsieh HF, Chen TW, Yu JC, Chou SJ. Ectopic pancreas presenting as ampulla of Vater tumor. Am J Surg. 2008;195:498-500. |
| 2. | Mizuno Y, Sumi Y, Nachi S, Ito Y, Marui T, Saji S, Matsutomo H. Acinar cell carcinoma arising from an ectopic pancreas. Surg Today. 2007;37:704-707. |
| 3. | Mulholland KC, Wallace WD, Epanomeritakis E, Hall SR. Pseudocyst formation in gastric ectopic pancreas. JOP. 2004;5:498-501. |
| 4. | Ishikawa O, Ishiguro S, Ohhigashi H, Sasaki Y, Yasuda T, Imaoka S, Iwanaga T, Nakaizumi A, Fujita M, Wada A. Solid and papillary neoplasm arising from an ectopic pancreas in the mesocolon. Am J Gastroenterol. 1990;85:597-601. |
| 5. | Tornóczky T, Kálmán E, Jáksó P, Méhes G, Pajor L, Kajtár GG, Battyány I, Davidovics S, Sohail M, Krausz T. Solid and papillary epithelial neoplasm arising in heterotopic pancreatic tissue of the mesocolon. J Clin Pathol. 2001;54:241-245. |
| 6. | Koh HC, Page B, Black C, Brown I, Ballantyne S, Galloway DJ. Ectopic pancreatic-type malignancy presenting in a Meckel’s diverticulum: a case report and review of the literature. World J Surg Oncol. 2009;7:54. |
| 7. | Tolentino LF, Lee H, Maung T, Stabile BE, Li K, French SW. Islet cell tumor arising from a heterotopic pancreas in the duodenal wall with ulceration. Exp Mol Pathol. 2004;76:51-56. |
| 8. | Abakar-Mahamat A, Rahili A, Saint-Paul MC, Chevallier P, Peroux JL, Schneider SM, Benchimol D, Hébuterne X. [Cancer of an ectopic pancreas in the duodenal wall] Gastroenterol. Clin Biol. 2005;29:201-203. |
| 9. | Jiang LX, Xu J, Wang XW, Zhou FR, Gao W, Yu GH, Lv ZC, Zheng HT. Gastric outlet obstruction caused by heterotopic pancreas: A case report and a quick review. World J Gastroenterol. 2008;14:6757-6759. |
| 10. | Rubbia-Brandt L, Huber O, Hadengue A, Frossard JL. An unusual case of gastric heterotopic pancreas. JOP. 2004;5:484-487. |
| 11. | Guillou L, Nordback P, Gerber C, Schneider RP. Ductal adenocarcinoma arising in a heterotopic pancreas situated in a hiatal hernia. Arch Pathol Lab Med. 1994;118:568-571. |
| 12. | Yasuda K, Cho E, Nakajima M, Kawai K. Diagnosis of submucosal lesions of the upper gastrointestinal tract by endoscopic ultrasonography. Gastrointest Endosc. 1990;36:S17-S20. |
| 13. | Motoo Y, Okai T, Ohta H, Satomura Y, Watanabe H, Yamakawa O, Yamaguchi Y, Mouri I, Sawabu N. Endoscopic ultrasonography in the diagnosis of extraluminal compressions mimicking gastric submucosal tumors. Endoscopy. 1994;26:239-242. |
| 14. | Matsushita M, Hajiro K, Okazaki K, Takakuwa H. Gastric aberrant pancreas: EUS analysis in comparison with the histology. Gastrointest Endosc. 1999;49:493-497. |