Published online Mar 14, 2010. doi: 10.3748/wjg.v16.i10.1274
Revised: January 9, 2010
Accepted: January 16, 2010
Published online: March 14, 2010
AIM: To investigate the relation of Fas and Fas ligand (FasL) protein expression with carcinogenesis and metastasis of gastric carcinoma.
METHODS: Immunohistochemistry was used to detect Fas and FasL protein expression in 64 gastric carcinoma tissue samples and 20 normal gastric tissue samples. Relation between FasL and Fas expression, age and gender of gastric cancer patients, and pathological subtype and lymph node metastasis of gastric cancer was analyzed.
RESULTS: The Fas expression level was significantly higher in normal gastric tissue samples than in gastric carcinoma tissue samples (85.0% vs 25.0%, P < 0.001), while the FasL expression level was significantly lower in normal gastric tissue samples than in gastric carcinoma tissue samples (30.0% vs 81.3%, P < 0.001). The Fas expression level was significantly higher in invasive lymph nodes than in non-invasive lymph nodes (82.9% vs 56.5%, P < 0.003) and in well-differentiated gastric carcinoma tissue samples than in poorly-differentiated gastric carcinoma tissue samples (50.0% vs 18.0%, P = 0.015). The FasL expression level was significantly lower in well-differentiated gastric carcinoma tissue samples than in poorly- differentiated gastric carcinoma tissue samples (42.9% vs 84.0%, P = 0.021). The Fas and FasL expression levels (25.0% and 81.3%) were significantly different in gastric carcinoma tissue samples (P < 0.001), but had a non-linear correlation (P = 0.575).
CONCLUSION: Abnormal Fas and FasL expressions in gastric carcinoma and lymph node tissues are involved in carcinogenesis and metastasis of gastric cancer.
- Citation: Li Q, Peng J, Li XH, Liu T, Liang QC, Zhang GY. Clinical significance of Fas and FasL protein expression in gastric carcinoma and local lymph node tissues. World J Gastroenterol 2010; 16(10): 1274-1278
- URL: https://www.wjgnet.com/1007-9327/full/v16/i10/1274.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i10.1274
Gastric carcinoma is one of the most common malignant tumors in the world. Currently, no effective treatment modalities are available for its metastasis and recurrence. Fas, a cell-surface receptor, activates the apoptosis signal pathway by binding to its ligand, FasL, resulting in cancer cell apoptosis[1,2]. It has been shown that Fas protein is abnormally expressed in esophageal, colon, and gallbladder carcinomas[3-5]. FasL is over-expressed in transformation and metaplasia of esophageal carcinoma[6].
By investigating the role of Fas and FasL expression in the carcinogenesis and metastasis of gastric carcinoma, several investigators have found that Fas expression in gastric carcinoma is decreased[7]. Bennett et al[8] showed that the high FasL expression level in gastric carcinoma can induce apoptosis of T lymphocytes infiltrating cancerous regions, suggesting that Fas and FasL induce apoptosis of activated lymphocytes, allowing cancer cells to escape immune attack.
However, no research is available on the clinical significance of Fas and FasL in local lymph nodes. To explore the relation between gastric carcinoma and regional lymph node metastasis, Fas and FasL protein expressions were detected using immunohistochemistry method in this study.
Specimens were obtained from 64 cases of gastric carcinoma admitted to the Department of Pathology, Second Affiliated Xiangya Hospital, Central South University, in May 2007-May 2008. Of these specimens, 38 were from male patients and 26 from female patients, (including 3 patients at the age of 20-29 years, 6 patients at the age of 30-39 years, 20 patients at the age of 40-49 years, 19 patients at the age of 50-59 years, and 16 patients at the age of over 60 years), 14 were from well-differentiated adenocarcinoma patients, and 50 were from poorly-differentiated adenocarcinoma patients. Lymph node metastasis was detected in 41 cases. The patients did not receive radiotherapy or chemotherapy before operation. Twenty normal gastric tissue samples were also obtained from the same hospital.
Specimens, fixed with 10% neutral formaldehyde solution and embedded in paraffin, were cut into 3-μm thick sections. Streptavidin-biotin complex (SABC) immunohistochemistry kit, primary antibodies: rabbit anti-human Fas and FasL, second antibody: biotin goat anti-rabbit IgG, and 3,3′-diaminobenzidine chromogenic kit were purchased from Wuhan Boster Company (China). PBS was used instead of primary antibody as a negative control following its manufacturer’s instructions.
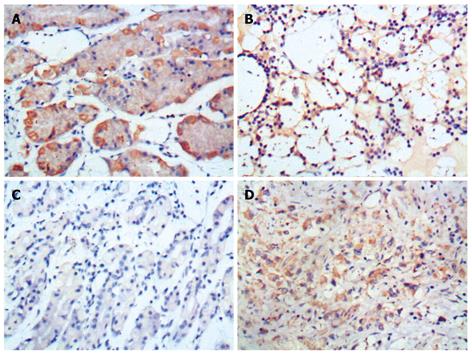
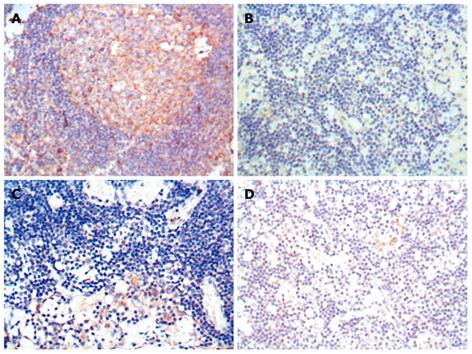
Intensity of staining was scored as 0: negative, 1: light yellow, 2: brown-yellow and 3: brown. Eight random high-power fields were observed under optical microscope and 4 high-power fields were recorded as the percentage of positive cells. Extent of staining was scored as 0: < 5%, 1: 5%-25%, 2: 26%-50%, 3: 51%-75% and 4: > 75%. The final score was determined by multiplying the scores of intensity and extent of staining, ranging 0-12. Scores 9-12 were defined as strong staining (+++), scores 4-8 as moderate staining (++), scores 1-3 as weak staining (+), and 0 as negative staining (-)[7].
Statistical analysis was performed using SPSS 15.0. The data were analyzed by rank-sum test and χ2 test. P < 0.05 was considered statistically significant.
The expression level of Fas protein was higher in normal gastric tissue samples than in gastric carcinoma tissue samples (85.0% vs 25.0%, Z = -4.780, P < 0.001). The FasL protein expression level was lower in normal gastric tissue samples than in gastric carcinoma tissue samples (30.0% vs 81.3%, Z = -4.115, P < 0.001) (Table 1 and Figure 1).
The expression level of Fas protein was higher in invasive lymph nodes than in non-invasive lymph nodes (82.9% vs 56.5%, Z = -2.954, P = 0.003). The expression level of FasL protein was lower in invasive lymph nodes than in non-invasive lymph nodes (56.1% vs 69.6%, Z = -0.593, P = 0.593) (Table 2 and Figure 2).
The Fas expression level was higher in well-differentiated gastric carcinoma tissue samples than in poorly-differentiated gastric carcinoma tissue samples (50.0% vs 18.0%, Z = -2.421, P = 0.015). The FasL expression level was lower in well-differentiated gastric carcinoma tissue samples than in poorly-differentiated gastric carcinoma tissue samples (42.9% vs 84.0%, Z = -2.307, P = 0.021) (Table 3).
The expression level of Fas and FasL protein in gastric carcinoma tissue samples was not significantly different in patients at different ages with or without lymph node metastasis (Table 4).
| Item (cases) | Fas | P value | FasL | P value | |||
| Positive | Negative | Positive | Negative | ||||
| Sex | |||||||
| Male (38) | 11 | 27 | χ2 = 0.777 | 29 | 9 | χ2 = 0.086 | |
| Female (26) | 5 | 21 | 0.378 | 19 | 7 | 0.769 | |
| Age (yr) | |||||||
| < 40 (9) | 3 | 6 | 6 | 3 | |||
| 40-49 (20) | 5 | 15 | 18 | 2 | |||
| 50-59 (19) | 6 | 13 | χ2 = 2.105 | 15 | 4 | χ2 = 5.891 | |
| > 60 (16) | 2 | 14 | 0.551 | 9 | 7 | 0.117 | |
| Lymph node metastasis | |||||||
| Yes (41) | 10 | 31 | χ2 = 0.023 | 29 | 12 | χ2 = 1.109 | |
| No (23) | 6 | 17 | 0.880 | 19 | 4 | 0.292 | |
The Fas and FasL expression rate was 25.0% and 81.3%, respectively, in gastric carcinoma tissue samples (Z = -4.780, P < 0.001). Rank correlation test showed that the Fas and FasL expression in gastric carcinoma tissue samples had a non-linear correlation (correlation coefficient: -0.425, P = 0.575).
The binding of Fas to its natural ligand, FasL, induces a signal transduction pathway leading to apoptosis[9]. Fas is a type I transmembrane protein belonging to the tumor necrosis factor (TNF) superfamily. Combination of Fas and FasL or a corresponding membrane-antibody, activates caspases, and is a major route to apoptosis[10].
Dysfunction of Fas or FasL will lead to inactivation of the Fas system and inhibit normal apoptosis, resulting in abnormal survival and proliferation of tumor cells, and may promote malignancies. It has been shown that inhibition of apoptosis plays a significant role in various stages of tumor formation and abnormal expressions of Fas and FasL protein can inhibit apoptosis[1,2].
In this study, the Fas expression level was lower in gastric carcinoma tissue samples than in normal gastric mucosa tissue samples, and the positive FasL expression in gastric carcinoma tissue samples had a trend to increase, which is considered to be the general mechanism by which tumor cells escape the Fas-mediated host immune system by reducing or inhibiting Fas expression, suggesting that Fas-mediated cytotoxic T cells (CTL) are unable to identify carcinoma cells, and carcinoma cells can thus avoid attack by T-lymphocytes[11].
Fas and FasL can be simultaneously expressed in tumor tissues. It has been reported that the susceptibility to Fas-mediated cell death is not positively correlated with the expression of tumor cell surface ligand FasL[12]. The molecular mechanism may be related to the expression of both FasL and decoy receptor 3 (DcR3) in gastric cancer cells. DcR3, a newly identified member of the tumor necrosis factor receptor (TNFR) family, binds to some specific protein ligands and may competitively inhibit Fas binding to FasL, thus blocking FasL and inhibiting apoptosis[13].
Although activated T lymphocytes and their ligand FasL can combine with Fas receptors in carcinoma cells, they do not induce apoptosis. However, membrane FasL in carcinoma cells can combine with Fas receptors of activated T lymphocytes, resulting in T lymphocyte apoptosis[14]. In this study, some invasive lymph nodes could over-express Fas, indicating that FasL can combine with over-expressed Fas receptors on T cells, resulting in T cell apoptosis and tumor proliferation. Since a high Fas expression level in lymphoid tissue signifies a higher susceptibility to tumor cells, the Fas/FasL system is involved in gastric carcinoma tumors evading the immune system. On the one hand, since Fas expression is reduced or lost in gastric carcinoma, anti-tumor immune cells expressing FasL are less able to clear them. On the other hand, gastric carcinoma cells expressing high FasL levels are able to attack tumor-infiltrating immune cells expressing high Fas levels by promoting apoptosis of immune cells, thus allowing gastric cancer cells to escape immune attack[15]. Kume et al[16] reported that a large number of lymphocytes in gastric carcinoma can express FasL. In our study, the Fas expression level was significantly higher in invasive lymph nodes than in non-invasive lymph nodes, suggesting that lymph nodes with a high Fas expression level may be more susceptible to self-apoptosis, thereby promoting lymph node metastasis.
In this study, the expression of Fas and FasL in gastric carcinoma tissue samples was not correlated with the sex and age of patients with or without lymph node metastasis, suggesting that the characteristics of Fas and FasL expression may have already formed in malignant transformation of gastric tumor cells[17].
In this study, the Fas and FasL expression rates were significantly different between well- and poorly-differentiated gastric carcinomas, which is consistent with the reported findings[7]. A possible mechanism is that a high FasL expression level in well-differentiated gastric carcinoma combines with Fas in an autocrine or paracrine manner to promote gastric carcinoma cell apoptosis and reduces tumor growth, while poorly-differentiated gastric carcinoma can produce soluble Fas (sFas), which competes with tumor cell membrane Fas receptor for FasL, leading to the inhibition of cancer cell apoptosis[18]. Meanwhile, sFas released from cancer cells plays an important role in the immunosuppressive effect. An elevated sFas level in hepatocellular carcinoma patients is negatively correlated with humoral (IgA, IgG) and cellular immune function parameters (e.g. lymphocyte transformation rate, natural and lymphokine-activated killer cell killing rate). Tumor cells produce sFas and combine with FasL expressed by T lymphocytes, a mechanism to evade immune attack[19], indicating that sFas may play an important role in carcinogenesis and cancer progression. However, how sFas generates and regulates such a mechanism is unclear, and needs to be further investigated.
In conclusion, abnormal expression of Fas and FasL is involved in carcinogenesis and metastasis of gastric carcinoma by escaping T lymphocyte attack and inducing lymphocyte apoptosis.
Gastric carcinoma is one of the most common malignant tumors in the world. Fas, a cell-surface receptor, activates the apoptosis signal pathway by binding to its ligand FasL, resulting in cancer cell apoptosis. Abnormal expressions of Fas and FasL are associated with carcinogenesis.
A high FasL expression level in gastric carcinoma can induce apoptosis of T lymphocytes infiltrating the tumor. However, no current research is available on the clinical significance of Fas and FasL protein expression in local lymph nodes. In this study, the authors showed that a high Fas expression level in lymphoid tissue might indicate a greater susceptibility to tumor cell invasion.
Binding of Fas to its natural ligand, FasL, induces the signal transduction pathway leading to apoptosis. This is the first study to report that Fas-mediated cell death susceptibility and expression of the tumor cell surface ligand FasL are not positively correlated. Furthermore, in the study, lymph nodes with a high Fas expression may be more susceptible to self-apoptosis, thus promoting lymph node metastasis.
By exploring the relation between the expressions of Fas and FasL in gastric carcinoma and lymph node tissues with regional lymph node metastasis, this study may convey a strategy for therapeutic intervention in gastric carcinoma patients.
It is a well written paper. The authors investigated the expression of Fas and FasL in gastric cancer, which helps to understand the pathogenesis of gastric cancer. The experimental procedure is quite well performed.
Peer reviewers: Dr. Luca Morelli, MD, Department of Anatomy and Histology, Hospital S. Clare, largo Medaglie d’Oro 9, Trento 38100, Italy; Mario M D’Elios, Professor, University of Florence, viale Morgagni 85, Florence 50134, Italy
S- Editor Wang JL L- Editor Wang XL E- Editor Ma WH
| 1. | Kase S, Osaki M, Adachi H, Kaibara N, Ito H. Expression of Fas and Fas ligand in esophageal tissue mucosa and carcinomas. Int J Oncol. 2002;20:291-297. |
| 2. | O’Connell J, Bennett MW, O’Sullivan GC, Collins JK, Shanahan F. Resistance to Fas (APO-1/CD95)-mediated apoptosis and expression of Fas ligand in esophageal cancer: the Fas counterattack. Dis Esophagus. 1999;12:83-89. |
| 3. | Shibakita M, Tachibana M, Dhar DK, Kotoh T, Kinugasa S, Kubota H, Masunaga R, Nagasue N. Prognostic significance of Fas and Fas ligand expressions in human esophageal cancer. Clin Cancer Res. 1999;5:2464-2469. |
| 4. | Zhu Q, Liu JY, Xu HW, Yang CM, Zhang AZ, Cui Y, Wang HB. Mechanism of counterattack of colorectal cancer cell by Fas/Fas ligand system. World J Gastroenterol. 2005;11:6125-6129. |
| 5. | Xu LN, Zou SQ, Wang JM. Action and mechanism of Fas and Fas ligand in immune escape of gallbladder carcinoma. World J Gastroenterol. 2005;11:3719-3723. |
| 6. | Younes M, Schwartz MR, Finnie D, Younes A. Overexpression of Fas ligand (FasL) during malignant transformation in the large bowel and in Barrett’s metaplasia of the esophagus. Hum Pathol. 1999;30:1309-1313. |
| 7. | Li YZ, Zhao P, Han WD. Clinicopathological significance of LRP16 protein in 336 gastric carcinoma patients. World J Gastroenterol. 2009;15:4833-4837. |
| 8. | Bennett MW, O’connell J, O’sullivan GC, Roche D, Brady C, Kelly J, Collins JK, Shanahan F. Expression of Fas ligand by human gastric adenocarcinomas: a potential mechanism of immune escape in stomach cancer. Gut. 1999;44:156-162. |
| 9. | Memon SA, Hou J, Moreno MB, Zacharchuk CM. Apoptosis induced by a chimeric Fas/FLICE receptor: lack of requirement for Fas- or FADD-binding proteins. J Immunol. 1998;160:2046-2049. |
| 10. | Fukuzawa K, Takahashi K, Furuta K, Tagaya T, Ishikawa T, Wada K, Omoto Y, Koji T, Kakumu S. Expression of fas/fas ligand (fasL) and its involvement in infiltrating lymphocytes in hepatocellular carcinoma (HCC). J Gastroenterol. 2001;36:681-688. |
| 11. | Compagni A, Christofori G. Recent advances in research on multistage tumorigenesis. Br J Cancer. 2000;83:1-5. |
| 12. | O’Connell J, Bennett MW, O’Sullivan GC, Collins JK, Shanahan F. The Fas counterattack: a molecular mechanism of tumor immune privilege. Mol Med. 1997;3:294-300. |
| 13. | Takahama Y, Yamada Y, Emoto K, Fujimoto H, Takayama T, Ueno M, Uchida H, Hirao S, Mizuno T, Nakajima Y. The prognostic significance of overexpression of the decoy receptor for Fas ligand (DcR3) in patients with gastric carcinomas. Gastric Cancer. 2002;5:61-68. |
| 14. | Yaqub S, Henjum K, Mahic M, Jahnsen FL, Aandahl EM, Bjørnbeth BA, Taskén K. Regulatory T cells in colorectal cancer patients suppress anti-tumor immune activity in a COX-2 dependent manner. Cancer Immunol Immunother. 2008;57:813-821. |
| 15. | Lee TB, Min YD, Lim SC, Kim KJ, Jeon HJ, Choi SM, Choi CH. Fas (Apo-1/CD95) and Fas ligand interaction between gastric cancer cells and immune cells. J Gastroenterol Hepatol. 2002;17:32-38. |
| 16. | Kume T, Oshima K, Yamashita Y, Shirakusa T, Kikuchi M. Relationship between Fas-ligand expression on carcinoma cell and cytotoxic T-lymphocyte response in lymphoepithelioma-like cancer of the stomach. Int J Cancer. 1999;84:339-343. |
| 17. | Zhao XH, Gu SZ, Tian HG, Quan P, Pan BR. Clinical significance of expression of apoptotic signal proteins in gastric carcinoma tissue. World J Gastroenterol. 2005;11:3846-3849. |
| 18. | Hallermalm K, De Geer A, Kiessling R, Levitsky V, Levitskaya J. Autocrine secretion of Fas ligand shields tumor cells from Fas-mediated killing by cytotoxic lymphocytes. Cancer Res. 2004;64:6775-6782. |