Published online Jan 7, 2010. doi: 10.3748/wjg.v16.i1.89
Revised: November 6, 2009
Accepted: November 13, 2009
Published online: January 7, 2010
AIM: To predict treatment success using only simple clinical data from peg-interferon plus ribavirin therapy for chronic hepatitis C.
METHODS: We analyzed the clinical data of 176 patients with chronic hepatitis and hepatitis C virus genotype 1 who received 48 wk standard therapy, derived a predictive formula to assess a sustained virological response of the individual patient using a logistic regression model and confirmed the validity of this formula. The formula was constructed using data from the first 100 patients enrolled and validated using data from the remaining 76 patients.
RESULTS: Sustained virological response was obtained in 83 (47.2%) of the patients and we derived formulae to predict sustained virological response at pretreatment and weeks 4, 12 and 24. The likelihood of sustained virological response could be predicted effectively by the formulae at weeks 4, 12 and 24 (the area under the curve of the receiver operating characteristic: 0.821, 0.802, and 0.891, respectively), but not at baseline (0.570). The formula at week 48 was also constructed and validation by test data achieved good prediction with 0.871 of the area under the curve of the receiver operating characteristic. Prediction by this formula was always superior to that by viral kinetics.
CONCLUSION: These results suggested that our formula combined with viral kinetics provides a clear direction of therapy for each patient and enables the best tailored treatment.
- Citation: Saito H, Ebinuma H, Ojiro K, Wakabayashi K, Inoue M, Tada S, Hibi T. On-treatment predictions of success in peg-interferon/ribavirin treatment using a novel formula. World J Gastroenterol 2010; 16(1): 89-97
- URL: https://www.wjgnet.com/1007-9327/full/v16/i1/89.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i1.89
Persistent hepatitis C virus (HCV) infection is the major chronic liver disease in Japan, and pegylated interferon-α (PEG-IFN) plus ribavirin (RBV) therapy is the current mainstay of treatment. The goal of treatment is a sustained virological response (SVR), which is defined as undetectable serum HCV RNA, according to a polymerase chain reaction-based assay, 6 mo after the cessation of therapy[1]. Patients who achieve SVR gain the benefits of regression of fibrosis, decreased incidence of hepatocellular carcinoma, and reduced morbidity and mortality. The genotype of HCV affects treatment efficacy and HCV RNA levels (viral load) also may have an effect. Only a small percentage of patients infected with HCV genotype 1b and high viral load achieved SVR with conventional IFN therapy for 6 mo[2]. Patients infected with HCV genotype 1 should receive 48 wk of PEG-IFN plus RBV, while 24 wk of treatment is recommended for patients with HCV genotype 2[3,4]. When PEG-IFN plus RBV is administered in this manner, around 50% of patients infected with HCV genotype 1 achieve SVR[5], which is a great improvement over the SVR associated with 24 wk conventional IFN-α therapy.
The likelihood of treatment success may also be predicted by viral kinetics on therapy as well as circulating core antigen and immune parameters[6,7]. In particular, recent studies have shown that SVR can be predicted by a rapid virological response (RVR), which is defined as an undetectable level of HCV RNA at 4 wk of treatment[8], and an early virological response (EVR), which is defined as either an undetectable level of HCV RNA or a drop in HCV RNA levels of at least 2 log10IU/mL after 12 wk of treatment[9].
Nevertheless, the current dosing regimens for PEG-IFN plus RBV could potentially under-treat some patients[10] and additional measurements of viral response are needed to facilitate individualization of therapy. Among predictive factors already reported[11-15], many are not readily available from daily clinical assessment, because they require genomic analyses and/or advanced experimental methods. There is increasing evidence to support extending the duration of treatment beyond 48 wk for patients with an HCV genotype 1 infection who display a slow virological response, which is defined by HCV RNA levels > 50 IU/mL at week 12 but undetectable at week 24. Several trials suggested that 72 wk of treatment with PEG-IFN plus RBV results in a better SVR rate than the same treatment for 48 wk[16-18]. However, it is difficult to accurately determine whether the individual should have their therapy extended at week 48, because the predictive value of a slow virological response may be insufficient alone. It would be very valuable to have a more accurate predictive marker of SVR at week 48, derived from clinically available measurements.
In this study, to try to make better prediction of patients who would or would not respond to 48 wk of PEG-IFN plus RBV therapy, we analyzed the clinical data of patients with chronic hepatitis C who received 48 wk of therapy, derived a predictive formula to assess the likelihood of an SVR for each individual patient using a logistic regression model and confirmed the validity of this formula.
The study was approved by the Ethics Committee of the Keio University, School of Medicine, and was performed in accordance with the internationally accepted ethical standards for human experimentation. The study was conducted by the Keio Association for the Study of Liver Diseases (KASLD). All patients received explanations of the purpose and protocol of the study and written informed consent was obtained from each patient.
One hundred and seventy-six patients with chronic hepatitis C infected with HCV genotype 1b were enrolled prospectively and received PEG-IFN plus RBV therapy from December 2004 to May 2007. All patients had HCV RNA levels ≥ 100 KIU/mL, measured by a quantitative polymerase chain reaction (PCR) assay (COBAS HCV Amplicor MONITORTM, sensitivity 500 IU/mL; Roche Diagnostic Systems, Inc., Tokyo, Japan). Pregnant women and women of childbearing potential, nursing mothers, male patients whose partner could have become pregnant, and those with anemia (hemoglobin concentration of 10 g/dL or less), leucopenia (1500 cells/μL or less), thrombocytopenia (80 000 cells/μL or less), severe dysfunction of organs other than the liver (these exclusion criteria are included in the instruction of the drug and provided by the manufacturer), infection with hepatitis B virus or human immunodeficiency virus, autoimmune hepatitis, primary biliary cirrhosis, and liver dysfunction caused by other etiologies were excluded. Some patients did not undergo a liver biopsy because not all of the centers could perform biopsies. All patients were treated for 48 wk and were followed for 24 wk after cessation of treatment. The formula was derived using data from the first 100 patients enrolled as selection data and validated using data from the additional 76 patients as test data. In this way it was possible to analyze the predictive accuracy and validity of the constructed formula.
PEG-IFN-α2b (Schering-Plough K.K., Osaka, Japan) was administered weekly in doses adjusted for body weight according to the manufacturer’s recommendations in Japan (45 kg or less, 60 μg; 46-60 kg, 80 μg; 61-75 kg, 100 μg; 76-90 kg, 120 μg; 91 kg or more, 150 μg). Similarly, RBV (Schering-Plough K.K.) was given in daily doses adjusted to body weight according to manufacturer’s instructions (60 kg or less, 600 mg/d; 61-80 kg, 800 mg/d; 81 kg or more, 1000 mg/d). Serum levels of HCV RNA were quantified and, when the level was below 500 IU/mL, HCV RNA was measured with the COBAS HCV Amplification and Detection version 2.0, sensitivity 50 IU/mL, Roche Diagnostic Systems). Blood cell counts and chemistry were analyzed at the beginning of treatment and every 4 wk thereafter. A questionnaire was used to review demographic data (gender, age, weight, height), previous treatment, histologic activity grade, and fibrosis stage, dose of PEG-IFN, dose of RBV, presence of diabetes, HCV RNA levels, SVR, white blood cell counts (WBC), neutrophil counts (NC), red cell counts (RBC), hemoglobin levels (Hb), platelet counts (PLT), serum aspartate aminotransferase levels (AST), and serum alanine aminotransferase levels (ALT).
The Mann-Whitney U-test was used to analyze continuous variables. Chi-squared and Fisher’s exact tests were used for analysis of categorical data. One of our goals was to predict SVR using only simple clinical data, so a database was created containing the following basic information: for all patients, baseline age, sex, body weight (kg), height (cm), dose of PEG-IFN (μg/kg), dose of RBV (mg/kg), HCV RNA levels (KIU/mL), SVR (+/-), WBC (/μL), NC (/μL), RBC (/μL), Hb (g/dL), PLT (/μL), AST (IU/L), and ALT (IU/L). Statistical analyses were performed using the Statistical Package of Services Solutions (SPSS; SPSS Inc., Chicago, IL, USA) software, version 11.0. First, factors that differed significantly between SVR and non-SVR groups were identified at every time point by univariate analyses. The independent discriminative value of markers for predicting SVR was then assessed by logistic regression analysis. The third step was to construct a formula that combined independent factors. The best index for discrimination was the logistic regression function that combined the most discriminatory independent factors. The predictive formula was logically constructed by following basal formula:
1/p = 1 + exp [-(β0 + β1X1 + β2X2 +......+ βrXr)]
Diagnostic values of indices and isolated factors were assessed by sensitivity, specificity, positive and negative predictive values (PPV and NPV), and receiver operating characteristic (ROC) curves.
Of the 176 patients, 101 (59.8%) were men and the median age was 56 years (18-77), which is greater than has been reported from Western countries. The median values of body weight and BMI were 61 kg (41.2-90.5) and 22.8 (15.7-32.0), respectively, which are lower than has been reported from Western countries. These conditions are characteristic of recent trends in Japanese patients; older and less obese patients. Ninety-four patients (66.2%) were treatment naïve and the median value of HCV RNA was 2165 KIU/mL (130 to > 5000). The pretreatment median values were as follows; RBC 464 cells/μL, Hb 14.4 g/dL, PLT 165 × 103 cells/μL, WBC 4775 cells/μL, NC 2549 cells/μL, AST 51 IU/L, and ALT 63 IU/L.
SVR was obtained in 83 (47.2%) patients and in 54 (54%) of the first 100 patients (selection data) enrolled in this study (Table 1). Of the 83, 43 were male; 60.6% of the male patients achieved SVR and there was a statistically significant gender difference (P = 0.020). The median age was significantly lower in the SVR group. There was no difference in body weight and BMI between the SVR group and non-SVR group for the patients used for selection data. The pretreatment HCV RNA level did not differ significantly between the SVR and non-SVR groups, while pretreatment RBC, Hb, PLT and ALT levels differed between the groups. The average cumulative dose of RBV administered up to the time point indicated was always much greater in the SVR group than in the non-SVR group. The average cumulative dosage of PEG-IFN differed between the groups at week 48.
| SVR | non-SVR | P value | |
| Number (%) | 54 (54.0) | 46 | |
| Gender (%) | |||
| Male | 43 (60.6) | 28 | 0.038 |
| Female | 9 (34.6) | 18 | |
| Age | |||
| Median | 53 | 57 | 0.0098 |
| Range | 18-72 | 37-77 | |
| Weight (kg) | |||
| Median | 65.1 | 60 | 0.138 |
| Range | 42.5-90.5 | 43.9-86.0 | |
| BMI (kg/m2) | |||
| Median | 23.5 | 22.9 | 0.834 |
| Range | 17.5-31.8 | 18.2-31.2 | |
| Previous + treatment | 22 | 19 | 1.000 |
| HCV RNA (KIU/mL) | |||
| Median | 1889 | 2263 | 0.554 |
| Range | 140 to < 5000 | 150 to < 5000 | |
| RBC (× 10-4/mL) | |||
| Median | 469 | 452 | 0.0041 |
| Range | 319-621 | 354-552 | |
| Hb (g/dL) | |||
| Median | 15.0 | 14.1 | 0.0059 |
| Range | 10.9-17.7 | 11.3-17.2 | |
| PLT (× 10-3/mL) | |||
| Median | 172 | 159 | 0.039 |
| Range | 84-292 | 62-270 | |
| WBC (/mL) | |||
| Median | 5000 | 4850 | 0.256 |
| Range | 3270-8900 | 2300-9200 | |
| NC (/mL) | |||
| Median | 2550 | 2549 | 0.978 |
| Range | 1066-4231 | 1184-5626 | |
| AST (IU/L) | |||
| Median | 54 | 50 | 0.898 |
| Range | 22-156 | 24-241 | |
| ALT (IU/L) | |||
| Median | 76 | 55 | 0.027 |
| Range | 36-311 | 15-278 | |
| PEG-IFN dose (48 wk) (μg/kg per day) | |||
| Median | 1.43 | 1.32 | 0.0043 |
| Range | 0.68-1.82 | 0.52-1.82 | |
| RBV dose (48 wk) (mg/kg per day) | |||
| Median | 10.83 | 8.39 | 0.0002 |
| Range | 3.42-14.55 | 2.75-12.64 |
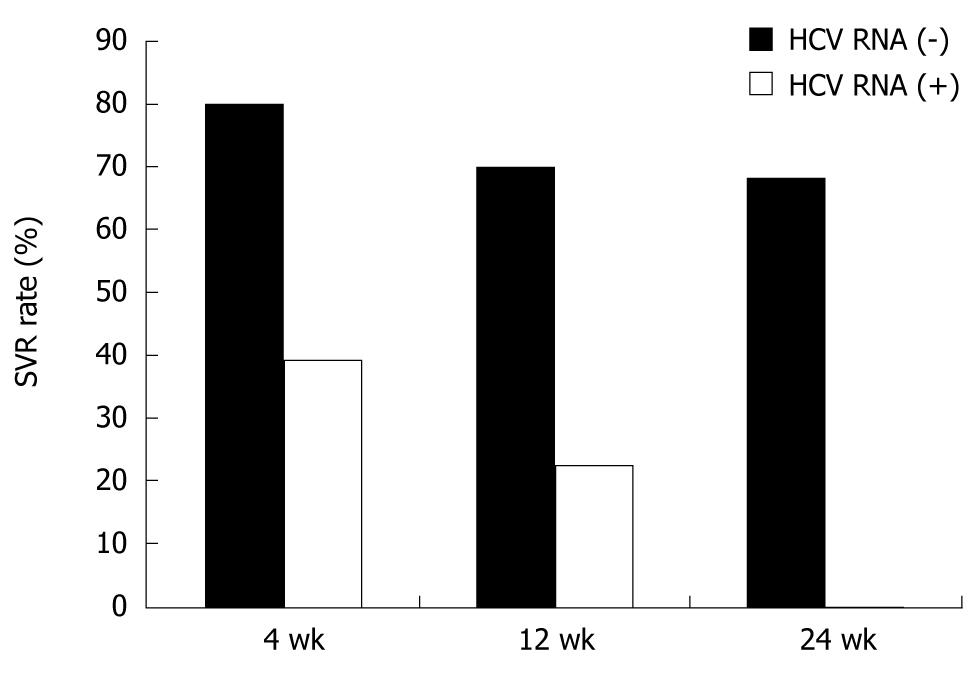
Among the first 100 patients, serum HCV RNA decreased 1 log or more at week 4 in 67 (67.0%) and became undetectable in 31 (RVR, 31.0%). SVR was attained in 25 of the 31 RVR patients (80.6%). Likewise, a complete EVR was attained in 66 patients, among whom 46 (69.7%) finally achieved SVR. SVR was achieved in 52 of 76 patients (68.4%) whose serum HCV RNA had disappeared by week 24. SVR also was achieved in patients whose serum HCV RNA had not disappeared until week 12. SVR was attained in 39.3%, 22.6% and 0% of patients who failed to achieve RVR, complete EVR and HCV RNA negativity at week 24, respectively (Figure 1). From these data, PPV and NPV determined by viral kinetics at week 4 were 80.6% and 60.7%, respectively. PPV at weeks 12 and 24 were 68.7% and 68.4% respectively, and NPV were 77.4% and 100%, respectively.
Multivariate analysis was performed to determine the factors contributing to SVR. Analysis was made pretreatment and at weeks 4, 12 and 24. Factors available from pretreatment until at the time point were all included, and those calculated as P < 0.1 by univariate analysis at each time point were analyzed using the logistic regression method. A statistical difference was found in gender, age, RBC, Hb, PLT and log (ALT 0 wk: ALT levels at week 0) at pretreatment by univariate analysis. The independent factor contributing to SVR was RBC (P = 0.024) at pretreatment. Among significant factors found by univariate analysis at week 4, log (ALT 0 wk) (P = 0.015), RVR (4 wk) (P = 0.0049), and log (AST 4 wk) (P = 0.042) were independent factors by multivariate analysis. Similarly, log (ALT 0 wk) (P = 0.0076), EVR (P = 0.0083), WBC (4 wk) (P = 0.035), and average cumulative RBV dose (P = 0.045) were significant factors at week 12. Independent contributing factors at week 24 were log (ALT 0 wk) (P = 0.0047), HCV RNA (-/+) (24 wk) (P = 0.005), WBC (4 wk) (P = 0.044), log (AST 12 wk) (P = 0.044) and average cumulative RBV dose (12 wk) (P = 0.040) (Table 2). It was intriguing in addition to RVR and EVR that baseline ALT level (log) always affected SVR prediction from pretreatment until week 24.
| Variables | Odds ratio | 95% CI | P value |
| At pretreatment | |||
| RBC (× 104) (0 wk) | 1.011 | 1.002-1.021 | 0.024 |
| PLT (× 103) (0 wk) | 1.085 | 0.986-1.193 | 0.095 |
| log (ALT 0 wk) | 3.509 | 0.727-16.934 | 0.118 |
| At week 4 | |||
| Age | 0.941 | 0.885-1.000 | 0.051 |
| log (ALT 0 wk) | 27.090 | 1.891-388.001 | 0.015 |
| RVR +/- | 6.543 | 1.766-24.243 | 0.0049 |
| log (AST 0 wk) | 0.036 | 0.001-0.886 | 0.042 |
| At week 12 | |||
| log (ALT 0 wk) | 39.331 | 2.648-584.144 | 0.0076 |
| RVR +/- | 3.015 | 0.694-13.100 | 0.141 |
| EVR +/- | 8.340 | 1.728-40.265 | 0.0083 |
| WBC (4 wk) | 1.001 | 1.000-1.002 | 0.035 |
| log (AST 12 wk) | 0.049 | 0.002-1.037 | 0.053 |
| RBV dose (12 wk) | 1.519 | 1.010-2.284 | 0.045 |
| At week 24 | |||
| log (ALT 0 wk) | 68.688 | 3.669 to < 999.999 | 0.0047 |
| RVR +/- | 3.329 | 0.819-13.529 | 0.093 |
| EVR +/- | 31.775 | 2.840-355.460 | 0.0050 |
| WBC (4 wk) | 1.001 | 1.000-1.002 | 0.044 |
| log (AST 12 wk) | 0.036 | 0.001-0.918 | 0.044 |
| RBV dose (12 wk) | 1.607 | 1.021-2.528 | 0.040 |
According to the method of logistic regression analysis, we derived four formulae to predict SVR of patients receiving 48-wk PEG-IFN plus RBV treatment in our cohort from significant factors selected by multivariate analysis at pretreatment and week 4, week 12 and week 24 as shown in Table 2. These formulae are as follows:
Pretreatment: 1/p = 1 + exp {-[-8.8065 - 0.0114 × RBC (× 104) 0 wk + 0.0812 × PLT (× 104) 0 wk + 1.2552 × log (ALT 0 wk)]}
At week 4: 1/p = 1 + exp [-(-1.8839 - 0.00607 × Age + 3.2992 × log (ALT 0 wk) + 1.8784 × RVR - 3.3364 × log (AST 4 wk)]
At week 12: 1/p = 1 + exp {-[-11.5278 + 3.672 × log (ALT 0 wk) + 1.1036 × RVR + 2.1211 × EVR + 0.000837 × WBC 4 wk - 3.0134 × log (AST 12 wk) + 0.418 × RBV dose 12 wk]}
At week 24: 1/p = 1 + exp {-[-14.5754 + 4.2296 × log (ALT 0 wk) + 1.2028 × RVR + 3.4587 × HCV RNA 24 wk + 0.0009 × WBC 4 wk - 3.3224 × log (AST 12 wk) + 0.4741 × RBV dose 12 wk]}
HCV RNA (-): 1, (+): 0.
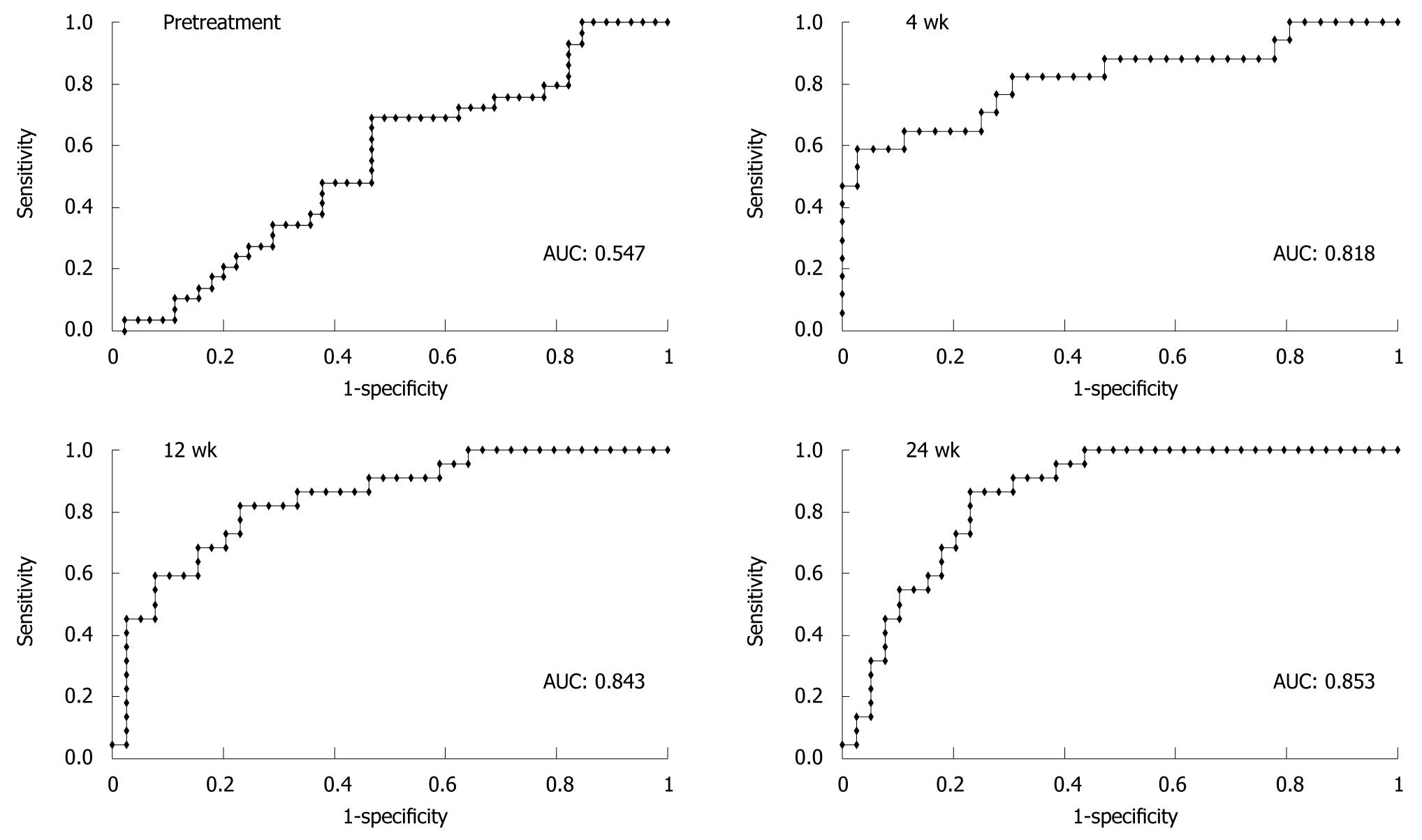
ROC curve analysis was conducted to evaluate the accuracy of each prediction using both selection data and test data. The area under the curve of the ROCs (AUCs) of multiple logistic regression analyses using selection data (n = 100) at pretreatment and at weeks 4, 12 and 24 were 0.710, 0.828, 0.889, and 0.933, respectively. The predictive value at pretreatment was insufficient, but those after weeks 4, 12 and 24 were satisfactory.
The validity of the predictive formulae was evaluated further using test data (n = 76). The AUCs of logistic regression analyses using test data were 0.547, 0.818, 0.843, and 0.853 at pretreatment and weeks 4, 12 and 24, respectively (Figure 2). Unlike prediction by viral kinetics, our formula is always applicable to all patients and the final predictive value is fairly high according to the ROC analysis, as described above. The median calculated values for patients who ultimately attained SVR were 0.545, 0.661, 0.589 and 0.656, at pretreatment and weeks 4, 12 and 24, respectively, and these values were always significantly higher than those of non-SVR cases (Table 3). The statistical difference of predictive values between SVR and non-SVR patients increased with the duration of therapy.
| Median (range) | Patients who attained SVR | Patients with non-SVR | P value |
| Pretreatment | 0.545 (0.286-0.926) | 0.507 (0.108-0.945) | 0.514 |
| At week 4 | 0.661 (0.139-0.998) | 0.251 (0.056-0.688) | 0.000 |
| At week 12 | 0.589 (0.079-0.994) | 0.146 (0.001-0.952) | 0.000 |
| At week 24 | 0.656 (0.038-0.996) | 0.026 (0.000-0.949) | 0.000 |
It has been suggested that the prolongation of treatment is effective for patients who do not achieve HCV RNA negativity at week 12. There are also patients who achieve RVR and/or EVR but whose serum HCV RNA reappears after cessation of the treatment. We evaluated whether we can select such patients for whom treatment should be lengthened to 72 wk, using the same procedure as for on-treatment prediction of SVR with 48 wk of treatment. The predictive formula at week 48 was constructed from the data of the 100 patients used for selection data. This formula included the parameter whether HCV RNA disappeared during therapy. When HCV RNA disappeared at week 4, 8, 12 and 24, each value, such as 4, 8, 12 and 24, was inserted into the formula. For non-SVR cases, 100 was inserted into the formula. The formula was determined as follows:
1/p = 1 + exp {-[-4.9107 - 0.0079 × Time of HCV RNA negative (wk) + 0.1477 × PLT 0 wk + 3.4941 × log (ALT 0 wk) - 1.7018 × log (AST 12 wk)]}
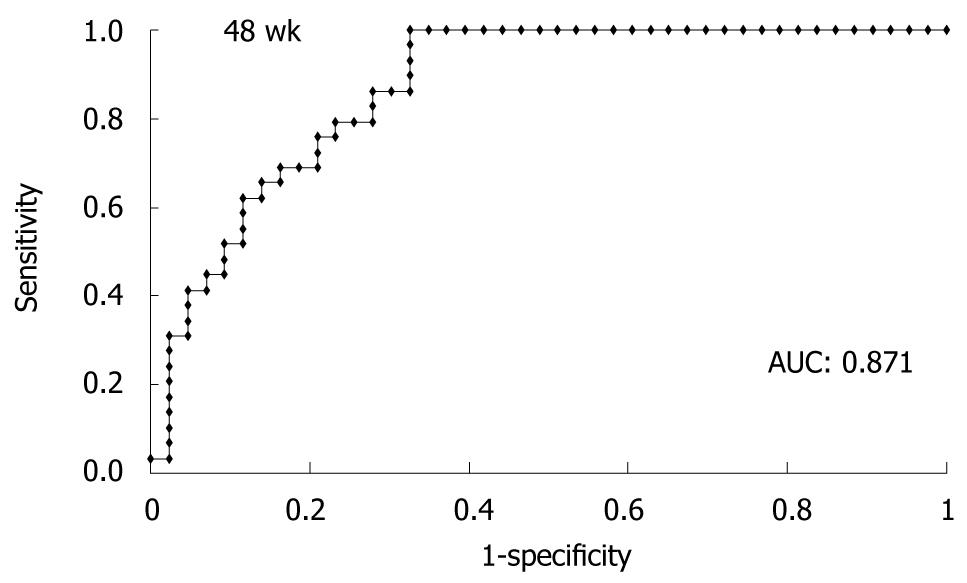
The ROC curve obtained from the test data of 76 patients is shown in Figure 3. The AUC derived from the test data (n = 76) by logistic regression analysis was 0.871, suggesting that patients who can stop treatment at week 48 are predicted accurately by this formula. The median calculated value of patients who attained SVR was 0.775 (0.237-0.999) and that of patients who did not achieve SVR was 0.004 (0-0.966, P < 0.00001). PPVs and NPVs calculated by the formula with various cut-off points are shown in Table 4. When we set the cut-off point at 0.2, NPV was 100% and 28 patients were included in this category.
| Cut-off point | PPV (%) | NPV (%) | P value |
| 0.2 | 65.9 | 100 | < 0.00001 |
| 0.3 | 66.7 | 96.7 | < 0.00001 |
| 0.4 | 66.7 | 86.1 | < 0.00001 |
| 0.5 | 68.8 | 82.5 | 0.000013 |
| 0.6 | 71.4 | 79.5 | 0.000024 |
| 0.7 | 77.3 | 76.0 | 0.000048 |
| 0.8 | 80.0 | 70.2 | 0.00074 |
There were 10 patients who relapsed after attaining complete EVR in 76 patients (4 cases achieved negativity of HCV RNA at week 8 and others achieved at week 12), and two of them could be pointed out as non-SVR by our calculation if the cut-off point was set at 0.5 (the value of one predicted person was 0.129 and another was 0.421).
We propose here a method using formulae for the prediction of SVR in patients with chronic hepatitis C treated for 48 wk with PEG-IFN plus RBV. The predictive potential was very high when judged by AUC analysis, which was more than 0.8 from week 4. In particular, the validity at week 24 was more than 0.85 of AUC. The simplest method of prediction of SVR may be viral kinetics and a response-based on-treatment prediction, such as RVR and EVR, which are the outcomes of totally integrated viral and host factors. The PPV of the formulae were better at weeks 12 and 24 than the prediction with viral kinetics, and the NPV of the formulae were better at weeks 4 and 12. Evaluation of the formulae using data from the test patients revealed a very high AUC value of more than 0.85. These results suggest that formulae based on simple clinical data are superior to prediction by viral kinetics. These formulae, however, cannot be permanent, and may vary among different groups of patients, so should be re-evaluated or re-constructed, even for our series when the number of patients has increased. The most important outcome of this study is that we can predict SVR of our patients accurately with 48-wk PEG-IFN plus RBV therapy using only “simple” clinical data. Individual tailoring of treatment duration may be an option in the future to reduce relapse rates in HCV type 1-infected patients. The concept that extension of treatment duration can reduce relapse rates should be adopted only for a limited proportion of type 1-infected patients because the possibility of SVR may be very low in those whose serum HCV RNA remains positive at week 24. The patients who will benefit most from prolongation of therapy include those whose serum HCV RNA is positive at week 12 but negative at week 24, including even patients with RVR and EVR. The formulae we suggest might be helpful for such patients who are expected to achieve SVR but did not do so. For those individuals, our method based on logistic regression analysis will show a clear direction of therapy in each case and enable the best tailored treatment. Further prospective studies should be performed to determine whether this approach really increases the SVR rate by selection of patients and extension of treatment duration up to week 72.
RVR may be valuable for determining treatment duration but is not sufficient for predicting the response to treatment[8]. When SVR is predicted by RVR, the confirmed SVR rate within whole patients may be low. In the study of Yu et al[19], SVR was achieved in 42 of 100 patients, and all the patients who attained RVR achieved SVR. However, SVR was also attained in patients who did not attain RVR, and another 30 SVR patients who were not included in the RVR group. If 100% of patients with RVR attain SVR, the final prediction of SVR at this point (week 4) is 53.2%. In the study of Jensen et al[20], 95 of 374 (25.4%) genotype 1 patients attained RVR. The SVR rate of these 95 patients was 82% compared to 20.8% among the 374 treated subjects. The PPV of EVR for SVR is estimated to be less robust, less than 70%, and the validity may decrease more when we predict SVR by RVR only. We could predict SVR patients with 57.9% if the cut-off point was set at 0.5, and with 64.7% if that was set at 0.6 at week 4. The potent SVR of patients who did not achieve RVR could be predicted by our formula and the combination with viral kinetics may further improve predictive value.
Ferenci et al[21] investigated response-guided treatment based on RVR and 78.8% of HCV genotype 1 patients with RVR attained SVR. Around 20% of patients with RVR failed to achieve SVR in their study. The SVR rate of patients with a complete EVR is around 80% and that of patients without an EVR is around 15%. Therefore, there are several unfortunate patients with RVR and EVR but in whom serum HCV RNA reappears after the cessation of treatment. On the other hand, it may be difficult to attain SVR for patients whose serum HCV RNA does not disappear until after week 24 (late responders), even if they are treated for more than 48 wk with PEG-IFN plus RBV. Recent studies suggested that the extension of treatment to 72 wk would help to achieve SVR in such “unfortunate” patients, who should have responded well to the 48 wk therapy. It is not realistic that all patients who attain RVR and EVR should receive 72 wk therapy to ensure SVR. Our method of deriving a formula, predicting success or failure of response to 48 wk treatment, may serve as a good compass to identify patients who require extended treatment to achieve SVR. Of course, further prospective study is necessary and there has been no evidence that prolongation of therapy really decreases relapse rate. However, using the formula of week 48, we could predict patients who will benefit from an additional 24 wk of treatment and achieve an SVR. In fact, we could recognise 2 of 10 EVR patients as non-SVR by our formula and they would be rescued if they receive additional 24 wk therapy.
Perhaps the rule of stopping the treatment of patients with a decrease of less than 2 log10 in HCV RNA level within the initial 12 wk of therapy should be reconsidered because the high NPV of this rule (98%-100%) could be confirmed only for the 48 wk treatment group and not for the 72 wk group. As seen in our series, Japanese patients with chronic hepatitis C are older and have thinner physiques than those in Western countries. Because the time of infection was approximately 60 years ago[22], and much earlier than elsewhere in the world, patients of an older age require treatment with PEG-IFN plus RBV[23]. However, these patients easily become anemic, probably because of their older age combined with their physical characteristics[24], and the adherence to RBV, which may be critical for attaining SVR, is usually low. An RBV dosage of 1000-1200 mg/d is administered rarely in Japanese studies. The higher dose of PEG-IFN and RBV in 48 wk therapy suggested by Fried et al[25], who studied patients with a mean age of approximately 47, is almost impossible in Japan. When older patients opt for PEG-IFN plus RBV therapy, it is a “one-chance-treatment” and they always endure patiently the side effects of therapy but rarely agree to re-treatment after the cessation of therapy. Therefore, the on-treatment prediction of SVR is very important for older patients and, if the probability of success is reasonable, they would choose prolongation of the therapy. In this case, our formula is a very useful tool to decide whether the patient should receive additional therapy at week 48.
Many factors affecting the SVR rate have been reported, including viral- and host-related factors. Among the viral factors, amino acid substitutions in the interferon sensitivity determining region (ISDR) located in HCV nonstructural region 5A[11] and the core region (71st and 90th codons)[12] are well established. We also reported amino acid substitutions in the RNA-dependent RNA polymerase (NS5B) region from our cohort study[26,27]. On the other hand, host factors, such as pretreatment intrahepatic CD8+ cell count[28], the T-helper type 1 and 2 (Th1/Th2) ratio[13,29,30] and T-helper activity[31], have also been demonstrated. Other factors, such as metabolic and diabetic factors, have been implicated in the efficacy of IFN therapy[32]. RBV plasma concentration at week 4[14] and a new index, named accordion index[33], have also been proposed. These significant viral and host factors, except for the metabolic factors, are difficult to examine and daily clinical assessment is not practical. Shirakawa et al[15] published an excellent report recently on the classification of patients according to their responsiveness to PEG-IFN plus RBV therapy. They predicted SVR successfully with pretreatment data, but the prediction included particular determinations, such as ISDR sequences and Th1/Th2 ratios, which are not easily available in clinics and are uneconomical. In contrast to their report, the formulae proposed in this study involve factors included among readily available data, and moreover, the validity was very high, especially at weeks 24 and 48.
In conclusion, our predictive formula, which is easily constructed in every institution with simple clinical data, would offer better prediction of SVR and non-SVR than the prediction by viral kinetics. Further study including extended protocol (72 wk treatment) and analysis with other measurement of HCV RNA, such as real-time PCR, should be evaluated in the future.
The likelihood of treatment success of 48 wk peg-interferon (PEG-IFN) plus ribavirin (RBV) therapy for chronic hepatitis C may be predicted by viral kinetics on therapy. In particular, recent studies have shown that sustained virological response (SVR) can be predicted by a rapid virological response (RVR), and an early virological response (EVR). Nevertheless, the current dosing regimens could potentially under-treat some patients and additional measurement of viral response is needed to facilitate individualization of therapy. Among predictive factors already reported, many are not readily available from daily clinical assessment, because they require genomic analyses and/or advanced experimental methods.
It is difficult to accurately determine whether the individual should have their therapy extended at week 48, because the predictive value of a slow virological response may be insufficient alone. It would be very valuable to have a more accurate predictive marker of SVR at week 48, derived from clinically available measurements. According to the method of logistic regression analysis, the authors of this study derived formulae to predict SVR of patients receiving 48 wk PEG-IFN plus RBV treatment in their cohort from significant factors selected by multivariate analysis at pretreatment and weeks 4, week 12 and week 24.
The most important outcome of this study is that it is possible to predict SVR accurately with 48 wk PEG-IFN plus RBV therapy by formulae using only “simple” clinical data.
This study may enable the best tailored treatment especially for patients with a high expectation of sustained virological response with 48 wk peg-interferon and ribavirin therapy for chronic hepatitis C but whose responses relapse. Prospective studies informed by this method will be of considerable value.
RVR is defined as an undetectable level of HCV RNA at 4 wk of treatment, and EVR is defined as either an undetectable level of HCV RNA or a drop in HCV RNA levels of at least 2 log10IU/mL after 12 wk of treatment. Recent studies reported that these on-treatment viral kinetics are useful for prediction of SVR.
Conclusively, establishing a new predictive formula to assess the likelihood of a SVR of the individual patient chronically infected with HCV is very important.
Peer reviewer: Dariusz M Lebensztejn, Ass. Professor, IIIrd Department of Pediatrics, Medical University of Bialystok, 17 Waszyngtona Str., Bialystok 15-247, Poland
S- Editor Wang YR L- Editor O’Neill M E- Editor Ma WH
| 1. | Veldt BJ, Heathcote EJ, Wedemeyer H, Reichen J, Hofmann WP, Zeuzem S, Manns MP, Hansen BE, Schalm SW, Janssen HL. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med. 2007;147:677-684. |
| 2. | Saito H, Tsuchimoto K, Miyaguchi S, Tada S, Sawaguchi K, Komatsu K, Kaneko K, Watanabe T, Morizane T, Ishii H. Keio multicenter trial in high-dose interferon-alpha 2b treatment for chronic hepatitis C. Keio Interferon-alpha 2b Study Group. Keio J Med. 1996;45:161-167. |
| 3. | Farrell GC. New hepatitis C guidelines for the Asia-Pacific region: APASL consensus statements on the diagnosis, management and treatment of hepatitis C virus infection. J Gastroenterol Hepatol. 2007;22:607-610. |
| 4. | Yee HS, Currie SL, Darling JM, Wright TL. Management and treatment of hepatitis C viral infection: recommendations from the Department of Veterans Affairs Hepatitis C Resource Center program and the National Hepatitis C Program office. Am J Gastroenterol. 2006;101:2360-2378. |
| 5. | Manns MP, Foster GR, Rockstroh JK, Zeuzem S, Zoulim F, Houghton M. The way forward in HCV treatment--finding the right path. Nat Rev Drug Discov. 2007;6:991-1000. |
| 6. | Takahashi M, Saito H, Higashimoto M, Atsukawa K, Ishii H. Benefit of hepatitis C virus core antigen assay in prediction of therapeutic response to interferon and ribavirin combination therapy. J Clin Microbiol. 2005;43:186-191. |
| 7. | Saito H, Ebinuma H, Satoh I, Miyaguchi S, Tada S, Iwabuchi N, Kumagai N, Tsuchimoto K, Morizane T, Ishii H. Immunological and virological predictors of outcome during interferon-alpha therapy of chronic hepatitis C. J Viral Hepat. 2000;7:64-74. |
| 8. | Poordad F, Reddy KR, Martin P. Rapid virologic response: a new milestone in the management of chronic hepatitis C. Clin Infect Dis. 2008;46:78-84. |
| 9. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL Jr, Häussinger D, Diago M, Carosi G, Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. |
| 10. | Zeuzem S, Pawlotsky JM, Lukasiewicz E, von Wagner M, Goulis I, Lurie Y, Gianfranco E, Vrolijk JM, Esteban JI, Hezode C. International, multicenter, randomized, controlled study comparing dynamically individualized versus standard treatment in patients with chronic hepatitis C. J Hepatol. 2005;43:250-257. |
| 11. | Enomoto N, Sakuma I, Asahina Y, Kurosaki M, Murakami T, Yamamoto C, Ogura Y, Izumi N, Marumo F, Sato C. Mutations in the nonstructural protein 5A gene and response to interferon in patients with chronic hepatitis C virus 1b infection. N Engl J Med. 1996;334:77-81. |
| 12. | Akuta N, Suzuki F, Kawamura Y, Yatsuji H, Sezaki H, Suzuki Y, Hosaka T, Kobayashi M, Kobayashi M, Arase Y. Prediction of response to pegylated interferon and ribavirin in hepatitis C by polymorphisms in the viral core protein and very early dynamics of viremia. Intervirology. 2007;50:361-368. |
| 13. | Masaki N, Fukushima S, Hayashi S. Lower th-1/th-2 ratio before interferon therapy may favor long-term virological responses in patients with chronic hepatitis C. Dig Dis Sci. 2002;47:2163-2169. |
| 14. | Maynard M, Pradat P, Gagnieu MC, Souvignet C, Trepo C. Prediction of sustained virological response by ribavirin plasma concentration at week 4 of therapy in hepatitis C virus genotype 1 patients. Antivir Ther. 2008;13:607-611. |
| 15. | Shirakawa H, Matsumoto A, Joshita S, Komatsu M, Tanaka N, Umemura T, Ichijo T, Yoshizawa K, Kiyosawa K, Tanaka E. Pretreatment prediction of virological response to peginterferon plus ribavirin therapy in chronic hepatitis C patients using viral and host factors. Hepatology. 2008;48:1753-1760. |
| 16. | Berg T, von Wagner M, Nasser S, Sarrazin C, Heintges T, Gerlach T, Buggisch P, Goeser T, Rasenack J, Pape GR. Extended treatment duration for hepatitis C virus type 1: comparing 48 versus 72 weeks of peginterferon-alfa-2a plus ribavirin. Gastroenterology. 2006;130:1086-1097. |
| 17. | Sánchez-Tapias JM, Diago M, Escartín P, Enríquez J, Romero-Gómez M, Bárcena R, Crespo J, Andrade R, Martínez-Bauer E, Pérez R. Peginterferon-alfa2a plus ribavirin for 48 versus 72 weeks in patients with detectable hepatitis C virus RNA at week 4 of treatment. Gastroenterology. 2006;131:451-460. |
| 18. | Pearlman BL, Ehleben C, Saifee S. Treatment extension to 72 weeks of peginterferon and ribavirin in hepatitis c genotype 1-infected slow responders. Hepatology. 2007;46:1688-1694. |
| 19. | Yu ML, Dai CY, Huang JF, Chiu CF, Yang YH, Hou NJ, Lee LP, Hsieh MY, Lin ZY, Chen SC. Rapid virological response and treatment duration for chronic hepatitis C genotype 1 patients: a randomized trial. Hepatology. 2008;47:1884-1893. |
| 20. | Jensen DM, Morgan TR, Marcellin P, Pockros PJ, Reddy KR, Hadziyannis SJ, Ferenci P, Ackrill AM, Willems B. Early identification of HCV genotype 1 patients responding to 24 weeks peginterferon alpha-2a (40 kd)/ribavirin therapy. Hepatology. 2006;43:954-960. |
| 21. | Ferenci P, Laferl H, Scherzer TM, Gschwantler M, Maieron A, Brunner H, Stauber R, Bischof M, Bauer B, Datz C. Peginterferon alfa-2a and ribavirin for 24 weeks in hepatitis C type 1 and 4 patients with rapid virological response. Gastroenterology. 2008;135:451-458. |
| 22. | Moriya T, Koyama T, Tanaka J, Mishiro S, Yoshizawa H. Epidemiology of hepatitis C virus in japan. Intervirology. 1999;42:153-158. |
| 23. | Yoshizawa H. Hepatocellular carcinoma associated with hepatitis C virus infection in Japan: projection to other countries in the foreseeable future. Oncology. 2002;62 Suppl 1:8-17. |
| 24. | Saito H, Tada S, Ebinuma H, Ishii H, Kashiwazaki K, Takahashi M, Tsukada N, Nishida J, Tanaka S, Shiozaki H. Role of erythrocytes as a reservoir for ribavirin and relationship with adverse reactions in the early phase of interferon combination therapy for chronic hepatitis C virus infections. J Clin Microbiol. 2006;44:3562-3568. |
| 25. | Fried MW, Jensen DM, Rodriguez-Torres M, Nyberg LM, Di Bisceglie AM, Morgan TR, Pockros PJ, Lin A, Cupelli L, Duff F. Improved outcomes in patients with hepatitis C with difficult-to-treat characteristics: randomized study of higher doses of peginterferon alpha-2a and ribavirin. Hepatology. 2008;48:1033-1043. |
| 26. | Kumagai N, Takahashi N, Kinoshita M, Tsunematsu S, Tsuchimoto K, Saito H, Ishii H. Polymorphisms of NS5B protein relates to early clearance of hepatitis C virus by interferon plus ribavirin: a pilot study. J Viral Hepat. 2004;11:225-235. |
| 27. | Nakamura M, Saito H, Ikeda M, Tada S, Kumagai N, Kato N, Shimotohno K, Hibi T. Possible molecular mechanism of the relationship between NS5B polymorphisms and early clearance of hepatitis C virus during interferon plus ribavirin treatment. J Med Virol. 2008;80:632-639. |
| 28. | Vrolijk JM, Kwekkeboom J, Janssen HL, Hansen BE, Zondervan PE, Osterhaus AD, Schalm SW, Haagmans BL. Pretreatment intrahepatic CD8+ cell count correlates with virological response to antiviral therapy in chronic hepatitis C virus infection. J Infect Dis. 2003;188:1528-1532. |
| 29. | Saito H, Tada S, Nakamoto N, Kitamura K, Horikawa H, Kurita S, Ebinuma H, Ishii H, Takahashi M, Tanaka S. Contribution of Irf-1 promoter polymorphisms to the Th1-type cell response and interferon-beta monotherapy for chronic hepatitis C. Hepatol Res. 2005;32:25-32. |
| 30. | Saito H, Tada S, Wakabayashi K, Nakamoto N, Takahashi M, Nakamura M, Ebinuma H, Ishii H. The detection of IRF-1 promoter polymorphisms and their possible contribution to T helper 1 response in chronic hepatitis C. J Interferon Cytokine Res. 2002;22:693-700. |
| 31. | Kobayashi K, Ueno Y, Kobayashi Y, Akahane T, Satoh S, Kikuchi K, Okamoto H, Ishii M, Shimosegawa T. Th1 response during ribavirin and interferon-alpha combination therapy in chronic hepatitis C. Hepatol Res. 2006;34:104-110. |
| 32. | Tarantino G, Conca P, Sorrentino P, Ariello M. Metabolic factors involved in the therapeutic response of patients with hepatitis C virus-related chronic hepatitis. J Gastroenterol Hepatol. 2006;21:1266-1268. |
| 33. | Satoh T, Masumoto A. Accordion Index: A new tool for the prediction of the efficacy of peg-interferon-alpha-2b and ribavirin combination therapy for chronic hepatitis C. Hepatol Res. 2008;38:315-318. |