Published online Jan 7, 2010. doi: 10.3748/wjg.v16.i1.48
Revised: November 6, 2009
Accepted: November 13, 2009
Published online: January 7, 2010
AIM: To investigate atrial natriuretic peptide (ANP) secretion from gastric mucosa and the relationship between the ANP/natriuretic peptide receptor type A (NPR-A) pathway and diabetic gastroparesis.
METHODS: Male imprinting control region (ICR) mice (4 wk old) were divided into two groups: control mice, and streptozotocin-induced diabetic mice. Eight weeks after injection, spontaneous gastric contraction was recorded by using physiography in control and streptozotocin-induced diabetic mice. The ANP-positive cells in gastric mucosa and among dispersed gastric epithelial cells were detected by using immunohistochemistry and flow cytometry, respectively. ANP and natriuretic peptide receptor type A (NPR-A) gene expression in gastric tissue was observed by using the reverse transcriptase polymerase chain reaction.
RESULTS: The frequency of spontaneous gastric contraction was reduced from 12.9 ± 0.8 cycles/min in the control group to 8.4 ± 0.6 cycles/min in the diabetic mice (n = 8, P < 0.05). However, the amplitude of contraction was not significantly affected in the diabetic group. The depletion of interstitial cells of Cajal in the gastric muscle layer was observed in the diabetic mice. ANP-positive cells were distributed in the gastric mucosal layer and the density index of ANP-positive cells was increased from 20.9 ± 2.2 cells/field in control mice to 51.8 ± 2.9 cells/field in diabetic mice (n = 8, P < 0.05). The percentage of ANP-positive cells among the dispersed gastric epithelial cells was increased from 10.0% ± 0.9% in the control mice to 41.2% ± 1.0% in the diabetic mice (n = 3, P < 0.05). ANP and NPR-A genes were both expressed in mouse stomach, and the expression was significantly increased in the diabetic mice.
CONCLUSION: These results suggest that the ANP/NPR-A signaling pathway is upregulated in streptozotocin-induced diabetic mice, and contributes to the development of diabetic gastroparesis.
- Citation: Qiu ZX, Mei B, Wu YS, Huang X, Wang ZY, Han YF, Lu HL, Kim YC, Xu WX. Atrial natriuretic peptide signal pathway upregulated in stomach of streptozotocin-induced diabetic mice. World J Gastroenterol 2010; 16(1): 48-55
- URL: https://www.wjgnet.com/1007-9327/full/v16/i1/48.htm
- DOI: https://dx.doi.org/10.3748/wjg.v16.i1.48
Gastroparesis is a chronic complication of diabetes, also called delayed gastric emptying, occurs in > 50% of patients with long-standing diabetes[1]. Symptoms of gastroparesis include heartburn, pain in the upper abdomen, vomiting, nausea, and early feeling of fullness, but the worst effect of gastroparesis is that it can make diabetes worse by making blood glucose control more difficult[2]. Besides, there are deterioration in glycemic control and incapacitating symptoms such as malnutrition, water and electrolyte imbalance, and aspiration. However, the pathophysiology of diabetic gastropathy and gastroparesis, such as the mechanism of impaired fundic, pyloric relaxation and impaired electrical pacemaking, are still not established[3,4]. It is generally believed that diabetic gastropathy and gastroparesis may be caused by visceral autonomic neuropathy, hyperglycemia, and degeneration of smooth muscle. Hyperglycemia itself can cause antral hypomotility, gastric dysrhythmia, and delayed gastric emptying in some patients[5]. Several physiological studies have reported that dysfunction of gastric smooth muscle in diabetes is associated with neural factors and intracellular signaling pathways[6,7].
Atrial natriuretic peptide (ANP) was isolated from the atrium by de Bold et al[8] in 1981. From then on, brain natriuretic peptide (BNP), C-type natriuretic peptide (CNP), dendroapsis natriuretic peptide (DNP), micrurus natriuretic peptide (MNP), and ventricular natriuretic peptide (VNP) have been found in succession. Three types of single transmembrane natriuretic peptide receptors (NPRs) for ANP, BNP and CNP have been identified[9,10], namely, NPR type A (NPR-A), type B (NPR-B), and type C (NPR-C). NPR-A and NPR-B have the membrane-bound particulate guanylate cyclase (pGC), which can catalyze the formation of cGMP from GTP[8-11]. NPR-A preferentially binds to ANP and BNP, but has a low affinity for CNP. NPR-B has a much higher affinity for CNP than either ANP or BNP[12]. Besides the heart, ANP is also distributed in other organs, for example, ANP can be secreted by gastric mucosa[13-15]. It is well known that ANP and other family members exert natriuretic-diuretic effects, vasorelaxation, and other functions including: decreasing blood pressure, and controlling electrolyte homeostasis. Some studies have demonstrated that the natriuretic peptide family plays an inhibitory role in regulating gastrointestinal motility, for example, in the chicken rectum[3], rat tenia coli[16] and guinea-pig cecum[17].
Our previous study also indicated that CNP relaxes gastric circular and longitudinal smooth muscles in human, rat and guinea-pig stomach, and NPRs are distributed in rat gastric smooth muscle layer[18-20]. Recently, we have also reported that the CNP-induced relaxation and the production of cGMP of gastric smooth muscle are potentiated in streptozotocin (STZ)-induced diabetic rats. As well as the activity of pGC, the expression of NPR-B gene in gastric smooth muscle is upregulated in STZ-induced diabetic rats[21]. These results suggest that the CNP/(NPR-B)/pGC/cGMP signaling pathway is involved in the pathogenesis of diabetes. Our previous studies have confirmed that ANP-synthesizing cells exist in different regions of the gastric mucosa in rats[15], therefore, ANP can be considered an endogenous natriuretic peptide of gastric mucosa. However, it is not clear whether the ANP/NPR-A signaling pathway is involved in the pathogenesis of diabetic gastroparesis.
In the present study, we investigated the relationship between the ANP signaling pathway and STZ-induced diabetic gastroparesis to confirm whether ANP contributes to the development of gastroparesis. The present study focused on whether the amount of ANP secretion from gastric mucosa was enhanced and the expression of the NPR-A gene in gastric smooth muscle was upregulated in a mouse model of STZ-induced diabetic gastroparesis.
STZ, TRIzol Reagent and chemicals were purchased from Sigma. C-kit antibody, ANP antibody and pronase were purchased from Santa Cruz Biotechnology and Roche. Other chemicals were purchased from Sangon Biological Company.
Male imprinting control region (ICR) mice (4 wk old) were purchased from the Experimental Animal Center of Shanghai Jiaotong University School of Medicine. A total of 80 mice were divided into two groups: control group and STZ-induced diabetic group. STZ-induced diabetes was created as follows: the mice were fasted overnight and intraperitoneally administered STZ solution (Sigma-Aldrich, St. Louis, MO, USA). STZ was diluted in 0.1 mol/L citrate buffer (pH = 4.0) and used at a dose of 200 mg/kg. Control mice were intraperitoneally administered with the same volume of 0.1 mol/L citrate buffer. The glucose concentration of blood was determined with One-touch Apparatus (Johnson & Johnson Medical Company). STZ-induced diabetic mice were confirmed by measuring glucose concentration from tail blood after fasting, and diabetes was defined when the blood glucose level was > 16 mmol/L. All experimental protocols included in this study were approved by the local Animal Care Committee and conformed to the Guide for the Care and Use of Laboratory Animals published by the Science and Technology Commission of P.R.C. (STCC Publication No. 2, revised 1988).
Eight weeks after treatment with STZ, the animals were euthanized by lethal dose of intraperitoneal pentobarbital sodium (50 mg/kg). The abdomen of each mouse was opened along the midline, and the intact stomach was removed and placed in pre-oxygenated Krebs solution (containing in 118.1 mmol/L NaCl, 4.7 mmol/L KCl, 1.0 mmol/L KH2PO4, 1.0 mmol/L MgSO4, 25.0 mmol/L NaHCO3, 2.5 mmol/L CaCl2, and 11.1 mmol/L glucose), which was equilibrated with 95% oxygen and 5% CO2. The connective tissue was removed and the pylorus was connected to a pressure transducer (Chengdu Equipment Factory, China) with a thin glass tubule, and the gastric cardia was tied with thin string. The stomach was incubated in a 15-mL organ bath filled with Krebs solution and gassed with 95% O2 and 5% CO2 at 37°C. The sensitivity of the pressure transducer was adjusted to an appropriate value and we recorded gastric motility by using the SMUP-E biological signal processing system (Chengdu Equipment Factory). The stomach was allowed to incubate for at least 60 min before the experiments were started, and we eliminated error by injecting an equal volume of Kreb’s solution into the stomach.
The mice were euthanized by lethal dose of intraperitoneal pentobarbital sodium (50 mg/kg), the stomach was removed and washed with saline, and then fixed in 4% paraformaldehyde (4°C, 24 h). The fixed tissue was washed with running water (room temperature, 2 h), immersed with 95% and 100% alcohol (room temperature, 2 × 2 h), xylene (2 × 20 min) and paraffin (68.5°C, 30/40/50 min). Sections of 6 μm thickness were cut and deparaffinized in xylene (4 × 30 min). The specimens were hydrated in graded concentrations of ethanol, and washed three times in PBS, and incubated in the blocking reagent for 30 min. The samples were incubated with polyclonal antibody against c-Kit protein (sc-5535; Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1:100 dilution). c-Kit protein was used as a marker of interstitial cells of Cajal (ICCs)[22,23] and with polyclonal antibody against ANP (sc-20158; Santa Cruz Biotechnology; 1:100 dilution) for 24 h at 4°C, followed by 3 h incubation in Rho-anti-rabbit and horseradish peroxidase-anti-rabbit second antibody, respectively. The negative control group was omitted by incubating with primary antibody against ANP. The slice was visualized and photographed under a fluorescence microscope (Olympus IX71, Tokyo, Japan).
Eight weeks after injection with STZ, the mice were euthanized by lethal dose of intraperitoneal pentobarbital sodium (50 mg/kg). The stomach was removed and washed with saline. After the stomachs were inverted to make mucosal-side-out stomachs, the stomachs were rinsed with saline, and pronase solution was injected into the stomach. The pronase was diluted into 1 mg/mL with MA solution (0.5 mmol/L NaH2PO4 , 1 mmol/L Na2HPO4, 20 mmol/L NaHCO3, 80 mmol/L NaCl, 5 mmol/L KCl, 50 mmol/L HEPES, 11 mmol/L glucose, 20 g/L BSA, 2 mmol/L EDTA; pH = 7.4). The stomach sacks were incubated in the oxygenated MA solution for 3 × 30 min, at 37°C equilibrated with 95% oxygen and 5% CO2, followed by gently stirring for 1 h in MB solution (0.5 mmol/L NaH2PO4, 1 mmol/L Na2HPO4, 20 mmol/L NaHCO3, 80 mmol/L NaCl, 5 mmol/L KCl, 50 mmol/L HEPES, 11 mmol/L glucose, 10 g/L BSA, 1 mmol/L CaCl2, 1.5 mmol/L MgCl2; pH = 7.4). MB solution that contained the gastric mucosal cells was collected, and filtered through a 200-mesh cellular sieve. The solution sample was centrifuged at 1500 ×g for 5 min. The centrifuged sample was re-suspended with MC solution (0.5 mmol/L NaH2PO4, 1 mmol/L Na2HPO4, 20 mmol/L NaHCO3, 80 mmol/L NaCl, 5 mmol/L KCl, 50 mmol/L HEPES, 11 mmol/L glucose, 1 mmol/L CaCl2, 1.5 mmol/L MgCl2, 1 mmol/L dithiothreitol; pH = 7.4) and centrifuged at 1500 ×g for 5 min again. The density of the cell suspension was adjusted to 3 × 106/mL. The cells were fixed with 75% cold alcohol (-20°C, 24 h), and washed with PBS, followed by 0.2% Triton X-100 for 10 min. The antigen was blocked by 10% goat serum diluted in PBS, and incubated with antibody against ANP (sc-20158; Santa Cruz Biotechnology; 1:50 dilution) overnight at 4°C. The cells were stained with FITC-conjugated goat anti-rabbit IgG and examined by flow cytometry (Becton Dickinson). Using Cellquest software, 104 cells were analyzed per sample.
The whole gastric tissue was quickly removed from the mouse. Total RNA was isolated from the tissue as recommended by the manufacturer of TRIzol Reagent (Sigma). RNA concentration was determined by absorbance reading at 260/280 nm. Reverse transcription was performed with a volume of 20 μL mixture that contained 11 μL mRNA, and 1 μL oligo dt18, which was incubated at 70°C for 5 min, and 4 μL 5 × reaction buffer, 2 μL dNTP, 1 μL RNase inhibitor, 1 μL M-MLV RT was added to the mixture, followed by incubation at 42°C for 1 h. The enzyme was inactivated by heating at 70°C for 10 min. cDNA samples were used for analyzing specific cDNA of ANP and NPR-A. One microliter of cDNA was added to 19 μL PCR reaction mixture that contained: 7 μL nuclease-free water, 10 μL 2 × reaction buffer, 1 μL sense primer, and 1 μL anti-sense primer. The following conditions were used for PCR amplification: for GAPDH, 95°C for 4 min; 95°C for 30 s; followed by 40 cycles at 52.9°C for 1 min; 72°C for 30 s; 72°C for 7 min; for ANP, 40 cycles at 54.6°C for 30 s; for NPR-A, 60°C for 30 s. RT-PCR was performed on an iCyclerTM Thermal Cycler (Bio-Rad, Hercules, CA, USA) using the Access RT-PCR System (Promega, Madison, WI, USA). Specific primers for murine GAPDH, ANP and NPR-A were synthesized by Sangon Biological Company (Shanghai, China). The primer sequences were as follows: GAPDH (sense) 5'TCAACGGCACAGTCAAGG3', GAPDH (antisense) 5'ACCAGTGGATGCAGGGAT3'; ANP (sense) 5'TCCTTCTCCATCACCCTG3', ANP (antisense) 5'CCTAGAGCACTGCCGTCT3'; NPRA (sense) 5'AGACGATGGGCAGGATAG3', NPRA (antisense) 5'GGATGTCAGGAGGTGGGT3'. The PCR products were size-fractionated by 1% agarose gel electrophoresis, and visualized under ultraviolet light with 0.5% ethidium bromide staining. GAPDH, ANP and NPR-A cDNAs were quantified by Gel Doc XR System and Quantity One software (Bio-Rad). Gene expression for ANP and NPR-A was normalized to that for GAPDH.
The data was expressed as the mean ± SE. We evaluated differences between the treatment groups using Student’s t test. Differences were considered to be significant at P < 0.05. The density index referred to the number of ANP-positive cell per field of vision.
We detected changes in blood glucose concentration. Mice were used at 8 wk after injection of STZ. At the time of the study, most STZ-treated mice exhibited hyperglycemia; their average blood glucose concentration was 26.7 ± 1.3 mmol/L (n = 32), which was significantly higher than that of the non-diabetic control mice (6.9 ± 0.6 mmol/L, n = 32; P < 0.01).
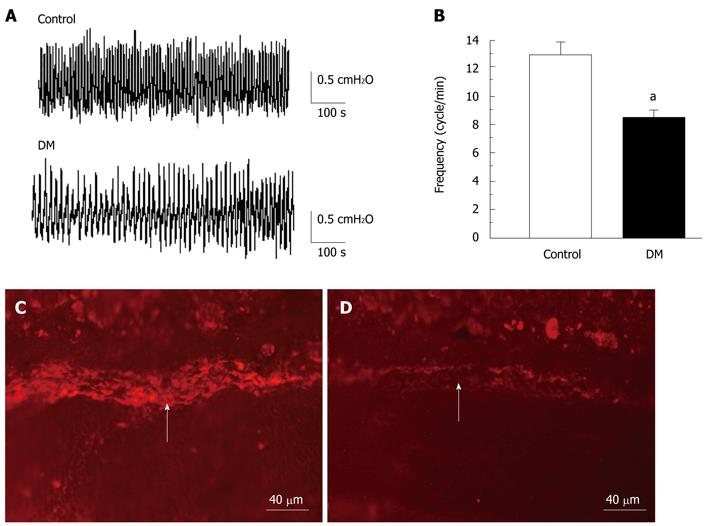
To determine whether diabetic gastropathy occurred, the amplitude and frequency of spontaneous gastric contraction were observed in normal and STZ-induced diabetic mice. The frequency of spontaneous gastric contraction decreased significantly from 12.9 ± 0.8 cycles/min in the control group to 8.4 ± 0.6 cycles/min in diabetic mice (n = 8, P < 0.05; Figure 1A and B). However, the amplitude of contraction was not changed in the diabetic group. Spontaneous rhythmic contraction of gastrointestinal smooth muscle is triggered by ICCs, which are also mediators of neuromuscular transmission in the gastrointestinal tract. Depletion of ICCs contributes to dysfunction of gastrointestinal motility in patients and animal models[24]. ICCs distributed in the gastric smooth muscle layer were observed by immunochemistry. c-Kit protein, an ICC marker, was detected in the inter-muscular layer of normal and diabetic mice (Figure 1C and D), but the expression was significantly decreased in the diabetic group (n = 6; Figure 1D). The results suggested that the STZ-induced diabetic mice exhibited gastric dysfunction, and the reduced frequency of gastric motility in diabetic mice might have been related to ICC depletion.
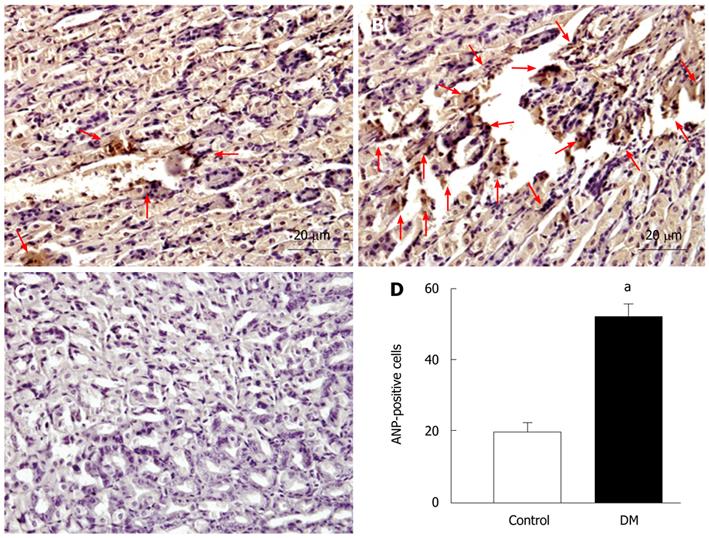
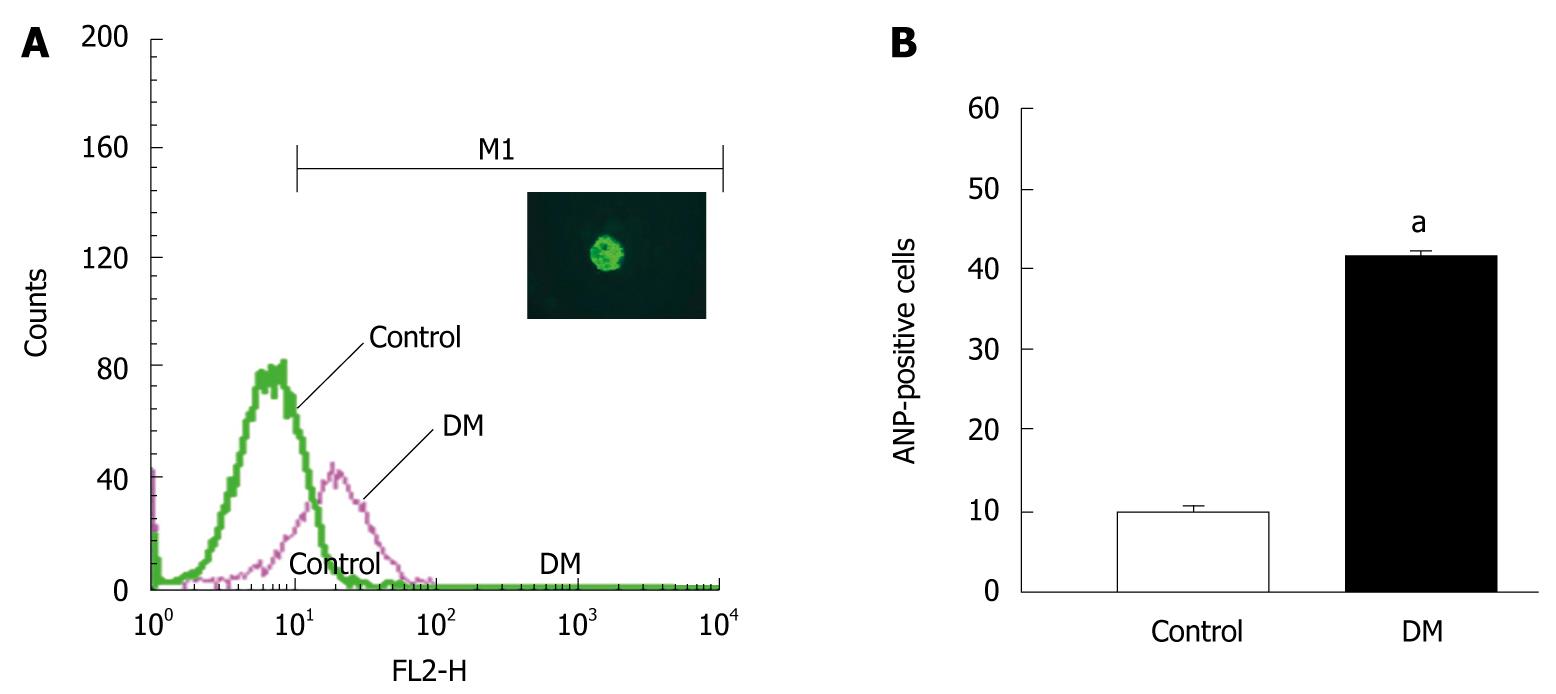
Besides the heart, the ANP family is also distributed in other organs, and some studies have found that ANP can be secreted from gastric mucosa[13-15]. To determine whether ANP secretion from gastric mucosa is enhanced in STZ-induced diabetic mice, ANP-positive cells in the gastric mucosa were observed in the control (Figure 2A) and STZ-induced diabetic (Figure 2B) mice. The number of ANP-positive cells was increased significantly in the gastric mucosa of diabetic mice, and the density index was enhanced from 20.9 ± 2.2 cells/field of vision in the controls to 51.8 ± 2.9 cells/field of vision in the diabetic group (n = 8, P < 0.05; Figure 2D). We further analyzed the number of ANP-positive cells in gastric epithelial cells by flow cytometry. The relative fluorescence intensity of the diabetic group was higher than that of the control group (Figure 3A). The number of ANP-positive cells was increased significantly in the diabetic group. The percentage of ANP-positive cells among dispersed epithelial cells was enhanced from 10.0% ± 0.9% in the control group to 41.2% ± 1.0% in the diabetic group (n = 3, P < 0.05; Figure 3B). The results suggested that ANP secretion from gastric mucosa was potentiated in the stomach of STZ-induced diabetic mice.
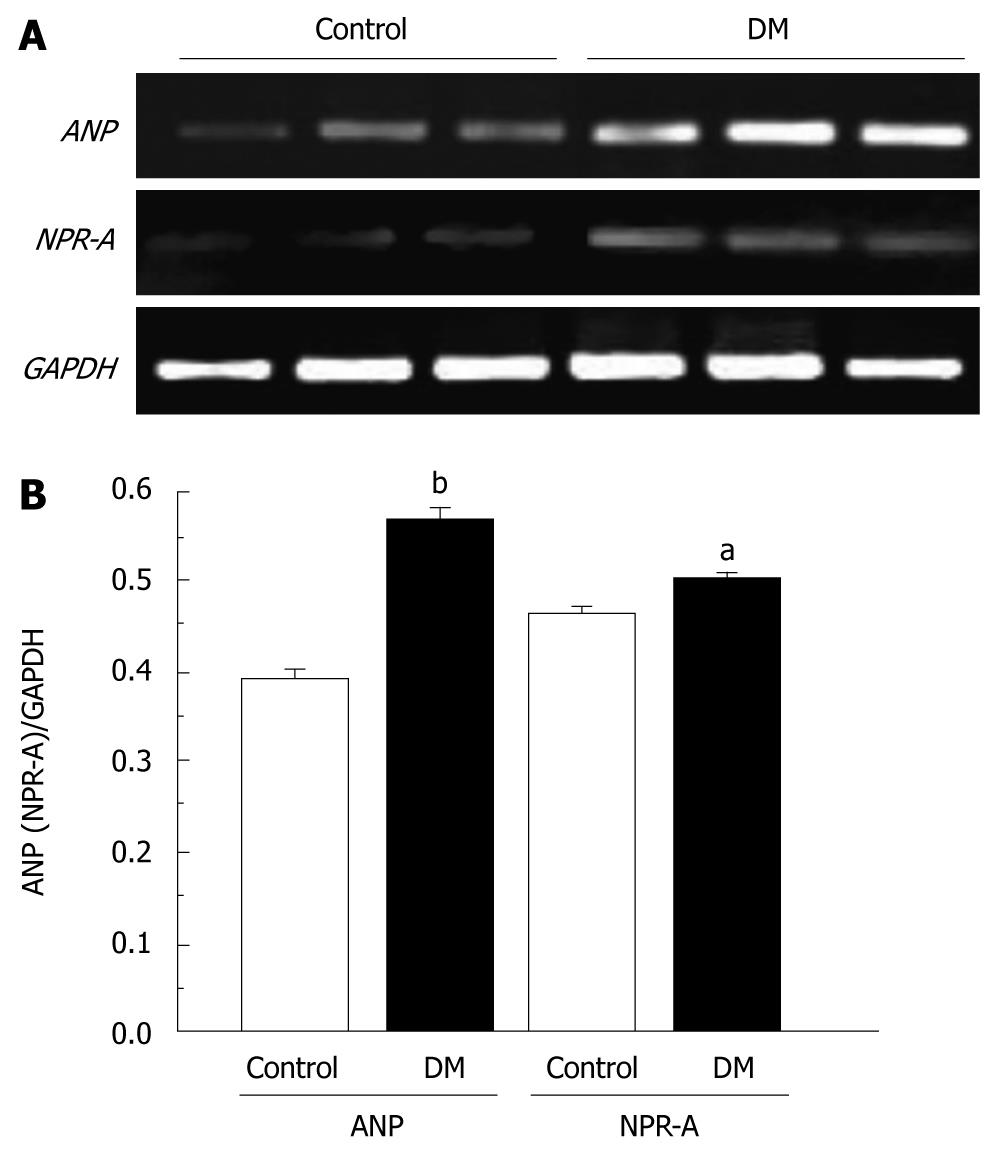
The number of ANP-positive cells was increased in the STZ-induced diabetic group, therefore, we investigated whether ANP and NPR-A gene expression was also increased. ANP and NPR-A gene expression was observed in gastric tissue by RT-PCR. ANP and NPR-A gene expression was increased in the diabetic group (n = 3; Figure 4A). The signal was normalized to that of GAPDH. The ratio of ANP/GAPDH and NPR-A/GAPDH was 0.54 ± 0.02 and 0.5 ± 0.01 and 0.39 ± 0.01 and 0.46 ± 0.02 in the STZ-induced diabetic and control group, respectively (n = 3, P < 0.05 and P < 0.01; Figure 4B).
Diabetic gastroparesis is defined as slow gastric emptying in the absence of mechanical obstruction, and constipation is considered to be the most significant clinical manifestation. It occurs in 30%-60% of diabetic patients observed at tertiary referral centers[25]. Many investigators view these changes as the consequences of irreversible autonomic neuropathy, which affects the vagus and sympathetic nerves primarily[2,5]. However, the cause of gastroparesis is multifactorial and also involves enteric neurons, mucosal endocrine cells, smooth muscle cells and ICCs[4]. The main conclusion drawn from the present study is that the ANP/NPR-A signaling pathway is upregulated and may contribute to the development of gastroparesis in STZ-induced diabetic mice, and then lead to gastric motility dysfunction.
In non-obese diabetic (NOD) mice, a well-established model of human type І diabetes mellitus, gastric emptying of solids is delayed after 6-8 wk of untreated diabetes[23]. In the present study, we established an STZ-induced diabetic model and gastric motility was observed in vitro after 8 wk of untreated diabetes. The frequency of spontaneous contraction in the STZ-induced diabetic group was decreased significantly, however, the amplitude of contraction was not significantly different between these two groups. The results suggest that diabetic gastropathy mostly exhibits slow rhythm and turbulence, however, the amplitude of spontaneous contraction is not affected significantly in the early stage of diabetes. ICCs play a key role in gastric motility, and damage to the ICC networks may contribute to the development of gastropathy and gastroparesis[26]. In the present study, we confirmed whether the number of ICCs in the gastric smooth muscle layer was changed, by using immunochemistry in the STZ-induced diabetic mice. c-Kit expression in the muscle layer was observed in normal and diabetic mice, however, c-Kit expression was decreased in diabetic mice. The results suggest that gastric dysfunction in STZ-induced diabetic mice may be related to ICC depletion.
Atrial myocytes are the main source of ANP, but ANP is also found in other tissues, such as the gastrointestinal tract[27]. ANP and its receptor NPR-A have been detected in the gastric antrum[28]. NPR-A has the same membrane-bound guanylate cyclase activity as NPR-B, which catalyzes the formation of cGMP from GTP[9,10]. Some studies have demonstrated that ANP has an inhibitory effect on the regulation of gastrointestinal motility; for example, in chicken rectum[16], rat tenia coli[17] and guinea-pig cecum[3]. Our previous study also has indicated that NPs relax gastric circular and longitudinal smooth muscles in human, rat and guinea-pig stomach, and NPRs are distributed in the rat gastric smooth muscle layer[18,19]. Another previous study has indicated that ANP release is augmented in the atrium of STZ-induced diabetic rats[29]. Recently, we have found that the expression of NPR-B gene is increased and NP-dependent guanylate cyclase/cGMP signaling is upregulated in STZ-induced diabetic rats[20,21]. ANP is also secreted from the gastric mucosa[15], therefore, ANP can be considered endogenous to the gastric mucosa. However, the relationship between the ANP/NPR-A signaling pathway and STZ-induced diabetic gastroparesis is not clear. In the present study, we confirmed that the expression of ANP in gastric mucosa was increased significantly in STZ-induced diabetic mice. We demonstrated that the percentage of ANP-positive cells was also enhanced significantly among the dispersed gastric epithelial cells, and the expression of the ANP gene in gastric tissue was upregulated in STZ-induced diabetic mice. These results suggest that ANP secretion from gastric mucosa is increased significantly in STZ-induced diabetes. The amount of ANP secretion is increased in diabetic mice, therefore, we investigated whether expression of NPR-A in the stomach was upregulated in STZ-induced diabetic mice. We demonstrated that expression of the NPR-A gene was also increased significantly in gastric tissue of diabetic mice. The results suggest that the ANP/NPR-A signaling pathway is upregulated in the stomach of STZ-induced diabetic mice.
Our previous study has demonstrated that the CNP/NPR-B signaling pathway is upregulated in STZ-induced diabetic rats[20,21], while the present study indicates that the ANP/NPR-A signaling pathway is also upregulated in STZ-induced diabetic mice. Previous studies have indicated that diabetes may also affect expression of the ANP gene; for example, ANP gene expression in heart and kidney are increased in STZ-induced diabetic rats[11,30] and plasma concentration of pro-ANP is increased in comparison with control rats[31]. Christoffersen et al[32] also have reported that diabetic mice show an increase in NPR-B gene expression in the heart, and have suggested that increased NPR-B signaling affects development of diabetic cardiomyopathy. The NP/NPR signaling pathway has an inhibitory effect on gastrointestinal motility, therefore, upregulation of this signaling pathway may be involved in development of gastroparesis in STZ-induced diabetic mice.
In summary, gastroenteropathy causes considerable morbidity in patients with diabetes mellitus and it has become a major healthcare burden. Current treatments are mainly symptomatic and frequently ineffective. Development of new therapeutic options is hampered because of poor understanding of the underlying pathological mechanisms. Our study demonstrates that the ANP/NPR-A signaling pathway is upregulated. The results suggest that ANP/NPR-A signaling is involved in the development of gastroparesis in STZ-induced diabetic mice.
Gastroparesis is a gastrointestinal complication of diabetes, which is also called delayed gastric emptying. The mechanism of gastroparesis is not clear, but a recent study has reported that the C-type natriuretic peptide-natriuretic peptide receptor B (CNP/NPR-B) pathway is upregulated in the stomach of diabetic rats. Atrial natriuretic peptide (ANP) has an inhibitory effect on motility of the gastrointestinal tract, but whether secretion of ANP is increased in the stomach of diabetic mice has not been reported.
NPs are located in several organs besides the heart. Recently, the authors have found that ANP and its receptor (NPR-A) are also present in the mouse stomach, and the CNP/cGMP pathway in diabetic gastroparesis was investigated in STZ-induced diabetic rats. This study was designed to investigate whether ANP secretion is altered in the stomach of STZ-induced diabetic mice.
ANP with an inhibitory effect on gastrointestinal motility is present in the stomach, and previous studies have focused on the relationship between the CNP/NPR-B pathway and gastric motility. This is believed to be the first study to investigate ANP secretion and NPR-A in the stomach of STZ-induced diabetic mice. The results suggest that ANP secretion and expression of NPR-A mRNA are both increased in the stomach of STZ-induced diabetic mice. This may be involved in stomach motility dysfunction in STZ-induced diabetic mice.
The dysfunction of stomach motility that occurs in diabetic gastroparesis may be related to ANP secretion and upregulation of NPR-A. This may contribute to the treatment and preventive intervention of diabetic gastroparesis in the future.
Gastroparesis also called delayed gastric emptying, and usually occurs in diabetes. Symptoms of gastroparesis include heartburn, pain in the upper abdomen, vomiting, nausea, and early feeling of fullness.
This study deals with questions about the mechanisms involved in gastroparesis in diabetes.
Peer reviewer: Giovanni Tarantino, MD, Professor, Department of Clinical and Experimental Medicine, Federico II University Medical School, VIA S. PANSINI, 5, Naples 80131, Italy
S- Editor Wang YR L- Editor Kerr C E- Editor Ma WH
| 1. | Koch KL. Diabetic gastropathy: gastric neuromuscular dysfunction in diabetes mellitus: a review of symptoms, pathophysiology, and treatment. Dig Dis Sci. 1999;44:1061-1075. |
| 2. | Kuo P, Rayner CK, Jones KL, Horowitz M. Pathophysiology and management of diabetic gastropathy: a guide for endocrinologists. Drugs. 2007;67:1671-1687. |
| 3. | Itaba S, Chijiiwa Y, Matsuzaka H, Motomura Y, Nawata H. Presence of C-type natriuretic peptide (CNP) in guinea pig caecum: role and mechanisms of CNP in circular smooth muscle relaxation. Neurogastroenterol Motil. 2004;16:375-382. |
| 4. | Ordog T. Interstitial cells of Cajal in diabetic gastroenteropathy. Neurogastroenterol Motil. 2008;20:8-18. |
| 5. | Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association medical position statement: diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1589-1591. |
| 6. | Bult H, Boeckxstaens GE, Pelckmans PA, Jordaens FH, Van Maercke YM, Herman AG. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature. 1990;345:346-347. |
| 7. | Endo K, Matsumoto T, Kobayashi T, Kasuya Y, Kamata K. Diabetes-related changes in contractile responses of stomach fundus to endothelin-1 in streptozotocin-induced diabetic rats. J Smooth Muscle Res. 2005;41:35-47. |
| 8. | de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Reprinted from Life Sci. 28:89-94, 1981. J Am Soc Nephrol. 2001;12:403-409; discussion 403-408, 408-409. |
| 9. | Maack T, Suzuki M, Almeida FA, Nussenzveig D, Scarborough RM, McEnroe GA, Lewicki JA. Physiological role of silent receptors of atrial natriuretic factor. Science. 1987;238:675-678. |
| 10. | Schulz S, Singh S, Bellet RA, Singh G, Tubb DJ, Chin H, Garbers DL. The primary structure of a plasma membrane guanylate cyclase demonstrates diversity within this new receptor family. Cell. 1989;58:1155-1162. |
| 11. | Shin SJ, Lee YJ, Tan MS, Hsieh TJ, Tsai JH. Increased atrial natriuretic peptide mRNA expression in the kidney of diabetic rats. Kidney Int. 1997;51:1100-1105. |
| 12. | Koller KJ, Goeddel DV. Molecular biology of the natriuretic peptides and their receptors. Circulation. 1992;86:1081-1088. |
| 13. | Gower WR Jr, McCuen RW, Arimura A, Coy DA, Dietz JR, Landon CS, Schubert ML. Reciprocal paracrine pathways link atrial natriuretic peptide and somatostatin secretion in the antrum of the stomach. Regul Pept. 2003;110:101-106. |
| 14. | Gower WR Jr, Salhab KF, Foulis WL, Pillai N, Bundy JR, Vesely DL, Fabri PJ, Dietz JR. Regulation of atrial natriuretic peptide gene expression in gastric antrum by fasting. Am J Physiol Regul Integr Comp Physiol. 2000;278:R770-R780. |
| 15. | Li CH, Yang ZW, Yin ZR, Jin Z, Xing DG, Piao LH, Kim YC, Xu WX. Relationship between atrial natriuretic peptide-immunoreactive cells and microvessels in rat gastric mucosa. Acta Pharmacol Sin. 2006;27:205-211. |
| 16. | Sudoh T, Minamino N, Kangawa K, Matsuo H. C-type natriuretic peptide (CNP): a new member of natriuretic peptide family identified in porcine brain. Biochem Biophys Res Commun. 1990;168:863-870. |
| 17. | Kim JH, Jeon GJ, Kim SZ, Cho KW, Kim SH. C-type natriuretic peptide system in rabbit colon. Peptides. 2001;22:2061-2068. |
| 18. | Guo HS, Cui X, Cui YG, Kim SZ, Cho KW, Li ZL, Xu WX. Inhibitory effect of C-type natriuretic peptide on spontaneous contraction in gastric antral circular smooth muscle of rat. Acta Pharmacol Sin. 2003;24:1021-1026. |
| 19. | Guo HS, Jin Z, Jin ZY, Li ZH, Cui YF, Wang ZY, Xu WX. Comparative study in the effect of C-type natriuretic peptide on gastric motility in various animals. World J Gastroenterol. 2003;9:547-552. |
| 20. | Cai YL, Xu DY, Li XL, Qiu ZX, Jin Z, Xu WX. C-type natriuretic-peptide-potentiated relaxation response of gastric smooth muscle in streptozotocin-induced diabetic rats. World J Gastroenterol. 2009;15:2125-2131. |
| 21. | Xu DY, Liu L, Cai YL, Li XL, Qiu ZX, Jin Z, Xu WX. Natriuretic Peptide-Dependent cGMP Signal Pathway Potentiated the Relaxation of Gastric Smooth Muscle in Streptozotocin-Induced Diabetic Rats. Dig Dis Sci. 2009;Epub ahead of print. |
| 22. | Li CH, Pan LH, Li CY, Zhu CL, Xu WX. Localization of ANP-synthesizing cells in rat stomach. World J Gastroenterol. 2006;12:5674-5679. |
| 23. | Ordog T, Takayama I, Cheung WK, Ward SM, Sanders KM. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49:1731-1739. |
| 24. | Horvath VJ, Vittal H, Ordog T. Reduced insulin and IGF-I signaling, not hyperglycemia, underlies the diabetes-associated depletion of interstitial cells of Cajal in the murine stomach. Diabetes. 2005;54:1528-1533. |
| 25. | Feldman M, Schiller LR. Disorders of gastrointestinal motility associated with diabetes mellitus. Ann Intern Med. 1983;98:378-384. |
| 26. | Horvath VJ, Vittal H, Lorincz A, Chen H, Almeida-Porada G, Redelman D, Ordog T. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of cajal in murine diabetic gastroparesis. Gastroenterology. 2006;130:759-770. |
| 27. | Gonzalez Bosc LV, Majowicz MP, Vidal NA. Effects of atrial natriuretic peptide in the gut. Peptides. 2000;21:875-887. |
| 28. | Guo HS, Cai ZX, Wu TH, Xu J, Qiu Y, Xu WX. Inhibitory effect of dendroaspis natriuretic peptide on spontaneous contraction in gastric antral circular smooth muscles of guinea pigs. Acta Pharmacol Sin. 2007;28:1797-1802. |
| 29. | Bai GY, Piao FL, Kim SY, Yuan K, Kim SZ, Kim SH. Augmentation of insulin-stimulated ANP release through tyrosine kinase and PI 3-kinase in diabetic rats. Peptides. 2006;27:2756-2763. |
| 30. | Matsubara H, Mori Y, Yamamoto J, Inada M. Diabetes-induced alterations in atrial natriuretic peptide gene expression in Wistar-Kyoto and spontaneously hypertensive rats. Circ Res. 1990;67:803-813. |
| 31. | Ortola FV, Ballermann BJ, Anderson S, Mendez RE, Brenner BM. Elevated plasma atrial natriuretic peptide levels in diabetic rats. Potential mediator of hyperfiltration. J Clin Invest. 1987;80:670-674. |
| 32. | Christoffersen C, Bartels ED, Nielsen LB. Heart specific up-regulation of genes for B-type and C-type natriuretic peptide receptors in diabetic mice. Eur J Clin Invest. 2006;36:69-75. |