INTRODUCTION
The stomach is the most common site of extranodal malignant lymphomas, constituting 30%-45% of all extranodal malignant lymphoma and 1%-7% of all gastric malignancies[1]. The most common gastric lymphomas are extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT), characterized by an infiltration of the mucosa by centrocyte-like B-cells associated with lymphoepithelial lesions, and diffuse large B cell lymphomas (DLBCL)[2]. This last terminology is commonly used to describe de novo DLBCL or DLBCL associated with a low grade component. Burkitt’s lymphoma (BL) is a highly aggressive non-Hodgkin lymphoma, often presenting in extranodal sites or as acute leukaemia. The incidence of gastric BL is low compared to other types of gastric lymphomas, although its true incidence is not yet defined[3].
There is a close link between Helicobacter pylori (H pylori) and gastric MALT lymphomas and probably also with gastric DLCBL[4,5]. But the possibility of an association between H pylori and gastric BL has only been suggested in occasional cases in children[6,7]. Eradication of H pylori is now well established as the first line treatment of gastric MALT lymphoma[8] and reports in the literature show 60%-80% remission rates[9-11]. In addition, several cases of complete remission of gastric DLBCL after H pylori eradication have been reported[12-16]. On the contrary, BL is known to require aggressive chemotherapy in order to induce remission, with systemic and intrathecal chemotherapy, and the impact of H pylori eradication on gastric BL has never been evaluated. We describe the first case of complete remission of a gastric BL in an adult patient following H pylori eradication.
CASE REPORT
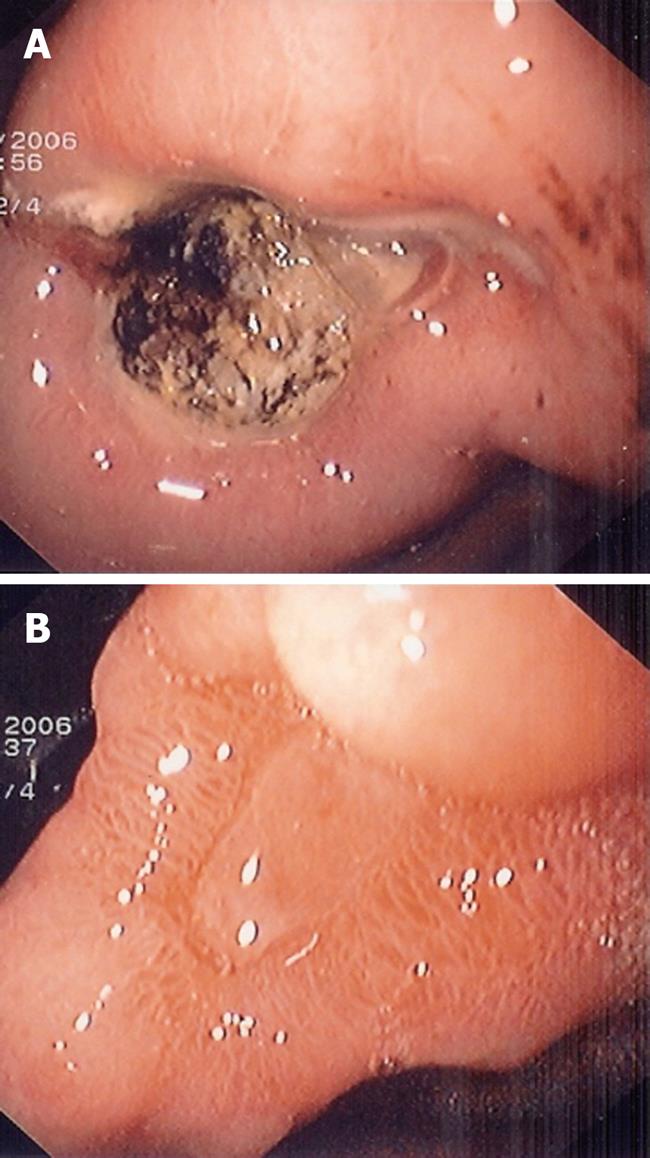
A 39-year old woman underwent a first gastroscopy in January 2006. She had complained of abdominal pain for 3 mo. She had no previous history of peptic ulcer disease. Examination revealed no palpable mass in the abdomen, ascites or superficial lymphadenopathy. A barium contrast radiography of the stomach showed an important ulcerated lesion of the antrum on the lesser curvature, suggesting malignancy. The patient underwent a gastroscopy that confirmed the ulcer at the angularis, measuring 5 cm × 5 cm (Figure 1A).
Figure 1 Endoscopic aspect of the gastric lesion before and after Helicobacter pylori (H pylori) eradication.
A: Oesophagogastroduodenoscopy shows a large ulcer at the angularis; B: Ulcer healing 3 mo after H pylori eradication.
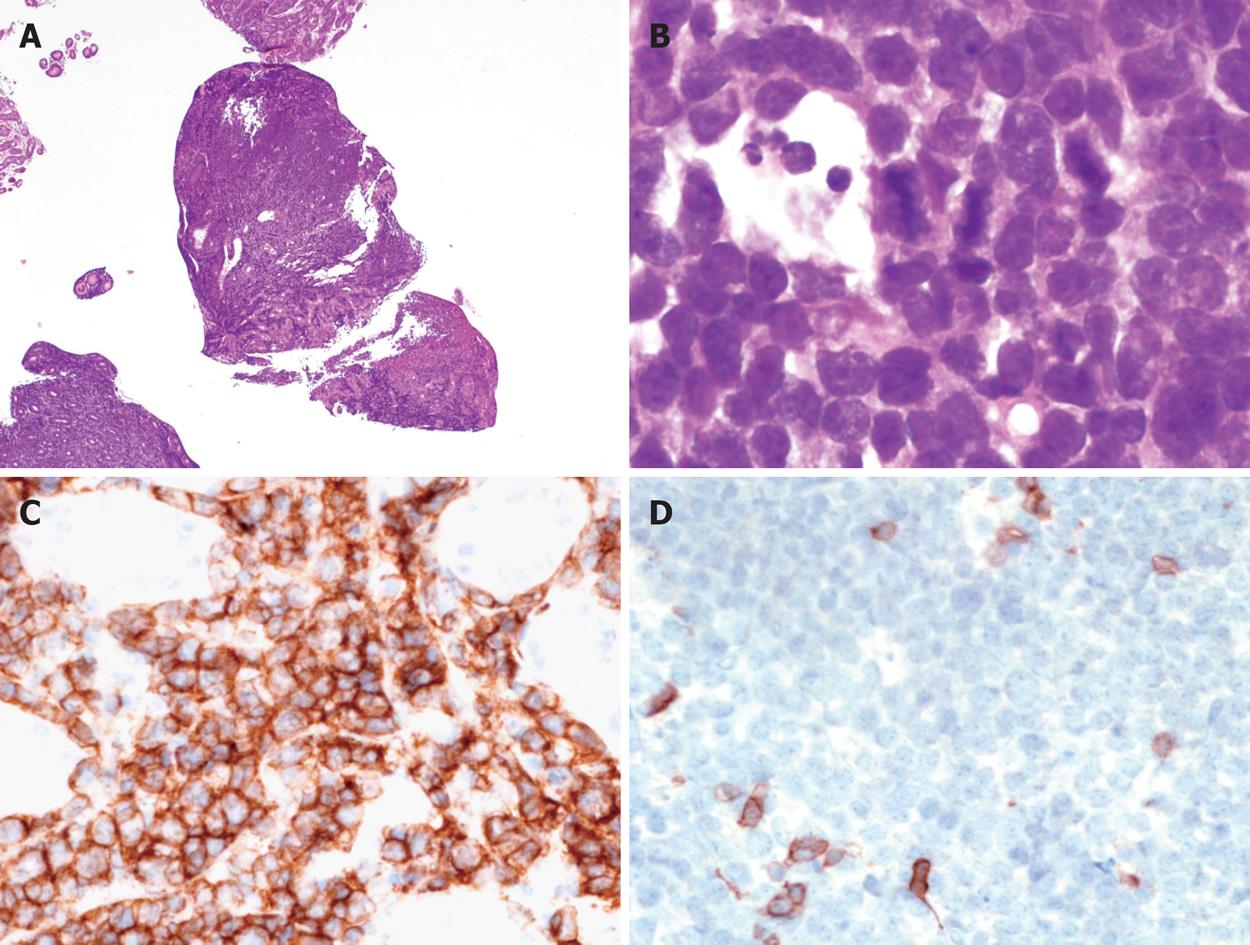
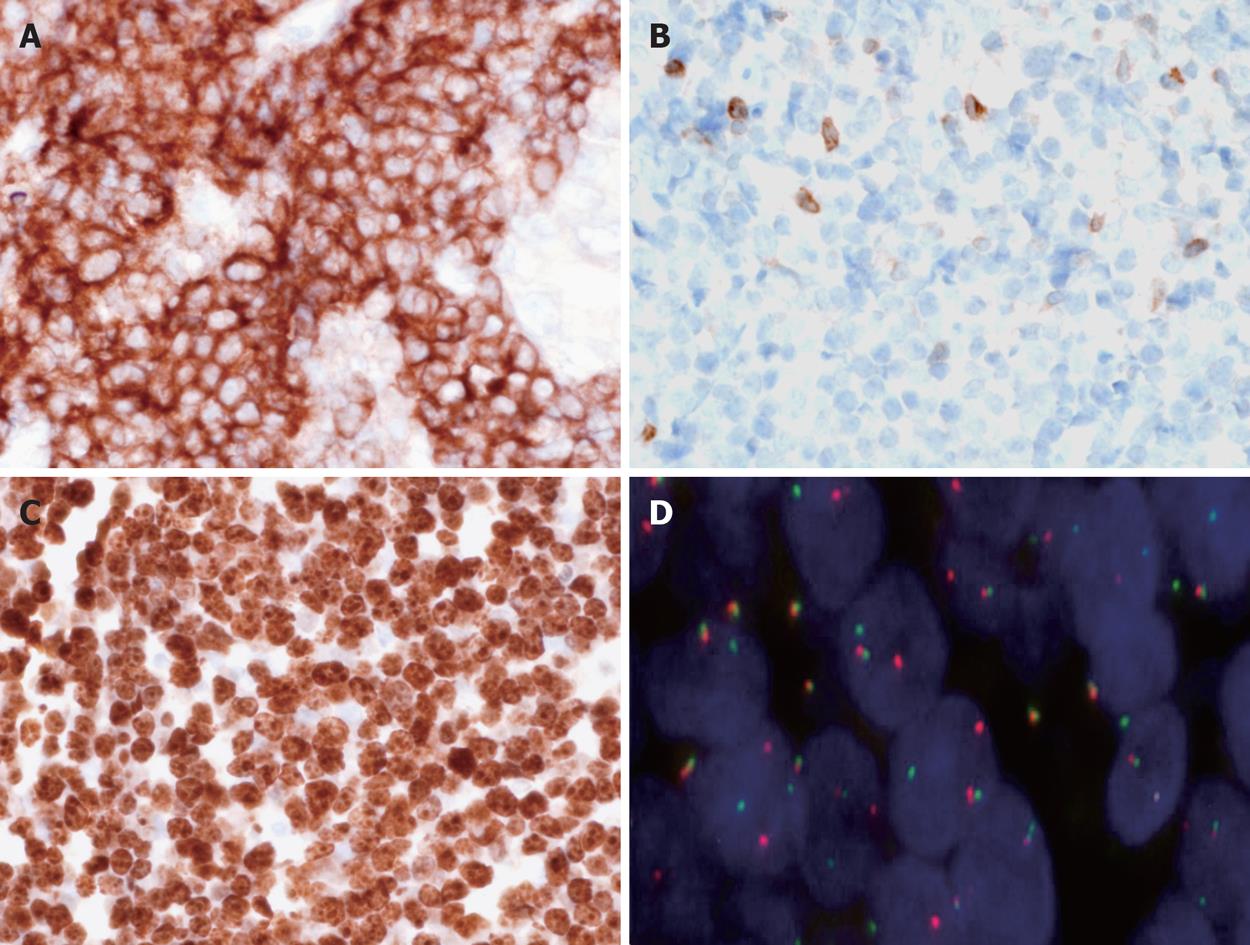
Histologic examination of gastric biopsies showed a diffuse infiltration of the mucosa by atypical lymphoid cells with a CD20+, CD3- immunophenotype and a diagnosis of “de novo DLBCL” was made initially by the local pathologist. H pylori corpuscles were observed histologically using modified Giemsa stain, and the patient was treated for H pylori eradication with pantoprazole 40 mg twice daily, amoxicillin 1 g twice daily and clarithromycin 500 mg twice daily for one week. Then she continued pantoprazole 40 mg once daily for three weeks and was referred to our gastroenterology unit four weeks later for further management of her disease. Initial gastric biopsies were reviewed by an expert hematopathologist (CCB) and additional immunostains and molecular studies were performed. The ulcerated mucosa was diffusely infiltrated by monotonous medium-sized lymphoid cells with round nuclei, scarce cytoplasm and starry sky pattern at low magnification (Figure 2A and B). Numerous mitoses were observed. H pylori were detected in biopsy specimens. Immunohistochemistry showed that the lymphoid cells were CD20+, CD5-, CD10+, BCL2-, BCL6+, MUM1-, P53+ and 100% of tumour cells were Ki67 positive (Figure 2C and D, and Figure 3A-C). In situ hybridization using EBERs probes was performed and EBV transcripts were not detected in tumour cells. Interphase fluorescence in situ hybridization (FISH) analysis was performed on formalin-fixed paraffin-embedded (FFPE) tissue sections using split signal FISH DNA probes for c-MYC, BCL2 and BCL6 (Dako AS, Glostrup, Denmark) according to the manufacturer’s recommendations (http://www.euro.fish.org). C-MYC gene rearrangement was observed in tumour cells without BCL2 or BCL6 gene rearrangement (Figure 3D). All these findings were in accordance with the diagnosis of BL.
Figure 2 Morphological and immunohistochemical features of the disease.
A: At low magnification, the gastric mucosa is diffusely infiltrated by medium sized lymphoid cells (HE, × 25); B: Tumour cells are medium sized with scarce cytoplasm, round nuclei and fine chromatin; numerous mitoses are observed; tangible body macrophages are present (HE, × 1000); C: Immunohistochemistry shows that the tumour cells are CD20+, × 400; D: CD5-, × 400.
Figure 3 Immunohistochemical and cytogenetic features of the disease.
A: Tumour cells are CD10+, × 400; B: BCL2-, × 400; C: 100% of tumour cells are Ki67+, × 400; D: Fluorescence in situ hybridization (FISH) performed with split signal FISH DNA probes for c-MYC revealed distinct red and green split signals in many nuclei, consistent with c-MYC gene rearrangement, beside yellow fusion signals for the non-rearranged alleles, × 1000.
Follow up endoscopy performed 6 wk after initiation of H pylori eradication therapy showed the ulcer size had decreased, measuring 3 cm in diameter, and histological analysis of gastric biopsies showed complete remission of the disease and absence of H pylori. Endoscopic ultrasonography showed a markedly increased thickness of the gastric wall (10 mm) with disappearance of the layer structure on the lesser curvature of the antrum and with a perigastric lymphadenopathy measuring 7 mm in diameter.
Appropriate investigations were performed to stage the lymphoma. Blood cell counts and biochemical parameters, including LDH and β2microglobulin level, and hepatic biology were normal. An EBV serology test showed IgG positive and IgM negative viral capsid antigen (VCA) and Epstein-Barr nuclear antigen (EBNA), reminiscent of “an old infection”. Human immunodeficiency virus serology was negative. Bone marrow biopsy and lumbar puncture were normal. Abdominal computed tomography showed an important increase in gastric wall thickness. As a perigastric lymphadenopathy was present at endoscopic ultrasonography, the patient was classified as stage IIE according to the Ann Arbor classification. The International Prognostic Index, including age (older or not than 60 years), LDH level (normal or elevated), tumour stage (I, II/III, IV), number of extranodal sites involved by disease (0 or 1/more than 1), patient performance status (less or more than 2), was scored 1 (low-risk group).
A new endoscopy with biopsy was performed one month later and showed complete healing of the ulcer (Figure 1B), and persistent histological remission of the disease. In spite of the absence of residual disease, taking into account the high risk of lymphoma recurrence, she then received systemic chemotherapy, which consisted of cyclophosphamide, vincristine, prednisone, doxorubicin and methotrexate, associated with intrathecal injection of methotrexate, until August 2006.The gastric endoscopic aspect with systematic biopsies was checked every 6 mo. The last outcome visit, in March 2008, with a new scanography and a new endoscopy and endoscopic ultrasonography confirmed the complete remission of the disease.
DISCUSSION
BL is a mature aggressive B-cell lymphoma, characterized by a high degree of proliferation of the neoplastic cells and deregulation of the c-MYC gene[17,18]. It is a distinct entity that occurs sporadically worldwide, sometimes associated with immunodeficiency or immunosuppression, and is endemic in central Africa (the lymphoma belt of Africa). Cells in both sporadic and endemic forms of BL are B lymphocytes that have clonal immunoglobulin (IG) gene rearrangement and 8q24/MYC translocation to the IG heavy chain region, 14q32 or less commonly at the lambda 22q11 or kappa 2p12 light chain loci. Epstein Barr virus and malaria are recognised as important cofactors of endemic BL. A combination of these two diseases seems to boost the incidence of endemic BL in the lymphoma belt by a factor of 100-150. But this disease can occur in the absence of both of these infections and arboviruses and plant tumour promoters are other possible local cofactors. There are probably other cofactors which are unknown.
The stomach is the most common site of extranodal malignant lymphomas. The incidence of gastric BL is low as compared to other types of gastric lymphomas, although its true incidence is not yet defined. Like other types of BL, gastric BL is known to have a highly aggressive clinical course. However, one study including 21 patients with a gastric BL, showed a better outcome for these patients as compared to 26 non-gastric BL with a long-term survival of 90%. An especially good outcome was observed when the disease was localized to the stomach and the authors concluded that, in this case, prognosis of BL and DLBCL could be similar. This observation suggested a common pathogenesis[3].
There is a close association between H pylori and gastric lymphomas[4]. But the link between H pylori and BL has not been demonstrated yet. H pylori plays an important role in the development and growth of MALT lymphoma and its eradication has been shown to achieve durable regression in 56% to 100% of patients with early stage low-grade gastric MALT lymphoma[8-11]. In vitro co-culture of H pylori with tumour B cells in the presence of non-neoplastic T cells proved that the bacteria were able to induce proliferative responses of MALT lymphocytes but not large B cells[19]. Therefore, DLBCL of the stomach were generally believed to be H pylori-independent, and unlikely to respond to antibiotic therapy. However, in addition to sporadic cases, one prospective study of 24 patients with DLBCL has shown that antibiotic therapy could also result in complete remission in patients with early stage gastric DLBCL with low grade components[15]. The same authors compared two prospective studies of the efficacy of H pylori eradication in the treatment of early-stage MALT lymphoma and DLBCL of the stomach and showed that the therapeutic efficacy of antibiotics for stage IE gastric high grade transformed MALT lymphoma was similar to their efficacy in stage IE low-grade lymphoma. It was suggested that the response was possible only in DLBCL with low grade component and more frequent in cases of superficial gastric wall involvement and in the absence of BCL10 protein nuclear expression[16-20].
Histologically, the distinction between diffuse large B cell lymphoma and BL is sometimes difficult, especially in adult patients. It is well known that these two entities may show overlapping morphological and immunohistochemical features[21,22]. Whether this case represents “a B cell lymphoma, unclassifiable, with features intermediate between diffuse large B cell lymphoma and Burkitt lymphoma”, a provisional entity defined in the recent 2008 World Health Organization (WHO) classification of lymphoid neoplasms may be discussed[2]. However, this case met all the characteristic morphological (starry sky appearance, monotonous medium sized tumour cells with round nuclei and scarce cytoplasm), immunohistochemical (CD10+, BCL2-, 100% Ki67 positivity) and cytogenetic (MYC translocation without BCL2 or BCL6 translocation) diagnosis criteria of BL according to the 2008 WHO classification, therefore excluding the hypothesis of de novo DLBCL.
In the present case, eradication of H pylori was performed before the definitive histological results were available. So, we had the opportunity to observe the first case of BL remission following H pylori eradication in an adult patient. This observation highlights the potential role of an infectious agent, notably H pylori, as a cofactor in the development of BL and suggests that H pylori eradication should be systematic in the context of H pylori positive gastric BL in association with conventional chemotherapy regimens.