Published online Nov 28, 2009. doi: 10.3748/wjg.15.5541
Revised: October 10, 2009
Accepted: October 17, 2009
Published online: November 28, 2009
AIM: To investigate the effects of macrophage migration inhibitory factor (MIF) on proliferation of human gastric cancer MGC-803 cells and expression of cyclin D1 and p27Kip1 in them, and further determine whether the effects are related to the PI3K/Akt signal transduction pathway.
METHODS: Gastric cancer MGC-803 cells were cultured and then treated with 50 μg/L recombinant human MIF (rhMIF) with and without a PI3K inhibitor, LY294002 (25 μmol/L). MTT assay was used to detect the proliferation of MGC-803 cells. Cell cycle was detected by flow cytometry. Expression of cyclin D1 and p27Kip1 mRNA was by reverse transcription-polymerase chain reaction. Protein expression of phosphorylated Akt (p-Akt), Akt, cyclin D1 and p27Kip1 was examined by immunocytochemistry and Western blotting.
RESULTS: rhMIF significantly stimulated the proliferation of MGC-803 cells and cell cycle progression from G1 phase to S phase in a concentration- and time-dependent manner. After the MGC-803 cells were treated with rhMIF for 24 h, the expression of cyclin D1 was significantly up-regulated compared with the cells not treated with rhMIF at both mRNA and protein levels (0.97 ± 0.02 vs 0.74 ± 0.01, P = 0.002; 0.98 ± 0.05 vs 0.69 ± 0.04, P = 0.003). The p27Kip1 was down-regulated but only statistically significant at the protein level. rhMIF significantly increased the expression of p-Akt, which reached the peak at 30 min, but did not affect the expression of Akt. However, LY294002 inhibited all the effects of rhMIF.
CONCLUSION: Macrophage MIF increases the proliferation of gastric cancer cells, induces the expression of cyclin D1 at the transcriptional level and inhibits the expression of p27Kip1 at the post-transcriptional level via the PI3K/Akt pathway.
-
Citation: Li GQ, Xie J, Lei XY, Zhang L. Macrophage migration inhibitory factor regulates proliferation of gastric cancer cells
via the PI3K/Akt pathway. World J Gastroenterol 2009; 15(44): 5541-5548 - URL: https://www.wjgnet.com/1007-9327/full/v15/i44/5541.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5541
Gastric cancer is the most common gastroenterological malignancy worldwide, with its incidence rate being the fourth among all cancers[1,2]. There are approximately 934 000 new cases, and 700 000 patients die of gastric cancer each year. Gastric cancer is the second leading cause of cancer-related death worldwide[3-5], and remains a disease of poor prognosis. The 5-year survival rate of gastric cancer patients is generally below 20%. The current major therapies for gastric cancer include surgery, chemotherapy combined with or without radiotherapy, but their efficacy is not satisfactory. It is, therefore, necessary to explore and investigate the tumorigenesis of gastric cancer and its novel therapy targets[6].
Macrophage migration inhibitory factor (MIF) is a multi-functional cytokine, which is associated with inflammation and tumorigenesis[7,8]. Recent studies have shown that MIF is related to the initiation and progression of gastric cancer, but its underlying mechanism is not very clear[9-11].
It has been reported that the effect of MIF on cell proliferation is relevant to cell cycle factors[12]. Cyclin Dl and 27Kip1 belong to the family of cyclin proteins and cyclin-dependent kinase inhibitors (CDKI), which regulates cell cycle progression from the G1 phase to the S phase[13,14]. Over-expression of cyclin D1 and aberrant expression of p27Kip can lead to the loss of control of cell proliferation and differentiation, thus promoting tumorigenesis[15].
The phosphoinositol-3-kinases (PI3K)/Akt pathway has been considered an important signaling pathway that is involved in the regulation of cell proliferation and differentiation[16]. It has been reported that NF-κB activates the transcription target, via PI3K/Akt signaling, to promote gastric tumorigenesis[17]. In a recent study with a gastric cancer cell line, AGS, Kim et al[18] reported that inhibition of the PI3K/Akt/PKB pathway could enhance the mitochondrial death pathway. Another recent study indicates that anti-cancer effects of celecoxib on gastric cancer cells are partly mediated by down-regulation of Akt signaling[19].
The aims of the present study were to investigate the effects of recombinant human MIF (rhMIF) on cell proliferation of human gastric cancer MGC-803 cells and cell cycle. Activity of Akt and expression of cyclin D1 and p27kip1 were examined. Whether the PI3K/Akt pathway is involved in the effects of rhMIF was further investigated, using a PI3K/Akt inhibitor, LY294002.
rhMIF and P13K specific inhibitor, LY294002, were purchased from PROSPEC (Rehovot, Israel) and Cell Signaling (Danvers, MA, USA), respectively. Primers for GAPDH, cyclin D1 and p27 were produced by Shanghai Sangon Biological Engineering Technology & Service Company, Ltd. (Shanghai, China). Mouse anti-human β-actin primary antibody was bought from Beyotime Institute of Biotechnology (Shanghai, China). Rabbit anti-human cyclin D1 primary antibody was purchased from Epitomics (Burlingame, CA, USA), and rabbit anti-human p27, rabbit anti-human phosphorylated Akt (p-Akt) and Akt primary antibodies were bought from Santa Cruz Biotechnology (CA, USA). Goat anti-mouse and goat anti-rabbit secondary antibodies were purchased from Beyotime Institute of Biotechnology (Shanghai, China) and BioDev-Tech (BioDev, Beijing, China), respectively. Epics-XL II flow cytometer was purchased from Beckman Coulter (Beckman, Fullerton, CA, USA).
Gastric cancer cell line, MGC-803, was provided by the Institute of Cancer Research, Nanhua University (China), and cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 100 μg/mL streptomycin and 100 μ/mL penicillin, and maintained at 37°C in a humidified atmosphere containing 5% CO2. The cultured cells were then used in different experiments as described below.
Approximately 2 × 103 cells/well were grown in 96-well microtiter plates and incubated overnight in 100 μL of the culture medium. Cells were starved without FBS for 24 h at 70%-80% confluence and then treated with rhMIF at different concentrations (25, 50 and 100 μg/L) with or without 1-h pretreatment with LY294002 (25 μmol/L), for 12, 24 and 48 h, respectively. Cells without any treatment were used as controls. Twenty microliters of 5 mg/mL MTT (Sigma, St Louis, MO) labeling reagent was added to the designated well, and cells were incubated at 37°C for 4 h. The supernatant was removed, and then 150 μL dimethyl sulfoxide (DMSO) was added to the designated well. After the plate was incubated at 37°C for 15 min, the absorbency was measured with a micro ELISA reader (Bio-Tek, Winooski, VT, USA) at a wavelength of 570 nm.
Cells were harvested after treated with rhMIF, with or without 1-h pretreatment with LY294002 for 24 h as described earlier, and fixed with 75% cold alcohol at 4°C overnight. After washed with phosphate buffered saline (PBS), propidium iodide (PI) was added and cells were incubated at 4°C for 30 min. Cell cycle distribution was detected with an Epics-XL II flow cytometer (Beckman Coulter, Inc., Fullerton, CA, USA).
Cells were harvested after treated with rhMIF, with or without 1-h pretreatment with LY294002 for 24 h as described earlier. Total RNA was extracted from cells with a total RNA kit (BioDev, Beijing, China). One microgram of RNA was reversely transcribed to cDNA with thermoscript RT system reagent (MBI Fermentas, Glen Burnie, USA) according to its manufacturer’s instructions. The primer sequences are as follows: cyclin D1, forward: 5'-CGTCCATGCGGAAGATC-3', reverse: 5'-CAGAGGGCAACGAAGGT-3' (406 bp); p27Kip1, forward: 5'-CGCAAGTGGAATTTCGATTT-3', reverse: 5'-ATGCGTGTCCTCAGAGTTAGC-3' (215 bp); GAPDH, forward: 5'-CGGAGTCAACGGATTTGGTCGTAT-3', reverse: 5'-AGCCTTCTCCATGGTGGTGAAGAC-3' (306 bp). PCR was performed according to the following conditions: denaturation at 94°C for 3 min, 40 cycles of denaturation at 94°C for 30 s, annealing at 57°C for 30 s and extension at 72°C for 2 min, followed by a final extension at 72°C for 10 min. Five microlitres of PCR product was used for agarose gel electrophoresis and analyzed using Alphalmager 2200 (Imaging System, CA, USA).
Whole cell lysates were prepared from cells with RIPA lysis buffer containing protease inhibitors (Beyotime, Shanghai, China). Protein concentration was measured with a BCA protein assay kit (Beyotime, Shanghai, China). An aliquot of 40 μg protein of each sample was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis. Then, the protein was transferred to a PVDF membrane (Millipore, Billerica, MA). Non-specific binding was blocked with 5% non-fat milk for 2 h at 37°C. The membrane was immunodetected with anti-cyclin D1 (1:500), anti-p27kip1 (1:500), anti-p-Akt (1:500), anti-Akt (1:500), anti-β-actin (1:1000) primary antibodies overnight at 4°C, and then with corresponding HRP-conjugated secondary antibodies (1:2000). Antigen-antibody complexes were visualized with a luminol reagent (Santa Cruz, CA, USA), and the results were analyzed with Alphalmager 2200. β-actin was detected as the loading control.
Cells were grown on slides at a concentration of 1.0 × 106 cells/mL, treated with rhMIF (50 μg/L) with or without 1-h pretreatment with LY294002 (25 μmol/L) for 24 h at 70%-80% confluence. Cells without any treatment were used as controls. The treated and control cells were fixed with 75% cold alcohol at 4°C overnight and then subjected to immunostaining using the streptavidin-peroxidase technique. In brief, endogenous peroxidase was blocked with 3% H2O2 in PBS, and then with a normal goat serum blocking solution for 20 min at room temperature. The slides were incubated with anti-p-Akt, anti-cyclin D1 and anti-p27kip1 primary antibodies (1:200 diluted with 0.01 mol/L PBS), respectively, for 60 min at room temperature. Slides that were incubated with PBS instead of primary antibodies were used as negative controls. After washed with PBS, the slides were incubated with corresponding HRP-conjugated secondary antibodies for 30 min. The color of immunostaining was developed with a diaminobenzidine solution and counterstained with hematoxylin.
Data were analyzed using SPSS 16.0 software. The results were expressed as mean ± SD. Student t-test was used to determine the difference in numeric parameters between different groups. P < 0.05 was considered statistically significant.
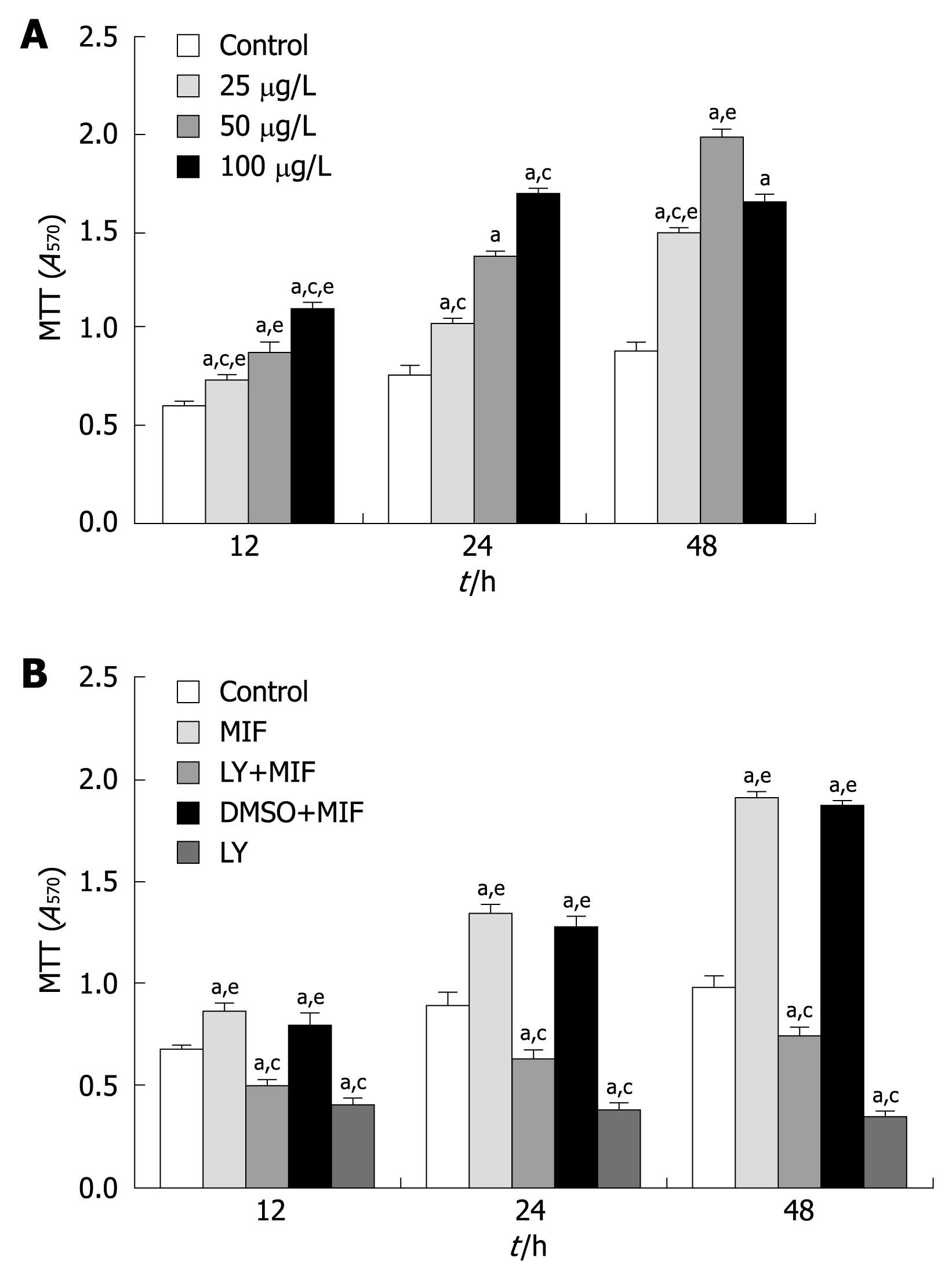
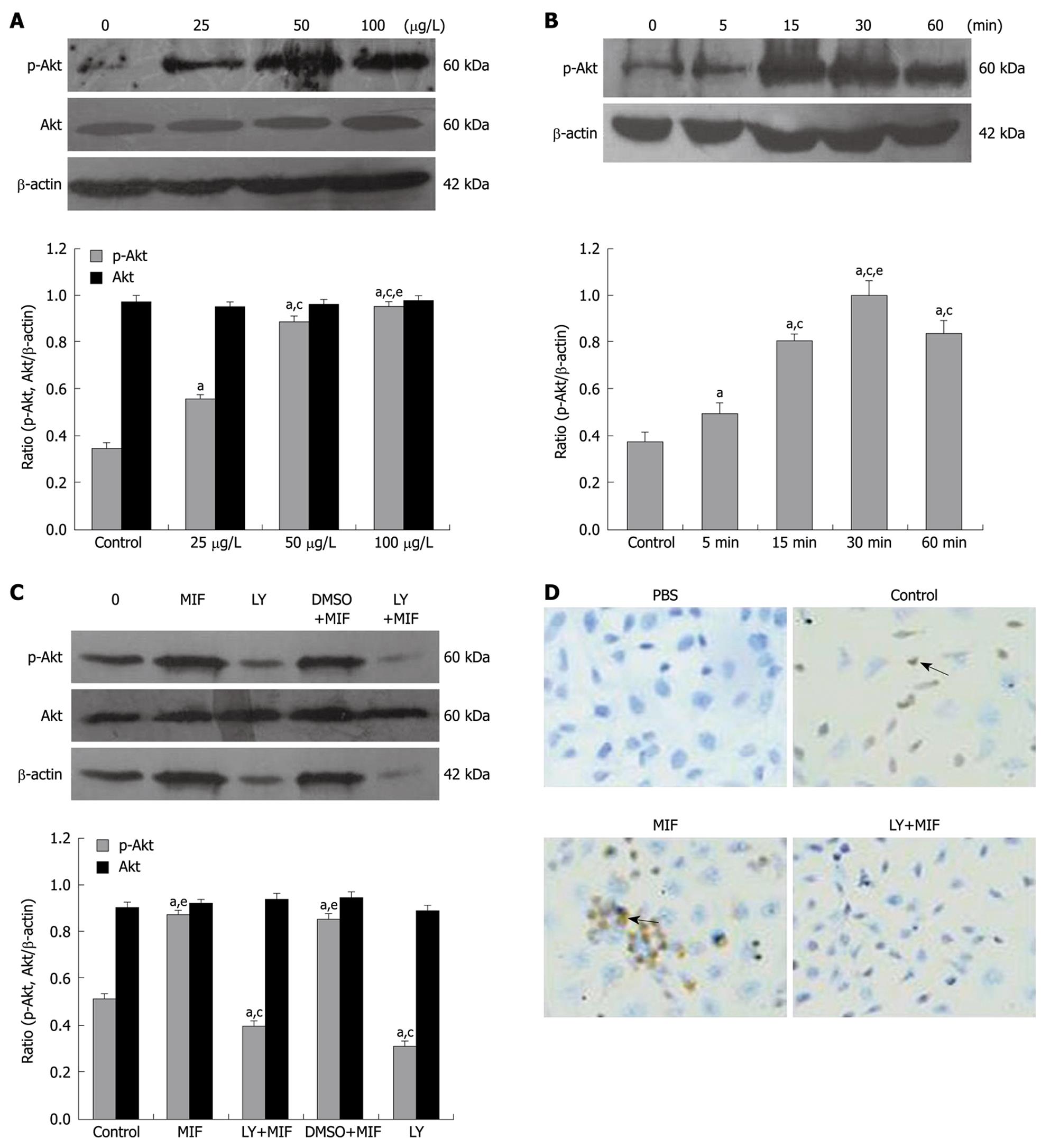
The MTT assay showed that treatment with rhMIF significantly increased the proliferation of MGC-803 cells in a dose- and time-dependent manner compared with the controls (P < 0.05). The proliferation of MGC-803 cells reached its peak (1.823 ± 0.04/0.647 ± 0.02, 282%) when they were treated with 50 μg/L rhMIF for 48 h (Figure 1A), which was significantly inhibited after 1-h pretreatment with 25 μmol/L LY294002 (P < 0.05, Figure 1B). DMSO (the menstruum of LY294002, less than 0.1%) had little effect on the proliferation of MGC-803 cells.
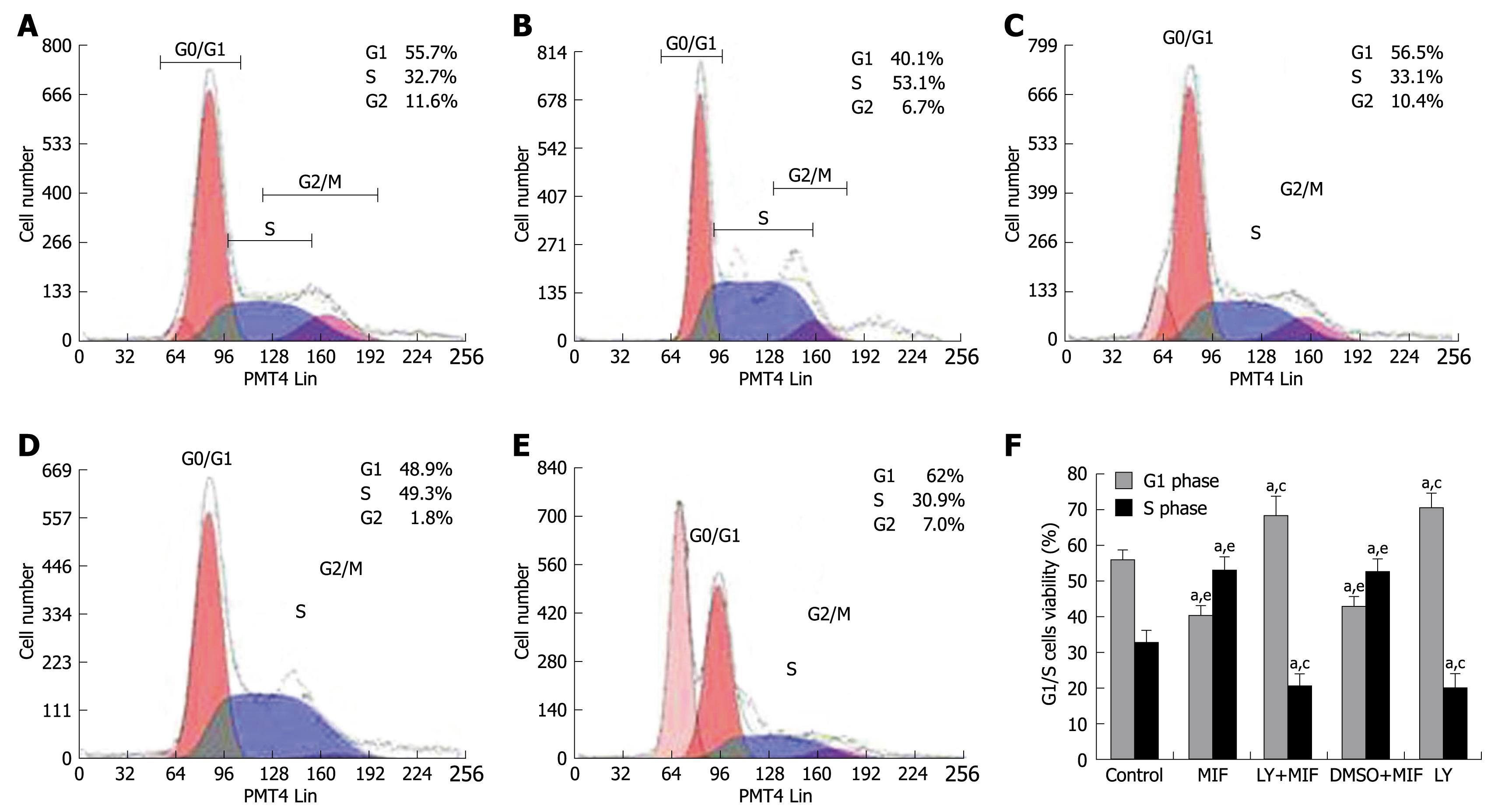
The effect of rhMIF on cell cycle distribution is shown in Figure 2. rhMIF (50 μg/L) significantly induced the progression of MGC-803 cells from G1 phase to S phase (P < 0.05), thus increasing the accumulation of cells in S phase. However, the cells treated with LY294002 were accumulated in G1 phase, namely G1 arrest, irrespective of rhMIF treatment (P < 0.05). DMSO had little impact on the rhMIF effect (Figure 2).
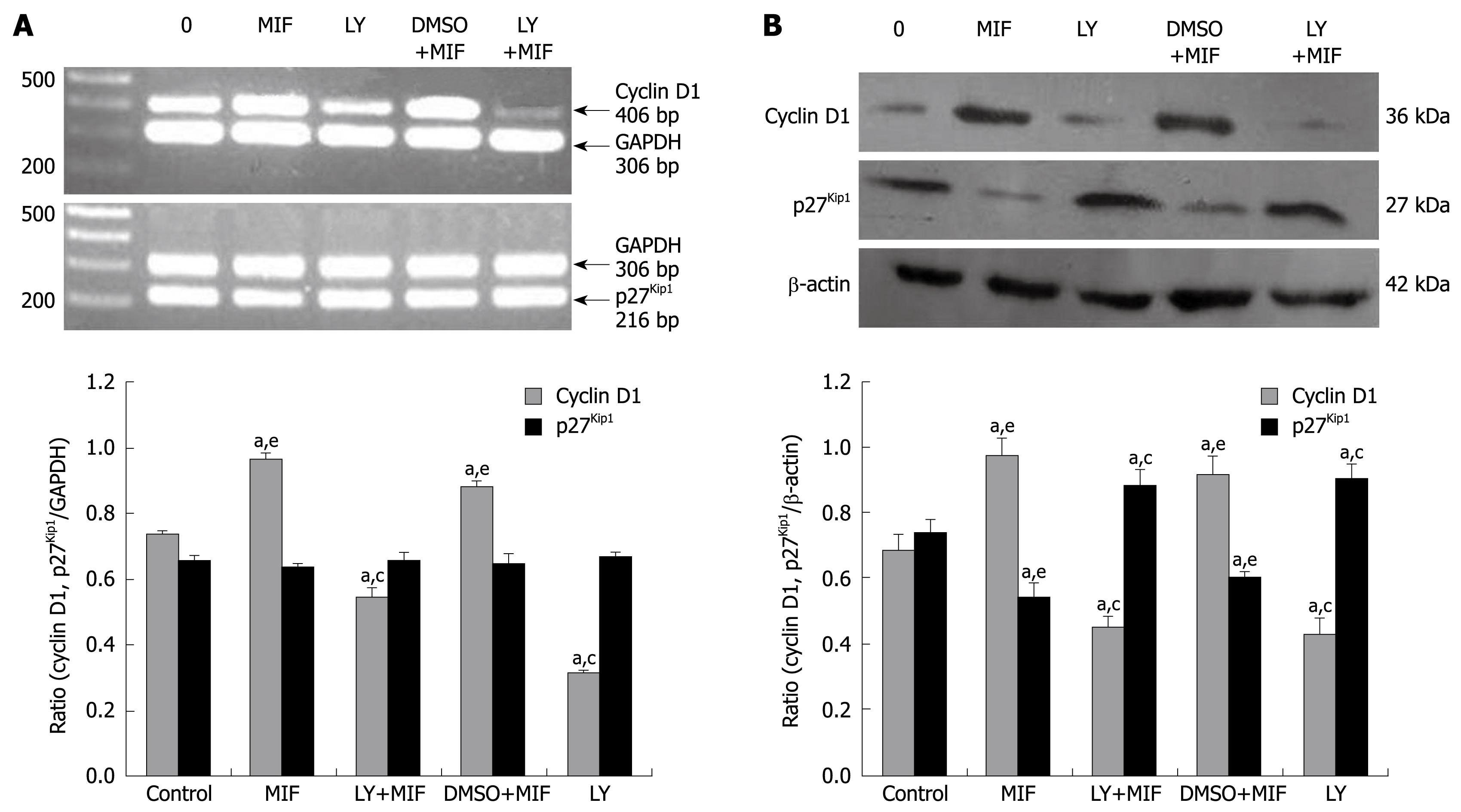
After treatment with 50 μg/L rhMIF for 24 h, the mRNA level of cyclin D1 was increased significantly (P < 0.05). However, 1-h pre-treatment with LY294002 reduced the mRNA expression irrespective of subsequent rhMIF treatment (P < 0.05, Figure 3A). DMSO had little impact on the rhMIF effect. Treatment with rhMIF or LY294002 had no effect on the mRNA level of p27kip1 (Figure 3A).
Western blotting showed that after treatment with 50 μg/L rhMIF for 24 h, the expression of cyclin D1 protein was significantly up-regulated, but the expression of p27kip1 protein was down-regulated (P < 0.05, Figure 3B). However, 1-h pretreatment with LY294002 prior to rhMIF treatment abrogated the effects of rhMIF on the expression of cyclin D1 and p27kip1 proteins (P < 0.05). Again, DMSO had little impact on the rhMIF effect (Figure 3B).
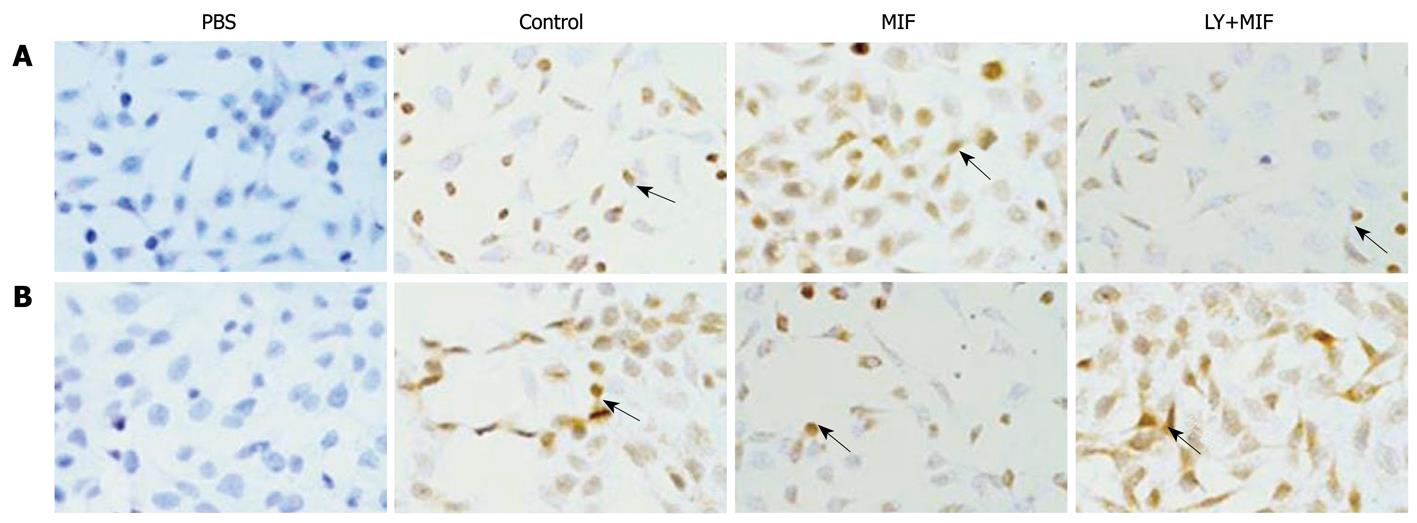
Immunocytochemistry demonstrated that the positive expression of cyclin D1 and p27kip1, in yellow or brown, was mostly located in the nuclei of MGC-803 cells, but rarely in the cytoplasm. rhMIF increased the expression of cyclin D1, but decreased the expression of p27kip1 (Figure 4). However, 1-h pretreatment with LY294002 down-regulated the expression of cyclin D1 (Figure 4A), but up-regulated the expression of p27kip1 (Figure 4B), which was consistent with the results obtained by Western blotting.
Western-blotting showed that after treatment with different concentrations of rhMIF (25, 50, 100 μg/L) for 30 min, the expression of p-Akt in MGC-803 cells was up-regulated in a dose-dependent manner (Figure 5A, P < 0.05). In addition, the cells treated with 50 μg/L rhMIF for different periods of time (5, 15, 30 and 60 min) showed that the expression of activated Akt in MGC-803 cells started to increase at 5 min, reached its peak at 30 min, then decreased but remained at a level higher than that in the untreated control cells (P < 0.05, Figure 5B). Pretreatment with LY294002 prior to rhMIF treatment, however, inhibited the expression of p-Akt irrespective of rhMIF treatment (Figure 5C). DMSO had little impact on the rhMIF effect. Both rhMIF and LY294002 had no effect on the expression of Akt (Figure 5A and C).
Immunocytochemistry showed that p-Akt was positively expressed in cytoplasm of the cells. rhMIF increased the expression of p-Akt. However, pre-treatment with LY294002 down-regulated the expression of p-Akt (Figure 5D).
MIF has multiple biological functions and plays an important role in inflammation as well as in carcinogenesis as a cytokine[20,21]. Increased expression of MIF has been reported in pre-cancerous, cancerous and metastatic tumors[10,22]. It has been shown that MIF-modulated cancer progress is associated with the ERK/MAPK, PI3K/Akt, and Rb-E2F pathways[22-25]. However, its detailed mechanism underlying inflammatory and malignant transformation as well as tumorigenesis needs to be further investigated.
In our study, rhMIF increased the MGC-803 cell proliferation in a dose- and time-dependent manner, and induced progression from G1 phase to S phase, leading to S phase arrest. It has been demonstrated that cyclin, cyclin-dependent kinase (CDK) and CDK inhibitor (CDKI) play a critical role in the orderly progression of cell cycle during the transition from G1 phase to S phase[26,27]. Since cyclin D1 is an important regulator of cell cycle progression, its increased expression contributes significantly to tumor development and malignant transformation[13,28]. p27Kip1, an inhibitor of CDK, functions as an integral brake of the cell cycle, thus also playing an important role in tumor suppression[29]. For example, deregulation of p27Kip1 has a profound effect on tumor progression in colorectal cancer and is suggested to be an accurate and independent prognostic marker[30]. Moreover, p27Kip1 may be a central target for growth regulation of the normal endometrium and for the pathogenesis of endometrial carcinoma[31]. In our study, rhMIF increased cyclin D1 mRNA expression, but had no effect on p27Kip1 mRNA expression. However, immunocytochemistry showed that rhMIF increased the expression of cyclin D1 protein and decreased the expression of p27Kip1 protein. These results were identified further by Western blotting. Therefore, our results indicate that rhMIF affects the cell cycle by inducing cyclin D1 and inhibiting p27Kip1, thus promoting the proliferation of MGC-803 cells. In addition, rhMIF up-regulates the transcriptional cyclin D1, which is in contrast with the posttranscriptional protein reduction of p27Kip1 in cells. This phenomenon may be related to the degradation by its ubiquitin ligase[32,33].
The PI3K/Akt pathway is crucial in tumorigenesis because the p-Akt can regulate the cell proliferation, apoptosis, angiogenesis and cell cycle by activating the downstream cell receptors or effectors[34-36]. Cinti et al[37] studied 50 cases of advanced gastric carcinoma using immunohistochemistry, showing a statistically significant direct correlation between p-Akt expression and depth of infiltration of the tumor, between the number of infiltrated lymph nodes and p34/cdc2 expression, and between prevalently nuclear p-Akt and cyclin D1 and cyclin E, but an inverse correlation between nuclear pAkt and apoptotic index, and between cytoplasmatic and nuclear pAkt and patient survival, suggesting that p-Akt may be considered an indicator of tumor progression and a prognostic factor for the survival of gastric cancer patients. Liang et al[38] reported that cellular prion protein up-regulates cyclin D1 at both mRNA and protein levels, thus promoting cell proliferation and transition from G1 phase to S phase in gastric cancer GC7901 and AGS cells, at least partially by activating the PI3K/Akt pathway. Moreover, a PI3K specific inhibitor, LY294002, can significantly suppress the effects of cellular prion protein. In the present study, immunocytochemistry showed that rhMIF increased the p-Akt expression, activated p-Akt in a dose- and time-dependent manner, and induced the maximum Akt phosphorylation at 30 min. However, the total expression level of Akt remained unchanged, indicating that rhMIF affects specifically the activated Akt, namely the p-Akt. To further validate the involved Akt pathway in the effect of rhMIF on MGC-803 cells, the cells were pretreated with a PI3K specific inhibitor, LY294002, showing that LY294002 reversed or abolished all the effects induced by rhMIF, including the increased proliferation, cell cycle S arrest, up-regulation of cyclin D1 and p-Akt, down-regulation of p27Kip1, suggesting that rhMIF can induce the proliferation and cell cycle progression of MGC-803 cells via the PI3K/Akt pathway.
In conclusion, MIF increases the proliferation of gastric cancer cells, induces the expression of cyclin D1 at the transcriptional level and inhibits the expression of p27Kip1 at the post-transcriptional level via the PI3K/Akt pathway. Further investigation is required to reveal whether macrophage MIF is a novel potential therapeutic target for the treatment of gastric cancer.
Gastric cancer is one of the most common gastroenterological malignancies worldwide and remains a disease of poor prognosis. It is necessary to investigate the gastric tumorigenesis and potential new therapies. Macrophage migration inhibitory factor (MIF) is a multi-function cytokine involved in inflammation, auto-immune disease and development of several cancers. Recent studies have shown that over-expression of MIF is associated with tumorigenesis of gastric cancer, but the underlying mechanism is unclear.
MIF induces cell proliferation through many signal pathways, but the mechanism underlying the regulation of immunoreaction and tumorigenesis is complicated. The PI3K/Akt signaling pathway plays an important role in the regulation of cell growth, proliferation and differentiation. Recent studies have shown that the proliferation of cancer cells is suppressed by blocking the PI3K/Akt pathway. In this study, we investigated the effects of rhMIF on the proliferation of gastric cells and cell cycle progression, and whether the expression of cyclin D1 and p27Kip1 and the regulation of PI3K/Akt pathway are involved in the effects of rhMIF.
The study found that MIF induced the proliferation of MGC-803 cells and promoted the cell cycle progression from G1 phase to S phase in gastric cancer MGC-803 cells, and that rhMIF executed its effects by up-regulating cyclin D1 and down-regulating p27Kip1 through the PI3K/Akt signal pathway.
The study explored the mechanism by which MIF induces the proliferation of gastric cancer MGC-803 cells and promotes cell cycle, indicating that MIF may be the potential target for gastric cancer therapy.
MIF, a T-cell-derived cytokine, was identified and named by Bloom and David in 1966. PI3Ks is involved in the regulation of cell proliferation, differentiation and apoptosis. The signal pathway through PI3K and its down-stream protein PKB or Akt are closely related to the human tumorigenesis.
In this study, the authors investigated whether the PI3K/Akt pathway is involved in the effect of rhMIF on the proliferation of MGC-803 cells, using a PI3K/Akt inhibitor, LY294002. Overall, the study is interesting and significant, and the data are presented in a clear and logical manner.
Peer reviewers: Lucia Malaguarnera, Associate Professor, MD, PhD, Department of Biomedical Sciences, University, Via E. De Amicis, 24 Trecastagni Catania, 95039, Italy; John M Luk, Associate Professor, Department of Surgery, The University of Hong Kong, 21 Sassoon Road, Pokfulam, Hong Kong, China
S- Editor Tian L L- Editor Wang XL E- Editor Lin YP
| 1. | Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354-362. |
| 2. | Gallo A, Cha C. Updates on esophageal and gastric cancers. World J Gastroenterol. 2006;12:3237-3242. |
| 3. | Inoue M, Tsugane S. Epidemiology of gastric cancer in Japan. Postgrad Med J. 2005;81:419-424. |
| 4. | Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581-592. |
| 5. | Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137-2150. |
| 6. | Sutter AP, Fechner H. Gene therapy for gastric cancer: is it promising? World J Gastroenterol. 2006;12:380-387. |
| 7. | Leng L, Bucala R. Macrophage migration inhibitory factor. Crit Care Med. 2005;33:S475-S477. |
| 8. | Bach JP, Rinn B, Meyer B, Dodel R, Bacher M. Role of MIF in inflammation and tumorigenesis. Oncology. 2008;75:127-133. |
| 9. | Shun CT, Lin JT, Huang SP, Lin MT, Wu MS. Expression of macrophage migration inhibitory factor is associated with enhanced angiogenesis and advanced stage in gastric carcinomas. World J Gastroenterol. 2005;11:3767-3771. |
| 10. | He XX, Yang J, Ding YW, Liu W, Shen QY, Xia HH. Increased epithelial and serum expression of macrophage migration inhibitory factor (MIF) in gastric cancer: potential role of MIF in gastric carcinogenesis. Gut. 2006;55:797-802. |
| 11. | Camlica H, Duranyildiz D, Oguz H, Oral EN, Yasasever V. The diagnostic value of macrophage migration inhibitory factor (MIF) in gastric cancer. Pathol Oncol Res. 2008;14:79-83. |
| 12. | Liu L, Ji C, Chen J, Li Y, Fu X, Xie Y, Gu S, Mao Y. A global genomic view of MIF knockdown-mediated cell cycle arrest. Cell Cycle. 2008;7:1678-1692. |
| 13. | Tashiro E, Tsuchiya A, Imoto M. Functions of cyclin D1 as an oncogene and regulation of cyclin D1 expression. Cancer Sci. 2007;98:629-635. |
| 14. | Zheng JY, Wang WZ, Li KZ, Guan WX, Yan W. Effect of p27(KIP1) on cell cycle and apoptosis in gastric cancer cells. World J Gastroenterol. 2005;11:7072-7077. |
| 15. | Ge JR, Ren GP, Yao HP. [Expression of cyclin D1 and p27kip1 in renal cell carcinoma and its significance]. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2007;36:483-487. |
| 16. | Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, Franklin RA, McCubrey JA. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17:590-603. |
| 17. | Kang MJ, Ryu BK, Lee MG, Han J, Lee JH, Ha TK, Byun DS, Chae KS, Lee BH, Chun HS. NF-kappaB activates transcription of the RNA-binding factor HuR, via PI3K-AKT signaling, to promote gastric tumorigenesis. Gastroenterology. 2008;135:2030-2042, 2042.e1-e3. |
| 18. | Kim JH, Go HY, Jin DH, Kim HP, Hong MH, Chung WY, Park JH, Jang JB, Jung H, Shin YC. Inhibition of the PI3K-Akt/PKB survival pathway enhanced an ethanol extract of Rhus verniciflua Stokes-induced apoptosis via a mitochondrial pathway in AGS gastric cancer cell lines. Cancer Lett. 2008;265:197-205. |
| 19. | Kim N, Kim CH, Ahn DW, Lee KS, Cho SJ, Park JH, Lee MK, Kim JS, Jung HC, Song IS. Anti-gastric cancer effects of celecoxib, a selective COX-2 inhibitor, through inhibition of Akt signaling. J Gastroenterol Hepatol. 2009;24:480-487. |
| 20. | Bucala R, Donnelly SC. Macrophage migration inhibitory factor: a probable link between inflammation and cancer. Immunity. 2007;26:281-285. |
| 21. | Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791-800. |
| 22. | Mitchell RA. Mechanisms and effectors of MIF-dependent promotion of tumourigenesis. Cell Signal. 2004;16:13-19. |
| 23. | Lue H, Kapurniotu A, Fingerle-Rowson G, Roger T, Leng L, Thiele M, Calandra T, Bucala R, Bernhagen J. Rapid and transient activation of the ERK MAPK signalling pathway by macrophage migration inhibitory factor (MIF) and dependence on JAB1/CSN5 and Src kinase activity. Cell Signal. 2006;18:688-703. |
| 24. | Lue H, Thiele M, Franz J, Dahl E, Speckgens S, Leng L, Fingerle-Rowson G, Bucala R, Lüscher B, Bernhagen J. Macrophage migration inhibitory factor (MIF) promotes cell survival by activation of the Akt pathway and role for CSN5/JAB1 in the control of autocrine MIF activity. Oncogene. 2007;26:5046-5059. |
| 25. | Petrenko O, Moll UM. Macrophage migration inhibitory factor MIF interferes with the Rb-E2F pathway. Mol Cell. 2005;17:225-236. |
| 26. | Santamaria D, Ortega S. Cyclins and CDKS in development and cancer: lessons from genetically modified mice. Front Biosci. 2006;11:1164-1188. |
| 27. | Johansson M, Persson JL. Cancer therapy: targeting cell cycle regulators. Anticancer Agents Med Chem. 2008;8:723-731. |
| 28. | Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24. |
| 29. | Belletti B, Nicoloso MS, Schiappacassi M, Chimienti E, Berton S, Lovat F, Colombatti A, Baldassarre G. p27(kip1) functional regulation in human cancer: a potential target for therapeutic designs. Curr Med Chem. 2005;12:1589-1605. |
| 30. | Hershko DD, Shapira M. Prognostic role of p27Kip1 deregulation in colorectal cancer. Cancer. 2006;107:668-675. |
| 31. | Lecanda J, Parekh TV, Gama P, Lin K, Liarski V, Uretsky S, Mittal K, Gold LI. Transforming growth factor-beta, estrogen, and progesterone converge on the regulation of p27Kip1 in the normal and malignant endometrium. Cancer Res. 2007;67:1007-1018. |
| 32. | Nakayama K, Nagahama H, Minamishima YA, Miyake S, Ishida N, Hatakeyama S, Kitagawa M, Iemura S, Natsume T, Nakayama KI. Skp2-mediated degradation of p27 regulates progression into mitosis. Dev Cell. 2004;6:661-672. |
| 33. | Lahav-Baratz S, Ben-Izhak O, Sabo E, Ben-Eliezer S, Lavie O, Ishai D, Ciechanover A, Dirnfeld M. Decreased level of the cell cycle regulator p27 and increased level of its ubiquitin ligase Skp2 in endometrial carcinoma but not in normal secretory or in hyperstimulated endometrium. Mol Hum Reprod. 2004;10:567-572. |
| 34. | Yu BH, Zhou XY. [Advances on PI3K/Akt/mTOR signalling pathway in malignancies]. Zhonghua Bing Li Xue Za Zhi. 2005;34:674-676. |
| 35. | Tokunaga E, Oki E, Egashira A, Sadanaga N, Morita M, Kakeji Y, Maehara Y. Deregulation of the Akt pathway in human cancer. Curr Cancer Drug Targets. 2008;8:27-36. |
| 36. | Michl P, Downward J. Mechanisms of disease: PI3K/AKT signaling in gastrointestinal cancers. Z Gastroenterol. 2005;43:1133-1139. |
| 37. | Cinti C, Vindigni C, Zamparelli A, La Sala D, Epistolato MC, Marrelli D, Cevenini G, Tosi P. Activated Akt as an indicator of prognosis in gastric cancer. Virchows Arch. 2008;453:449-455. |