Published online Oct 21, 2009. doi: 10.3748/wjg.15.4976
Revised: September 7, 2009
Accepted: September 14, 2009
Published online: October 21, 2009
The origin of myofibroblasts or myofibroblastic cells in the desmoplastic stroma associated with carcinoma invasion has been controversial. In the early invasive area of a rectal adenocarcinoma reported here, an obvious transition between the muscularis mucosa and the bundles of eosinophilic stromal cells observed in the carcinomatous stroma was demonstrated both in morphology and in their cytoskeletal phenotype, which conceivably suggests that the smooth muscle cells of the muscularis mucosa could convert to the eosinophilic stromal cells, namely myofibroblasts. Moreover, type I procollagen was demonstrated in both protein and mRNA levels in the areas of eosinophilic stromal cells with a lesser degree of differentiated smooth muscle phenotype that showed a transition from the muscularis mucosa, implying that the myofibroblastic cells converted from smooth muscle cells of the muscularis mucosa could be responsible for type I collagen production. These findings suggest that the muscularis mucosae may not be a passive barrier through which colorectal carcinomas infiltrate into the submucosa, but may play an active role in the formation and remodeling of tumor stroma.
- Citation: Ban S, Shimizu M. Muscularis mucosae in desmoplastic stroma formation of early invasive rectal adenocarcinoma. World J Gastroenterol 2009; 15(39): 4976-4979
- URL: https://www.wjgnet.com/1007-9327/full/v15/i39/4976.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4976
The invasion of some carcinomas is characterized by the induction of so-called desmoplastic stroma. The desmoplastic stroma is comprised of activated fibroblastic cells and the deposition of extracellular matrix (ECM) components[1-3]. The activated fibroblastic cells commonly show some morphological and phenotypic features of smooth muscle cells, and are referred to as myofibroblasts or myofibroblastic cells[4-6]. They would modulate the microenvironment and contribute to tumor invasion through production of extracellular matrices, proteases, and soluble factors[1,3,7,8].
The origin of myofibroblasts or myofibroblastic cells has been controversial[4,6]. With regard to myofibroblastic cells in the desmoplastic stroma, one experimental study of an organotypic assay using breast carcinoma cells and breast stromal cells stressed the local fibroblasts as the most frequent candidate for myofibroblasts, while it also observed the recruitment of vascular smooth muscle cells and pericytes[9]. However, in contrast to those local resident precursors, bone marrow-derived circulating fibrocytes have recently been proposed to be myofibroblastic precursors[10,11].
This case study of an early invasive rectal adenocarcinoma conceivably demonstrates that one of the precursors of myofibroblastic cells in the carcinomatous desmoplastic stroma is smooth muscle cells of the muscularis mucosa, and they are responsible for production of an ECM component around the invasive carcinomatous glands, reemphasizing the importance of local resident cells in the desmoplastic reaction, and giving a hint as to the implication and a role of the muscularis mucosa in the early invasive process of colorectal carcinomas.
A rectal tumor was transanally removed from a 59-year-old Japanese male patient. The tumor was a broad-based sessile polypoid lesion of 20 mm in the largest diameter, which was fixed in 10% formalin, embedded in paraffin blocks, and stained with haematoxylin and eosin (HE) routinely. Serial sections were also made for immunohistochemistry and in situ hybridization (ISH).
Immunohistochemical staining was performed by an established indirect method using the following monoclonal antibodies (clone; source; dilution in parenthesis): anti-α-smooth muscle actin; α-SMA (1A4; Dako, Glostrup, Denmark; 1:25), anti-desmin (D33; Immunotech, Marseilles, France; 1:25), anti-high molecular weight caldesmone; h-CD (h-CD; Dako; 1:50), and anti-type I procollagen (M58; Chemicon, Temecula, CA, USA; 1:500). Antigens were retrieved by autoclaving at 121°C for 5 min in a citrate buffer before immunostaining for desmin and h-CD, and by 1% trypsin digestion for 20 min at room temperature for procollagen I. Negative control sections were incubated without primary antibodies. Submucosal arteries and part of the proper muscle layer could be available for internal positive controls for α-SMA, desmin, and h-CD. As a positive control for procollagen I, we also immunostained scar tissues which were fixed in formalin and embedded in paraffin in the same way as the present case.
For ISH to detect type I procollagen mRNA, the tissue sections were deparaffinized, rehydrated, and treated with 0.3% hydrogen peroxide in methanol for 30 min at room temperature. Then they were treated with protease K (Dako, Glostrup, Denmark) for 60 min followed by depurination in 0.2 N HCl for 20 min both at room temperature, dehydrated, and air dried. Hybridization was performed using a cocktail of two synthetic DNA oligonucleotide probes[12] labeled with digoxygenin at the 3’-end in a solution consisting of 50% formamide, 10 mmol/L Tris-HCl pH 7.6, 200 μg/mL yeast tRNA, 100 μg/mL sonicated salmon sperm DNA, 1X Denhardt’s solution, 10% dextran sulfate, 600 mmol/L NaCl, 0.25% SDS, 1 mmol/L EDTA pH 8.0, and 10 μg/mL of each probe, at room temperature overnight. After posthybridization washes (two brief washes in 2X SSC at 47°C, two washes in 1X SSC at 47°C for 30 min each, one wash in 0.5X SSC at 47°C for 30 min, one wash in 0.1X SSC at 47°C for 60 min, and one wash in 0.05 mol/L Tris-HCl pH 7.6 with 0.1% Tween 20 at room temperature for 5 min), incubation with horseradish peroxidase-conjugated anti-digoxygenin antibody was performed at room temperature for 30 min followed by detection with TSA™ Biotin System (PerkinElmer, Wellesley, MA, USA). As controls, prehybridization digestion with RNAse and hybridization using the hybridization solution with non-labeled probes or without probes were performed.
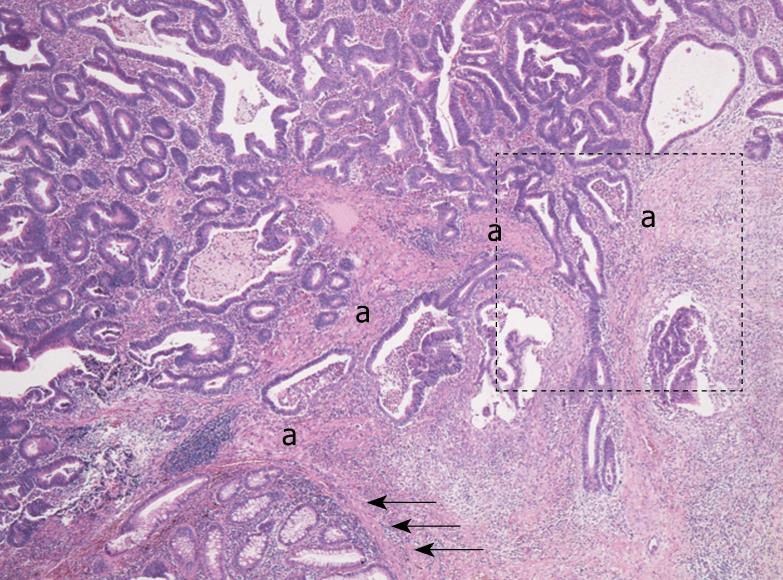
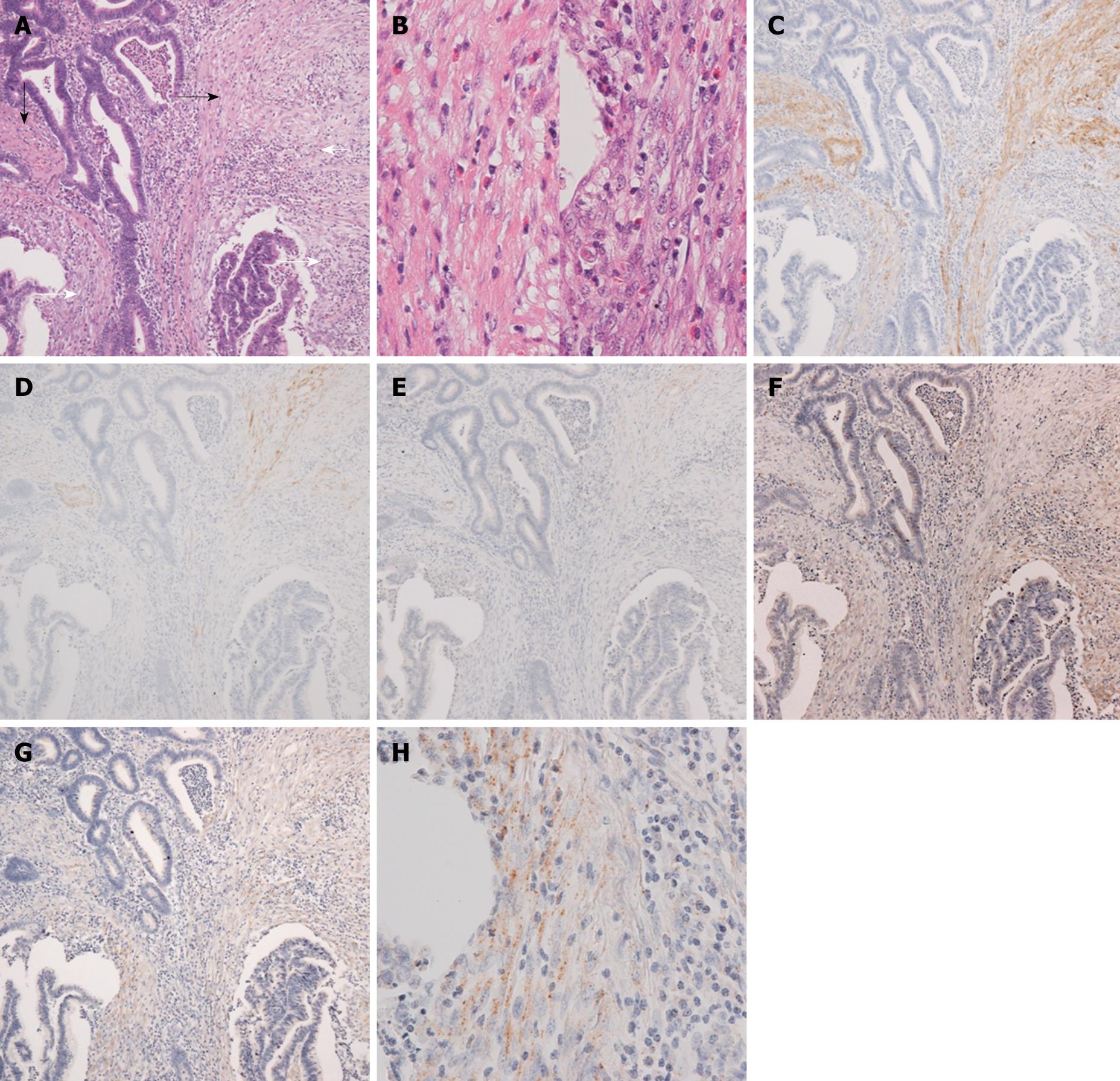
Histologically, the rectal tumor was a well-differentiated adenocarcinoma showing papillotubular growth, microscopically infiltrating into the submucosal layer beyond the muscularis mucosa. In the stroma of the invasive area, continuing to the muscularis mucosa of the adjacent normal mucosa, bundles of eosinophilic stromal cells were seen, and it was not easy to determine whether they were disarrayed muscularis mucosa remains or stromal cells simulating muscularis mucosa (Figure 1). When observed in detail, the bundles of eosinophilic stromal cells were not morphologically homogeneous but different in parts, namely, from those more similar to the smooth muscle cells of the muscularis mucosa to those composed of less eosinophilic spindle cells with plumper nuclei, showing a morphological transition from the former to the latter (Figure 2A and B).
Immunohistochemical examination of the cytoskeletal phenotype revealed that the former was more constantly positive for α-SMA, whilst the latter was positive for α-SMA less constantly or in a few cells (Figure 2C). The former was positive in a few cells or almost negative for h-CD and desmin, and the latter was negative for both (Figure 2D and E). The cytoskeletal feature of the muscularis mucosa was that of differentiated smooth muscle cells, namely, positive for α-SMA, desmin, and h-CD. Therefore, corresponding to the morphological transition, a continuous transition apart from the differentiated smooth muscle phenotype of the muscularis mucosa was revealed in the bundles of eosinophilic stromal cells. The presence of type I procollagen, both in protein level by immunohistochemistry and in mRNA level by in situ hybridization, was demonstrated in the less eosinophilic stromal cells with a lesser degree of differentiated smooth muscle phenotype, that is, less constantly positive for α-SMA and negative for desmin or h-CD (Figure 2F-H).
In our experience of routine histopathological diagnosis of early invasive colorectal adenocarcinomas, we commonly see bundles of eosinophilic stromal cells, which are confused with disarrayed muscularis mucosa remains around invasive carcinomatous glands. In the present case, an obvious transition between the muscularis mucosa and the bundles of eosinophilic stromal cells (both in morphology and in their cytoskeletal phenotype) was demonstrated, which we think conceivably suggests that the smooth muscle cells of the muscularis mucosa could convert to the eosinophilic stromal cells in the stroma of invasive colorectal adenocarcinomas. Moreover, considering their eosinophilic and α-SMA positive features, the eosinophilic stromal cells could be referred to as myofibroblasts or myofibroblastic cells, so we could paraphrase that the muscularis mucosa could convert to myofibroblasts or myofibroblastic cells, making it one of the sources of myofibroblasts in the stroma of invasive colorectal adenocarcinomas. This concept is supported by a ultrastructural morphological study that showed the smooth muscle cells of muscularis mucosae or muscularis propria as a possible source of myofibroblasts in the stroma of invasive colorectal adenocarcinomas[13].
The stromal change associated with carcinoma invasion has been called desmoplasia or desmoplastic reaction, which is characterized by modifications in the composition of stromal cells and ECM components, the latter being an excessive deposition of ECM components such as collagen, fibronectin, and proteoglycan, and type I collagen is one of the ECM components which deposits in desmoplastic tumor stroma[1-3]. In the present case, type I procollagen was demonstrated in both protein and mRNA expression in the areas of eosinophilic stromal cells that showed a transition from the muscularis mucosa and a lesser degree of differentiated smooth muscle phenotype, implying that the myofibroblastic cells converted from smooth muscle cells of the muscularis mucosa could be responsible for type I collagen production and play a role in the formation of desmoplastic stroma. This notion is supported by an in vitro study that showed the induction of proteoglycan production of smooth muscle cells derived from the colonic wall by colon carcinoma cells[14].
Two different sources of myofibroblasts or myofibroblastic cells have been proposed, i.e. bone marrow-derived circulating fibrocytes and local resident cells such as fibroblasts[8], the former being stressed recently[15]. However, the result of this case study seems to reemphasize roles of local resident cells in the desmoplastic reaction associated with carcinoma invasion. During the early invasive process of colorectal carcinomas, the muscularis mucosae may not be a passive barrier through which colorectal carcinomas infiltrate into the submucosa. They may, rather, play an active role in the formation and remodeling of tumor stroma and facilitate the invasive process of carcinoma cells.
Peer reviewer: Jennifer D Black, PhD, Professor and Member, Director of Cell Analysis, Department of Pharmacology and Therapeutics, Roswell Park Cancer Institute, Elm and Carlton Streets, Buffalo, NY 14263, United States
S- Editor Cheng JX L- Editor O’Neill M E- Editor Yin DH
| 1. | Noël A, Emonard H, Polette M, Birembaut P, Foidart JM. Role of matrix, fibroblasts and type IV collagenases in tumor progression and invasion. Pathol Res Pract. 1994;190:934-941. |
| 2. | Kunz-Schughart LA, Knuechel R. Tumor-associated fibroblasts (part I): Active stromal participants in tumor development and progression? Histol Histopathol. 2002;17:599-621. |
| 3. | Desmoulière A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol. 2004;48:509-517. |
| 4. | Schürch W, Seemayer TA, Hinz B, Gabbiani G. Myofibroblast. Histology for Pathologists 3rd edition. Philadelphia: Lippincott-Williams & Wilkins 2007; 124-164. |
| 5. | Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol. 1999;277:C1-C9. |
| 6. | Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807-1816. |
| 7. | Martin M, Pujuguet P, Martin F. Role of stromal myofibroblasts infiltrating colon cancer in tumor invasion. Pathol Res Pract. 1996;192:712-717. |
| 8. | De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123:2229-2238. |
| 9. | Rønnov-Jessen L, Petersen OW, Koteliansky VE, Bissell MJ. The origin of the myofibroblasts in breast cancer. Recapitulation of tumor environment in culture unravels diversity and implicates converted fibroblasts and recruited smooth muscle cells. J Clin Invest. 1995;95:859-873. |
| 10. | Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71-81. |
| 11. | Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556-7562. |
| 12. | Riaz Y, Cook HT, Wangoo A, Glenville B, Shaw RJ. Type 1 procollagen as a marker of severity of scarring after sternotomy: effects of topical corticosteroids. J Clin Pathol. 1994;47:892-899. |
| 13. | Ohtani H, Sasano N. Stromal cell changes in human colorectal adenomas and carcinomas. An ultrastructural study of fibroblasts, myofibroblasts, and smooth muscle cells. Virchows Arch A Pathol Anat Histopathol. 1983;401:209-222. |
| 14. | Iozzo RV, Sampson PM, Schmitt GK. Neoplastic modulation of extracellular matrix: stimulation of chondroitin sulfate proteoglycan and hyaluronic acid synthesis in co-cultures of human colon carcinoma and smooth muscle cells. J Cell Biochem. 1989;39:355-378. |
| 15. | Ishii G, Sangai T, Oda T, Aoyagi Y, Hasebe T, Kanomata N, Endoh Y, Okumura C, Okuhara Y, Magae J. Bone-marrow-derived myofibroblasts contribute to the cancer-induced stromal reaction. Biochem Biophys Res Commun. 2003;309:232-240. |